Published online Jul 21, 2016. doi: 10.3748/wjg.v22.i27.6287
Peer-review started: January 27, 2016
First decision: March 7, 2016
Revised: March 18, 2016
Accepted: March 30, 2016
Article in press: March 30, 2016
Published online: July 21, 2016
Processing time: 171 Days and 13.1 Hours
AIM: To identify suitable biomarkers of response to bevacizumab (BV) - it remains an open question. The measurement of serum vascular endothelial growth factor (VEGF) has been proposed as a predictive factor for this drug, even if literature data are contradictory.
METHODS: We prospectively evaluated the role of BV, total and not BV-bound VEGF and angiopoietin-2 (Ang-2) serum levels as potential predictive factors of response for BV in combination with an oxaliplatin-based chemotherapy. BV, Ang-2, total and not BV-bound VEGF levels were measured at baseline, before 2nd and 5th cycle of oxaliplatin-based chemotherapy in 20 consecutive metastatic colorectal cancer patients.
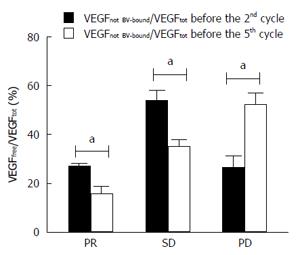
RESULTS: Results were correlated to response to treatment. Variability in BV levels have been found, with decreased level in less responding patients. In particular, the concentration of BV increased of 3.96 ± 0.69 folds in serum of responsive patients after 3 more cycles of therapy compared to those with stable or progressive disease with a 0.72 ± 0.25 and 2.10 ± 0.13 fold increase, respectively. The determination of free and total VEGF demonstrated that the ratio between the two values, evaluated immediately before the 2nd and the 5th cycle of therapy, decreased from 26.65% ± 1.33% to 15.50% ± 3.47% in responsive patients and from 53.41% ± 4.75 to 34.95% ± 2.88% in those with stable disease. Conversely, in those with progression of disease, the ratio showed the opposite behavior coming up from 25.99% ± 5.23% to 51.71% ± 5.28%. The Ang-2 levels did not show any relationship.
CONCLUSION: Our data show that the ratio of not BV-bound VEGF to total VEGF serum and BV plasma concentrations for predicting the response to BV plus oxaliplatin-based chemotherapy could be a promising biomarker of response to BV.
Core tip: In the main topic of the identification of possible reliable markers to predict response to antiangiogenic therapy, our paper represents an original contribution describing the role of not bevacizumab (BV)-bound vascular endothelial growth factor (VEGF)/total VEGF ratio and of bevacizumab serum level as predictors of response in a consecutive series of patients with metastatic colorectal cancer. In this paper the rediscovery of the predictive role of traditional biomarkers as not BV-bound VEGF/total VEGF plasma ratio together with the results of the bevacizumab pharmacokinetic in response, stable disease and progression settings of patients with metastatic colorectal cancer treated with bevacizumab plus oxaliplatin based chemotherapy supported our hypothesis.
- Citation: Azzariti A, Porcelli L, Brunetti O, Del Re M, Longo V, Nardulli P, Signorile M, Xu JM, Calabrese A, Quatrale AE, Maiello E, Lorusso V, Silvestris N. Total and not bevacizumab-bound vascular endothelial growth factor as potential predictive factors to bevacizumab-based chemotherapy in colorectal cancer. World J Gastroenterol 2016; 22(27): 6287-6295
- URL: https://www.wjgnet.com/1007-9327/full/v22/i27/6287.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i27.6287
Colorectal carcinoma (CRC) is the second leading cause of death from cancer in Europe and North America, with approximately one million new cases and half a million deaths per year worldwide[1]. In recent years treatment of metastatic disease has undergone a major evolution with the introduction of biologic drugs alone or in combination with chemotherapy regimen[2]. In particular, the addition of bevacizumab (BV), a humanized recombinant monoclonal antibody anti-vascular endothelial growth factor-A (VEGF-A), to standard chemotherapy regimens resulted associated to survival with an improvement in overall survival (OS) with respect to chemotherapy alone in patients with metastatic disease[3,4]. Nevertheless, differently from the anti-epidermal growth factor receptor monoclonal antibodies, for which the RAS state is a validated predictive biomarker[5,6], to date, there is no evidence of predictive markers of response to BV. Identification of predictive biomarkers would allow selection of patients most likely to benefit from treatment with BV, thereby avoiding toxicities. Several potential biomarkers have been studied and proposed as predictors of response to BV[7,8], among them the VEGF. VEGF is overexpressed in 40%-60% of CRC and is correlated with intratumoral vascular density[9]. Indeed, a metanalysis published by Des Guetz et al[10] reported the prognostic biomarker value of VEGF: the analysis of published studies relating intratumoural microvessel density (MVD) or VEGF expression, highlighted that high MVD and VEGF expression significantly predict poor progression free survival (PFS) and OS. However, pre-treatment tumor VEGF expression or circulating VEGF levels are not predictive of response to BV, but data obtained from a study that retrospectively analysed samples from two randomized phase III studies (HORIZON II and III), evaluating the prognostic and/or predictive value of VEGF signaling showed that high baseline VEGF levels were associated with worse outcomes for PFS and OS independent of treatment[11]. Anyway, these experimental findings that did not find any association between VEGF and response to treatment, could be related to a lack of differentiation between total VEGF (including BV-bound VEGF and not BV-bound VEGF) and not BV-bound VEGF. According to these data, a decrease of not BV-bound VEGF levels during treatment with BV has been observed using immunodepleted plasma samples[12]. On this regard, Loupakis et al[13] analysed plasma not BV-bound VEGF in metastatic CRC (mCRC) patients during BV treatment, after an immunodepletion procedure able to eliminate, among the other immunoglobulins, BV and BV-bound VEGF, suggesting that the anti-VEGF antibody significantly reduces not BV-bound and biologically active VEGF concentrations.
Angiopoietin-2 (Ang-2), an inhibitory ligand of the Tie-2 receptor, stored in the Weibel-Palade bodies of endothelial cells[14], has been described as an opponent of vascular normalization that prevents blood vessels from becoming structurally and functionally stabilized[15,16]. High levels of Ang-2 contrasting the normalization of tumor vessels mediated by BV may reduce the delivery of chemotherapeutic drugs into tumor tissues[17,18]. Moreover, BV has a high inter-individual variability in terms of pharmacokinetic, with a half-life ranging between 11 and 50 d. Blood concentrations of BV above and below the median have been reported to correlate with its toxicity and efficacy profile, respectively[19].
In the present study, we prospectively measured not BV-bound VEGF, Ang-2 and plasma BV levels of mCRC patients treated with BV in combination with oxaliplatin-based chemotherapy regimen, in order to find predictive biomarkers of response to BV-therapy.
Consecutive patients aged ≥ 18 years, with histologically confirmed mCRC, an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2, measurable disease, adequate hepatic and renal function, and no contraindications to BV therapy were included from December 2012 until January 2014. These patients received BV (5 mg/kg) and chemotherapy, consisting of FOLFOX-4 (leucovorin 200 mg/m2 per day as a 2-h infusion followed by bolus 5-fluorouracil 400 mg/m2 per day and a 22-h infusion of 5-fluorouracil 600 mg/m2 per day repeated for 2 consecutive days every 2 wk, with the addition of oxaliplatin 85 mg/m2 on day 1) or XELOX-2 (oxaliplatin 100 mg/m2 per day followed by oral capecitabine 1000 mg/m2 twice daily on days 1 through 7 of a 14-d cycle) as first or second line of treatment[20,21]. Response to treatment was evaluated between the 4th and the 5th cycle according to the Response Evaluation Criteria in Solid Tumors (RECIST) definition 1.1[22]. To exclude that the associated chemotherapeutic regimen could affect VEGF and Ang-2 levels, four patients in adjuvant treatment were included as a control arm. The study was approved by the Ethics Committee of the National Cancer Research Centre “Istituto Tumori Giovanni Paolo II” of Bari and informed consent was obtained from all patients enrolled in the study.
Venous blood was drawn at day 1 (baseline), and immediately before the 2nd and 5th cycle of chemotherapy. For control arm, venous blood was drawn only at the baseline and immediately before the 2nd cycle of therapy.
Plasma preparation: Whole blood was collected into commercially available EDTA-treated tubes and cells removed from plasma by centrifugation for 15 min at 2000 ×g at 4 °C, depleting also platelets. The plasma fractions were divided in aliquots, frozen and stored at -80 °C until assayed.
Serum preparation: After allowing the blood to clot by leaving it undisturbed at room temperature for 30 min, the clot was removed by centrifuging at 2000 ×g for 15 min at 4 °C. The serum fractions were divided in aliquots, frozen and stored at -80 °C until assayed.
Plasma VEGF, not BV-bound VEGF and Ang-2 detection: VEGF and Ang-2 plasma levels were measured by means of the ELISA Quantikine DVE00 and DANG 20 Kits (R&D Systems, Minneapolis, MN, United States), respectively. The optical density was determined using the multilabel plate reader Victor 3 (Perkin Elmer) set to 450 nm, with a wavelength correction set to 540 nm. To measure not BV-bound VEGF concentrations, plasma samples were immunodepleted as described by Loupakis et al[13]. Briefly, plasma samples were immunodepleted using Protein G-Sepharose 4 Fast Flow beads (Pharmacia Biotech, Uppsala, Sweden). Preliminarily, the beads were washed twice in PBS, then, these was reconstituted to 50% (v/v) protein G-sepharose in PBS. To deplete plasma samples of BV plus BV-bound VEGF, 100 μL of protein G slurry was added to 200 μL of plasma samples and incubated at 4 °C for 4 h. After centrifugation (2 min at 10000 rpm), 200 μL of plasma supernatants was removed and the immunodepletion was repeated by the addition of 100 μL of protein G slurry and overnight incubation at 4 °C. Each plasma sample was than assayed for human VEGF concentrations using the ELISA kit.
BV detection: The serum concentration of BV was measured with a home-made enzyme-linked immunosorbent assay (ELISA) kit[23]. Briefly, microwell plates (Immuno 96 Micro Cell solid plates; Nunc, Roskilde, Denmark) were coated with 100 μL/well recombinant human 1.0 μg/mL VEGF165 (R&D Systems, Minneapolis, MN)at a concentration of 1.0 μg/mL overnight at 4 °C. After three wash steps with PBS plus 0.05% Tween-20, the blocking of the wells was done with 3% BSA/PBS overnight at 4 °C (200 μL/well) to reduce non-specific binding. After five wash steps with PBS plus 0.05% Tween-20, 50 μL/well of each serum sample (diluted 1:1000 in PBS) and 50 μL/well of different concentrations of the standard were added to the plates. Incubation was overnight at 4 °C. A standard curve was prepared with BV ranging from 1ng/mL to 5000 ng/mL. The bound BV was made detected with 1 μg/mL of horseradish peroxidase-goat anti-human IgG (H + L) conjugate (Invitrogen Corporation, Carlsbad, CA) after an incubation of wells for 3 h at room temperature. After five wash steps with PBS plus 0.05% Tween-20, the substrate used was BM Blue POD substrate (Roche, United States) stopped with 1 mol/L HCl (100 μL). Absorbance was read at 450 nm on a multilabel plate reader Victor 3 (Perkin Elmer). In the plot, the BV serum accumulation is expressed as a ratio between drug concentration before the 5th cycle and before the 2nd cycle.
All samples determinations were performed in triplicate, and results have been expressed as the mean ± SD, unless otherwise indicated. Statistical differences in vitro data were assessed by the Student-Newman-Keuls test. P values lower than 0.05 were considered significant.
Twenty mCRC patients were evaluated for the changes of BV, not BV-bound VEGF, total VEGF and Ang-2 plasma concentrations in function of time of BV plus chemotherapy administration. Eleven patients were male and 9 were female with a median age of 64.2 years (range: 28-75 years). Thirteen patients had 3 or more metastatic sites and the majority received a first line therapy. Five of them had progression disease (PD), while 9 and 6 had stable disease (SD) and partial response (PR), respectively at first evaluation (Table 1).
| Number of patients (n = 20) | |
| Sex | |
| Male | 11/20 |
| Female | 9/20 |
| Age | |
| mean | 64.2 |
| range | 28-80 |
| Number of metastatic sites | |
| 1-2 | 7/20 |
| ≥ 3 | 13/20 |
| Tumor differentiation | |
| G1-G2 | 12/20 |
| G3 | 8/20 |
| Associated chemotherapy | |
| Folfox4 | 6/20 |
| Bi-weekly XELOX | 14/20 |
| Line of therapy | |
| First | 17/20 |
| Second | 3/20 |
| Response to treatment | |
| PR | 6/20 |
| SD | 9/20 |
| PD | 5/20 |
| Median number of cycles | 9 (range: 5-14) |
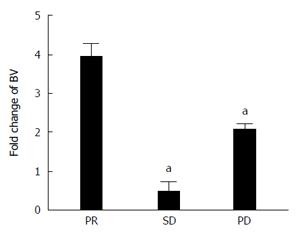
Assessment of BV serum levels showed that immediately before the 2nd cycle the level was quite similar between all patients, measuring 2.61 ± 1.10, 5.51 ± 5.28 and 2.33 ± 0.94 μg/mL in PR, SD and PD, respectively (Table 2). Conversely, the modification of the drug serum concentration before the 5th cycle, reported as the ratio between BV total amount, determined before the 5th and before the 2nd cycle, was different among patients with PR compared to the others (Figure 1 and Table 2). In particular, the first showed a 3.96 ± 0.69-fold increase in BV serum level after three more cycles of therapy compared to those with SD and PD with a 0.72 ± 0.25 and 2.10 ± 0.13-fold increase, respectively.
| Time of blood withdrawal | PR | SD | PD | |
| Before 2nd cycle | BV (μg/mL) | 2.61 ± 1.10 | 5.51 ± 5.28 | 2.33 ± 0.94 |
| Before 5th cycle | BV (μg/mL) | 10.34 ± 2.72 | 3.98 ± 3.14 | 4.89 ± 1.98 |
| Ratio BV (5th/2nd) | 3.96 ± 0.69 | 0.72 ± 0.25 | 2.10 ± 0.13 | |
| VEGF basal (pg/mL) | 376.39 ± 221.97 | 308.80 ± 177.76 | 395.98 ± 283.00 | |
| Before 2nd cycle | VEGF total (pg/mL) | 457.80 ± 46.84 | 310.18 ± 179.17 | 434.93 ± 12.90 |
| VEGF not BV-bound (pg/mL) | 122.01 ± 18.54 | 165.67 ± 74.61 | 113.03 ± 25.75 | |
| VEGF not BV-bound/VEGF total (%) | 26.65 ± 1.33 | 53.41 ± 4.75 | 25.99 ± 5.23 | |
| Before 5th cycle | VEGF total (pg/mL) | 694.97 ± 19.17 | 304.61 ± 186.16 | 300.55 ± 74.28 |
| VEGF not BV-bound (pg/mL) | 107.75 ± 27.08 | 106.46 ± 186.16 | 155.42 ± 31.68 | |
| VEGF not BV-bound/VEGF total (%) | 15.50 ± 3.47 | 34.95 ± 2.88 | 51.71 ± 5.28 |
A preliminary control step was the measurement of VEGF and Ang-2 levels in both immune depleted and not immunodepleted plasma from 4 CRC patients treated with adjuvant FOLFOX-4 regimen. These results allowed us to exclude that the associated chemotherapeutic regimens could affect VEGF and Ang-2 levels. Similar results, obtained in all pairs of samples, demonstrated that neither the immunodepletion procedure nor the chemotherapy affected the measurement of VEGF and of Ang-2 (Table 3).
| VEGF (pg/mL) | Ang-2 (pg/mL) | |||
| Total | After immunodepletion | Total | After immunodepletion | |
| Patient No. 1 | 200.24 | 205.62 | 242.10 | 270.48 |
| Patient No. 2 | 126.25 | 140.36 | 307.18 | 312.01 |
| Patient No. 3 | 188.75 | 182.50 | 662.65 | 733.45 |
| Patient No. 4 | 255.15 | 232.34 | 388.20 | 477.20 |
VEGF basal levels have been determined into plasma of all the patients. VEGF basal levels were 376.39 ± 221.97 pg/mL, 308.80 ± 177.76 pg/mL and 395.98 ± 283.00 pg/mL in PR, SD and PD respectively. The determination of VEGF as a total protein showed that its plasma levels are different in the population (Table 2) without a correlation with the response to therapy, in agreement with data reported in literature[24].
Moreover, assessment of VEGF as total and not BV-bound was carried out in plasma samples of each patient before the 2nd cycle of therapy and before the 5th cycle. Ratio of median value of VEGF not BV-bound /VEGF total was calculated for all patients. In particular, VEGF not BV-bound /VEGF total ratios determined before the 2nd cycle were 26.65 ± 1.33, 53.41 ± 4.75 and 25.99 ± 5.23 for PR, SD and PD, respectively. Interestingly, the VEGF not BV-bound /VEGF total ratios before the 5th cycle were 15.50 ± 3.47, 34.95 ± 2.88 and 51.71 ± 5.28% (Table 2 and Figure 2). The coefficient percentage decreasing is statistically significant in patients with PR and SD (P < 0.05), and its increasing is statistically significant in PD patients (P < 0.05). In particular, the ratio of not BV-bound VEGF to total VEGF before the 2nd cycle of therapy and before the 5th cycle decreased from 26.65% to 15.5% in PR group and from 53.41% to 34.95% in SD group. Conversely, in patients who showed a PD the ratio was higher than before the 2nd cycle, i.e., 51.71% vs 25.99% (Table 2 and Figure 2).
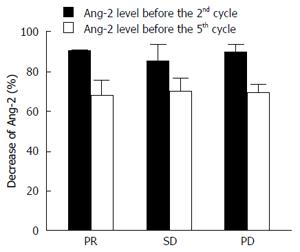
The analysis of Ang-2 levels conducted at the same time did not show any relation with either VEGF or therapy response. Only a slight, not statistically different reduction was found between the levels of Ang-2 before the 2nd and the 5th cycle of therapy, and no differences were evident among the three groups of patients (Figure 3).
To date, a number of different circulating biomarkers of response to antiangiogenic therapy have been investigated, including serum levels of pro-angiogenic factors such as VEGF, soluble VEGFR, collagen IV[7,8] and not pro-angiogenic factors such as LDH and fibrinogen[25], even if none have been validated for use in clinical practice. In particular, baseline concentration of serum VEGF (before BV treatment) has been proposed as a prognostic and/or predictive factor. However, no definitive results are available regarding VEGF modulation after BV therapy in CRC patients[24]. In our study, we observed that the plasma levels of not BV-bound and total VEGF varies much fold among patients, with high standard deviations. Therefore we tried to normalize all values by performing the ratio between not BV-bound VEGF and total VEGF. As showed in Table 2, standard deviations of this ratio expressed as percentage is lower than the absolute data. In particular, we saw that not BV-bound VEGF /total VEGF ratio significant decreased in patients with PR or SD during the treatment. At the same time, not BV-bound VEGF/total VEGF ratio in patients with PD before the 2nd cycle was similar to that of PR patients, but it strongly increased before the 5th cycle. In a comprehensive evaluation of total circulating VEGF-A in randomized phase III trials it was reported that short PFS and OS after BV treatment seemed to be associated with higher baseline circulating VEGF levels, even if the Authors excluded a predictive role for that angiogenic factor[26]. Changes in VEGF concentration related to treatment have also been investigated. Keskin et al[27] reported results of a preliminary evaluation of serum VEGF and basic fibroblast growth factor (bFGF) pre- and post-treatment levels as predictive of treatment response. In 33 mCRC patients treated with BV in combination with irinotecan-based chemotherapy, serum levels of VEGF and bFGF were higher than in healthy controls and pre-treatment low serum level of VEGF was associated with a significantly longer OS[27]. Interestingly, previous studies showed a paradoxical increase of VEGF during BV treatment, probably due to assays that do not discriminate between not BV-bound and bevacizumab-bound VEGF. Loupakis et al[13] reported that not BV-bound VEGF decreases after BV administration in 5 mCRC patients. Later, the same Authors confirmed that BV induce a prolonged and significant reduction of plasma active not BV-bound VEGF concentration in a larger cohort of CRC patients. Whereas, VEGF concentrations remained lower also at the time of PD suggesting other mechanisms of tumor resistance[13].
Brostjan et al[28] showed that the level of not BV-bound VEGF dropped significantly in 19 CRC patients treated with combination chemotherapy and BV in the neoadjuvant setting. In this study, VEGF level was measured in the plasma of all mCRC patients enrolled before starting therapy, and in agreement with results reported in literature no correlation was evident between the baseline amount of this factor and the response to the subsequent therapy regimen including BV[28-31]. Furthermore, the comparison of VEGF before and after chemotherapy plus BV also showed an absence of correlation between this molecule and the response to therapy, in agreement with data from Loupakis et al[13] study[27].
The amount of BV in serum was also determined using a previously designed immunoassay[23]. In agreement with a previous study analyzing BV pharmacokinetic in glioblastoma and breast cancer patients[19], we showed that BV concentration was markedly increased in patients which achieved a PR than patients with SD and PD. In particular, Nugue et al[19] demonstrate that low serum BV levels were associated with a PD, while high levels were associated with side effects. Comparison of median levels confirmed that the concentrations were significantly different in this groups (P > 0.05) and serum BV levels could to be used as clinical pharmacodynamic/predictive biomarker.
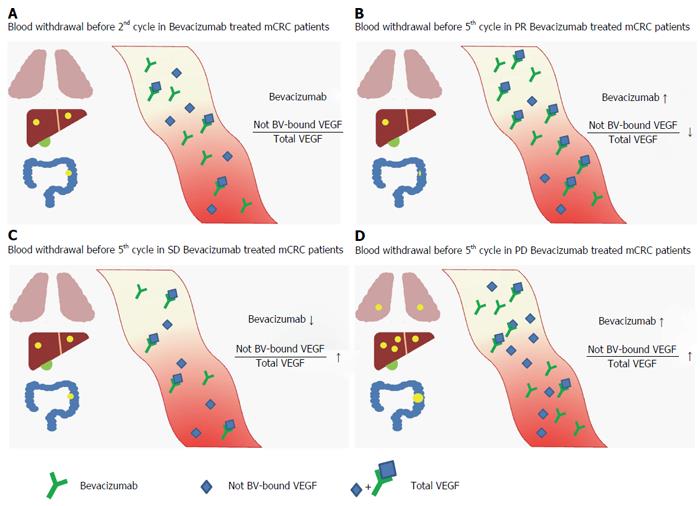
Interestingly in this study, serum levels of BV, significantly higher in PR compared to SD and PD patients, suggest that lower values favor inefficacy of this drug. However, fold increase level of the drug in patients with PD was inconsistent with the idea of positive correlation between drug levels and therapeutic efficacy. A possible explanation for this could lie in the metabolism of BV. Recently, Panoilia et al[32] analyzed the pharmacokinetics of BV and its relationship with VEGF in patients with mCRC receiving BV in combination with chemotherapy. They described a pharmacokinetic model that characterizes the in vivo interaction of BV with its soluble ligand, VEGF. Different levels of BV in patients were attributed to a possible role of VEGF polymorphisms. Although dosages of VEGF polymorphisms led no results in the kinetics alteration of BV, it would be interesting correlate different trends of BV serum in the different responses to treatment to the various polymorphisms of VEGF that could led to mechanisms of resistance to BV. In order to discover these mechanisms, we also analyzed the plasma level of Ang-2, hoping to highlight a correlation with clinical response and suggesting it as an additional predictive biomarker. Unfortunately, Ang-2 was not differently modulated in our samples in function of therapy response. Finally we have graphically represented the results of our study (Figure 4).
In conclusion, although basal VEGF level in the plasma of patients does not predict response to BV combined with chemotherapy, our data pointed out that the measurement of the ratio of not BV-bound VEGF to total VEGF could be relevant in monitoring the response to BV plus oxaliplatin-based chemotherapy. Moreover, this pilot study had the aim of finding a methodology which can standardize in all patients the response to anti-angiogenic therapy in function of VEGF and this ratio would appear to be very effective. An evaluation of these data in a larger series is advisable.
However, even if the high fold increased levels of BV in the serum of patients could play a role in determining the response to therapy, we have no pharmacokinetic data which emphasize the real predictive role of BV in mCRC. Hence, we suggest that both measurements of not BV-bound VEGF vs total VEGF and of BV serum levels should be taken into account while evaluating the clinical outcome of such patients.
We would like to thank Caroline Oakley for language revision of the manuscript.
To date, a number of different circulating biomarkers of response to antiangiogenic therapy have been investigated, including serum levels of pro-angiogenic factors such as vascular endothelial growth factor (VEGF), there is no evidence of predictive markers of response to bevacizumab (BV). Identification of predictive biomarkers would allow selection of patients most likely to benefit from treatment with BV, thereby avoiding toxicities. The identification of suitable biomarkers of response to BV is still an open question. The measurement of serum VEGF has been proposed as a predictive factor for this drug, even if literature data are contradictory.
Baseline concentration of serum VEGF (before BV treatment) has been proposed as a prognostic and/or predictive factor. However, no definitive results are available regarding VEGF modulation after BV therapy in CRC patients.
The authors observed that the plasma levels of not BV-bound and total VEGF varies much fold among patients, with high standard deviations. Therefore they tried to normalize all values by performing the ratio between not BV-bound VEGF and total VEGF, the rediscovery of the predictive role of traditional biomarkers as not BV-bound VEGF/total VEGF plasma ratio together with the results of the bevacizumab pharmacokinetic in response, stable disease and progression settings of patients with metastatic colorectal cancer treated with bevacizumab plus oxaliplatin based chemotherapy supported our hypothesis.
Although the study has been done in a small subset of patents treated with chemotherapy plus bevacizumab in first and second-line therapy and it’s exploratory it’s interesting because shows that patients with response to therapy are those with a higher decrease of free VEGF (not bound to bevacizumab). The study is well-written and designed.
P- Reviewer: Maurel J, Sameer AS, Zhang XC S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2486] [Article Influence: 248.6] [Reference Citation Analysis (0)] |
| 2. | Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015;33:1809-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 377] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 3. | Giampieri R, Scartozzi M, Del Prete M, Fulli A, Faloppi L, Bianconi M, Maccaroni E, Cascinu S. The “angiogenetic ladder”, step-wise angiogenesis inhibition in metastatic colorectal cancer. Cancer Treat Rev. 2014;40:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Fontanella C, Ongaro E, Bolzonello S, Guardascione M, Fasola G, Aprile G. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med. 2014;2:123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 101] [Reference Citation Analysis (0)] |
| 5. | Silvestris N, Tommasi S, Santini D, Russo A, Simone G, Petriella D, Maiello E, Tonini G, Colucci G. KRAS mutations and sensitivity to anti-EGFR monoclonal antibodies in metastatic colorectal carcinoma: an open issue. Expert Opin Biol Ther. 2009;9:565-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Silvestris N, Vincenzi B, Brunetti AE, Loupakis F, Dell’Aquila E, Russo A, Scartozzi M, Giampieri R, Cascinu S, Lorusso V. Pharmacogenomics of cetuximab in metastatic colorectal carcinoma. Pharmacogenomics. 2014;15:1701-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31:1219-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 8. | Jahangiri A, Aghi MK. Biomarkers predicting tumor response and evasion to anti-angiogenic therapy. Biochim Biophys Acta. 2012;1825:86-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Yao X, Ge J, Hu F, Zhao Y. Can vascular endothelial growth factor and microvessel density be used as prognostic biomarkers for colorectal cancer? A systematic review and meta-analysis. ScientificWorldJournal. 2014;2014:102736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823-1832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Jürgensmeier JM, Schmoll HJ, Robertson JD, Brooks L, Taboada M, Morgan SR, Wilson D, Hoff PM. Prognostic and predictive value of VEGF, sVEGFR-2 and CEA in mCRC studies comparing cediranib, bevacizumab and chemotherapy. Br J Cancer. 2013;108:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Gordon MS, Margolin K, Talpaz M, Sledge GW, Holmgren E, Benjamin R, Stalter S, Shak S, Adelman D. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843-850. [PubMed] |
| 13. | Loupakis F, Falcone A, Masi G, Fioravanti A, Kerbel RS, Del Tacca M, Bocci G. Vascular endothelial growth factor levels in immunodepleted plasma of cancer patients as a possible pharmacodynamic marker for bevacizumab activity. J Clin Oncol. 2007;25:1816-1818. [PubMed] |
| 14. | Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150-4156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 562] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 15. | Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 300] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Reiss Y, Knedla A, Tal AO, Schmidt MH, Jugold M, Kiessling F, Burger AM, Wolburg H, Deutsch U, Plate KH. Switching of vascular phenotypes within a murine breast cancer model induced by angiopoietin-2. J Pathol. 2009;217:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Goede V, Coutelle O, Neuneier J, Reinacher-Schick A, Schnell R, Koslowsky TC, Weihrauch MR, Cremer B, Kashkar H, Odenthal M. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br J Cancer. 2010;103:1407-1414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Kim S, Dobi E, Jary M, Monnien F, Curtit E, Nguyen T, Lakkis Z, Heyd B, Fratte S, Cléau D. Bifractionated CPT-11 with LV5FU2 infusion (FOLFIRI-3) in combination with bevacizumab: clinical outcomes in first-line metastatic colorectal cancers according to plasma angiopoietin-2 levels. BMC Cancer. 2013;13:611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Nugue G, Bidart M, Arlotto M, Mousseau M, Berger F, Pelletier L. Monitoring monoclonal antibody delivery in oncology: the example of bevacizumab. PLoS One. 2013;8:e72021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [PubMed] |
| 21. | Fedele P, Di Maggio G, Leo S, Nanni L, Giuliani F, Biglietto M, Lorusso V, Cinieri S, Colucci G, Maiello E. Bi-weekly administration of capecitabine oxaliplatin (XELOX-2) in first-line treatment of advanced colorectal cancer: a phase II study of the Gruppo Oncologico dell’Italia Meridionale (GOIM). Proc Am Soc Clin Oncol. 2009;27:15066 abstract. |
| 22. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21564] [Article Influence: 1347.8] [Reference Citation Analysis (1)] |
| 23. | Lin YS, Nguyen C, Mendoza JL, Escandon E, Fei D, Meng YG, Modi NB. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther. 1999;288:371-378. [PubMed] |
| 24. | Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, Adinin R, Overman MJ, Valero V, Wen S. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 377] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 25. | Silvestris N, Scartozzi M, Graziano G, Santini D, Lorusso V, Maiello E, Barni S, Cinieri S, Loupakis F, Pisconti S. Basal and bevacizumab-based therapy-induced changes of lactate dehydrogenases and fibrinogen levels and clinical outcome of previously untreated metastatic colorectal cancer patients: a multicentric retrospective analysis. Expert Opin Biol Ther. 2015;15:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Hegde PS, Jubb AM, Chen D, Li NF, Meng YG, Bernaards C, Elliott R, Scherer SJ, Chen DS. Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin Cancer Res. 2013;19:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 27. | Keskin M, Ustuner Z, Dincer M, Etiz D, Celik HE, Gulbas Z. Importance of serum VEGF and basic FGF levels in determining response to treatment and survival in patients with metastatic colorectal cancer. J Clin Oncol. 2012;30:e21050. |
| 28. | Brostjan C, Gebhardt K, Gruenberger B, Steinrueck V, Zommer H, Freudenthaler H, Roka S, Gruenberger T. Neoadjuvant treatment of colorectal cancer with bevacizumab: the perioperative angiogenic balance is sensitive to systemic thrombospondin-1 levels. Clin Cancer Res. 2008;14:2065-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2267] [Article Influence: 133.4] [Reference Citation Analysis (0)] |
| 30. | Giantonio BJ. Bevacizumab in the treatment of metastatic colorectal cancer (mCRC) in second- and third-line settings. Semin Oncol. 2006;33:S15-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539-1544. [PubMed] |
| 32. | Panoilia E, Schindler E, Samantas E, Aravantinos G, Kalofonos HP, Christodoulou C, Patrinos GP, Friberg LE, Sivolapenko G. A pharmacokinetic binding model for bevacizumab and VEGF165 in colorectal cancer patients. Cancer Chemother Pharmacol. 2015;75:791-803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |