Published online Jun 7, 2016. doi: 10.3748/wjg.v22.i21.5012
Peer-review started: January 19, 2016
First decision: February 18, 2016
Revised: March 4, 2016
Accepted: March 18, 2016
Article in press: March 18, 2016
Published online: June 7, 2016
Processing time: 132 Days and 18.8 Hours
AIM: To investigate the photodynamic effect of CdSe/ZnS quantum dots (QDs) on pancreatic cancer cells and elucidate the probable mechanisms.
METHODS: The pancreatic cancer cell line SW1990 was treated with different concentrations of CdSe/ZnS QDs (0, 0.5, 1.0, 1.5, 2.0, 2.5 μmol/L), with or without illumination. The viability of SW1990 cells was tested using the Cell Counting Kit-8 (CCK-8) assay. The ultrastructural changes of SW1990 cells were observed by transmission electron microscopy. Apoptosis was detected by nuclear staining and flow cytometry (FCM). Reactive oxygen species (ROS) were measured by dichlorofluorescein diacetate via fluorescence microscopy. Expression of Bax, Bcl-2 and caspase-3 was measured by real-time polymerase chain reaction (PCR) and protein immunoblotting 24 h after SW1990 cells were treated with CdSe/ZnS QDs and illuminated.
RESULTS: The CCK-8 assay results showed that both CdSe/ZnS QDs with and without illumination suppressed SW1990 cell proliferation. Cell viability was significantly lower when illuminated or with a longer incubation time and a higher light dose. CdSe/ZnS QDs with illumination caused ultrastructural changes in SW1990 cells, such as organelle degeneration and chromatin condensation and aggregation at the periphery of the nucleus. Fluorescence microscopy and FCM showed that CdSe/ZnS QDs (1.5 μmol/L) with illumination increased SW1990 cell apoptosis (53.2%) and ROS generation compared with no illumination. Real-time PCR showed that expression of Bax and caspase-3 was upregulated and Bcl-2 was downregulated. Immunoblotting results were consistent with real-time PCR results. Inhibition of ROS and apoptosis both attenuated QD-photodynamic-therapy-induced cell death.
CONCLUSION: CdSe/ZnS QDs can be used as a photosensitizer to inhibit SW1990 cell proliferation through ROS generation and apoptotic protein expression regulation.
Core tip: This study showed that quantum dots (QDs) may be a potential photosensitizer for photodynamic therapy (PDT) to treat pancreatic cancer by inhibiting SW1990 cell proliferation and inducing apoptosis through reactive oxygen species (ROS) generation. QD-PDT may induce apoptosis through ROS-, caspase-3-mediated apoptotic pathways, with upregulation of apoptosis signaling molecules such as Bax and downregulation of Bcl-2. These findings provide a new application for PDT in pancreatic cancer. However, more preclinical and clinical trials should be undertaken before further clinical application.
- Citation: He SJ, Cao J, Li YS, Yang JC, Zhou M, Qu CY, Zhang Y, Shen F, Chen Y, Li MM, Xu LM. CdSe/ZnS quantum dots induce photodynamic effects and cytotoxicity in pancreatic cancer cells. World J Gastroenterol 2016; 22(21): 5012-5022
- URL: https://www.wjgnet.com/1007-9327/full/v22/i21/5012.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i21.5012
Pancreatic cancer is a malignant neoplasm with a very poor prognosis. The 5-year survival is < 5% and medium survival is about 6 mo[1]. Surgical resection is the first-choice treatment for pancreatic cancer, however, 80% of patients may already have locally advanced or metastatic cancer when diagnosed, and only 10%-15% are eligible for surgery[2]. The majority of pancreatic cancer patients have to undergo radiotherapy or chemotherapy, although the survival rates of these nonsurgical patients are similar. Thus, there is an urgent need to identify a novel effective therapy.
Photodynamic therapy (PDT) is an innovative method that utilizes a photosensitizing agent or photosensitizer (PS) followed by light exposure to treat various diseases. Reactive oxygen species (ROS) are generated when PSs are activated by illumination and subsequently destroy cancer cells[3]. In PDT, only cells in contact with the PS, light and oxygen are affected, thus PDT is more selective than conventional chemotherapy and radiotherapy[4]. Most PSs are based on a tetrapyrrole structure. The first PS used clinically for cancer therapy was hematoporphyrin derivative, a water-soluble mixture of porphyrins. As hematoporphyrin derivative is purified from porfimer sodium, it has some disadvantages, such as instability in aqueous solution, long-lasting skin photosensitivity, and weak absorption at the therapeutic wavelength of 630 nm[5]. 5-aminolevulinic acid is a second-generation PS. It is a biosynthetic precursor of protoporphyrin IX that needs to be converted to protoporphyrin as an active PS[6]. However, its skin photosensitivity is still an unresolved problem[7]. Therefore, it is necessary to develop new PSs to confer survival benefits with fewer side effects.
Quantum dots (QDs) are colloidal semiconductors and mainly composed of group II-VI or group III-V elements[8]. QDs are of interest to many researchers due to their unique optical properties. QDs possess several characteristics such as large absorption spectra, narrow and symmetric emission bands, and a high molar extinction coefficient, which make them superior to conventional PSs in PDT[9,10]. Recently, many studies have shown the potential applications of QDs for PDT. With illumination, the QD conduction-band electron can be transferred to surrounding O2 and produce ROS, thus making QDs a potential PS for PDT[11,12].
In this study, we prepared water-dispersible CdSe/ZnS QDs with an extensive absorption in the UV-visible region and a strong emission peaking at 560 nm. We investigated the photodynamic effects of CdSe/ZnS QDs on pancreatic cancer cells, and analyzed the possible molecular mechanism involved in this procedure.
Nanocrystals with a CdSe core and ZnS shell were synthesized by Professor Zhang et al at the Department of Materials Science and Engineering, Shanghai University. Trioctylphosphine oxide, CdO and tetradecylphosphonic acid were heated to 180 °C under argon, exsiccated and exhausted under a vacuum. When the reaction temperature reached 330 °C, selenium precursor solution was added to trioctylphosphine and mixed together until the temperature decreased to 240 °C. ZnS stock solution was added along with dimethyl zinc solution and vigorously stirred until the molar ratio of Cd/Se: Zn/S reached 1:4. The mixture was cooled to room temperature and settled with anhydrous methanol, centrifuged (4000 rpm) and washed three times with anhydrous methanol to remove residua such as trioctylphosphine oxide and unreacted reagents. The precipitate was suspended in phosphate-buffered saline (PBS). The morphology of QDs was observed using a Morgagni 268(D) transmission electron microscope (FEI, Hillsboro, OR, United States). The UV-Vis spectra of the QDs suspensions were scanned within the wavelength range of 200-800 nm at 22 °C and automatically corrected for the suspension, using an Avantes UV-Vis spectrophotometer (Apeldoorn, The Netherlands).
The human pancreatic cancer cell line SW1990 was purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). The SW1990 cells were cultured in RPMI 1640 media with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin, in humidified air containing 5% CO2 at 37 °C. Cells in the exponential growth phase were used in the following experiments.
The cytotoxicity induced by QDs and PDT was determined using the Cell Counting Kit-8 (CCK-8) assay. The cells were seeded on a 96-well plate at 8 × 103 cells/well and incubated overnight before QDs were added. The cells were divided into two groups (A with illumination and B without illumination). The cells in each group were treated with different concentrations of QDs (0, 0.5, 1.0, 1.5, 2.0, 2.5 μmol/L) for 1 h. After incubation, all cells were washed with PBS to remove excess QDs and fresh media were added. Cells in Group A were irradiated using ZF-20D Ultraviolet Analyzing Equipment at a wavelength of 365 nm and power of 19 mW cm-2, and then incubated in RPMI 1640 medium with 10% FBS for a further 24 h at 37 °C in a humidified 5% CO2 atmosphere. However, the medium in Group B was removed and replaced with RPMI 1640 medium with 10% FBS, and then the cells were incubated in humidified air containing 5% CO2 at 37 °C for a further 24 h. After 24 h incubation, CCK-8 dye (Dojindo Laboratories, Kamimashiki-gun, Kumamoto, Japan) was added to each well, and the 96-well plates were put into a constant temperature incubator (37 °C) for 1 h. The absorbance of the solution was measured at 450 nm using an ELISA reader (Thermo Fisher Scientific, MA, United States). Cell viability was calculated as a percentage of the treated samples relative to untreated controls.
Transmission electron microscopy (TEM) was used to investigate the intracellular localization and subcellular structural targets of QDs in SW1990 cells. The cells were seeded on six-well plates at 4 × 105 cells/well and cultured overnight. Twenty-four hours later, after treatment [A: normal SW1990 cells; B: CdSe/ZnS QDs (1.5 μmol/L, 3 h) and illumination; C: CdSe/ZnS QDs (2 μmol/L, 3 h) and illumination (20 J/cm2)], the cells were collected and washed three times with cold PBS, pelleted using centrifugation (1000 rpm), and fixed in 2.5% glutaraldehyde for 2 h. Cell pellets were washed in PBS, postfixed with 1% osmium tetroxide, and dehydrated with an ascending series of alcohols. The specimens were cut into ultrathin sections (50-70 nm), placed onto copper grids, and stained with uranyl acetate and lead citrate for ultrastructural analysis using a JEM-1011EX transmission electron microscope (Jeol, Tokyo, Japan).
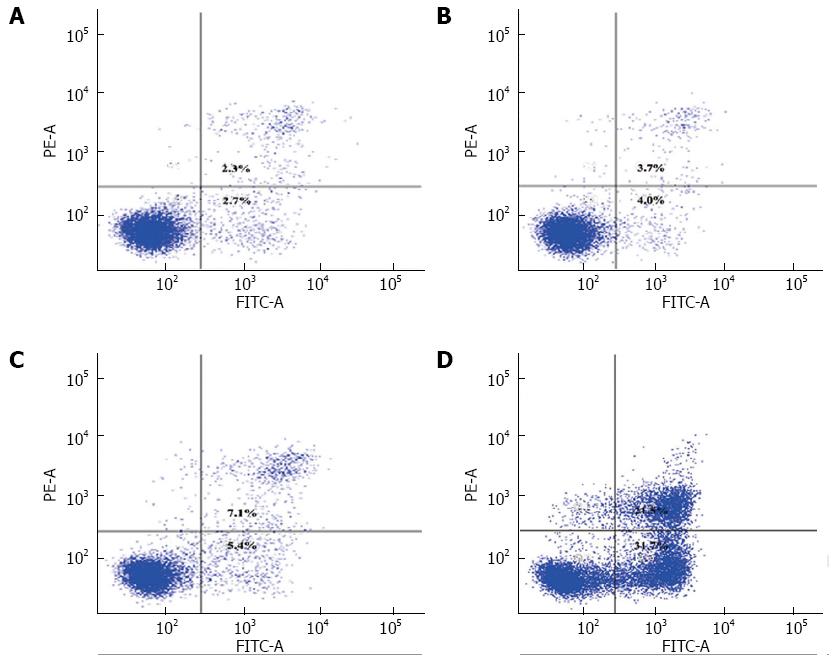
The Annexin V-FITC Apoptosis Detection Kit (BD Pharmingen, San Jose, CA, United States) was used to detect QD-induced apoptosis of SW1990 cells. Cells (2 × 105) were seeded in six-well plates and allowed to adhere overnight. The cells were treated [A: normal SW1990 cells; B: SW1990 cells with illumination (20 J/cm2); C: SW1990 cells treated with CdSe/ZnS QDs (1.5 μmol/L, 3 h); D: SW1990 cells treated with CdSe/ZnS QDs (1.5 μmol/L, 3 h) with illumination (20 J/cm2)], collected and washed twice with cold PBS. The cell pellets were resuspended in binding buffer, and incubated with staining solution [annexin V/propidium iodide (PI) = 1:1] in the dark for 15 min at room temperature. Fluorescence-activated cell sorting (FACS) analysis was performed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, United States).
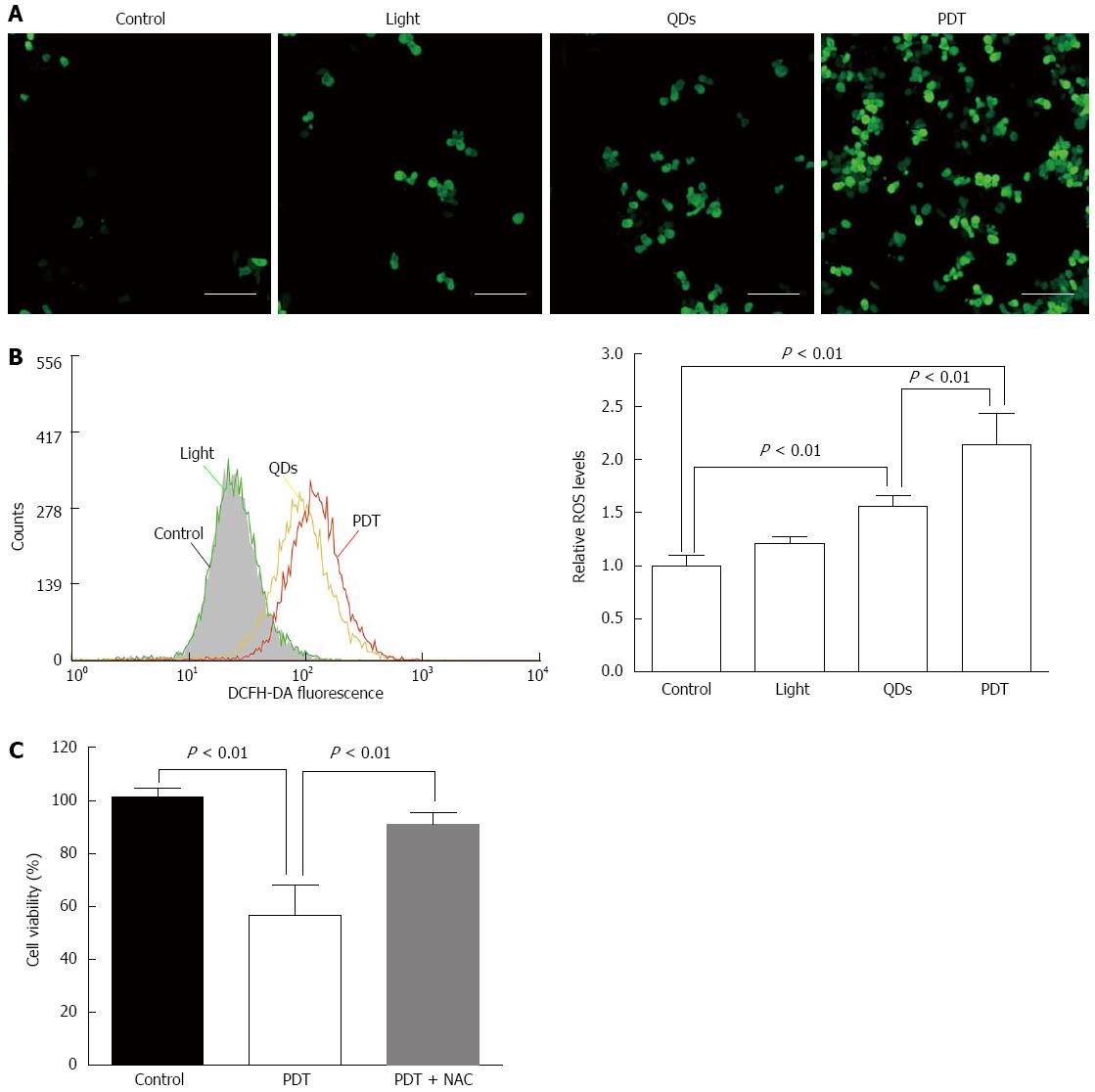
SW1990 cells were seeded on six-well plates at 4 × 105 cells/well and exposed to QDs as for flow cytometry (FCM) after the cells adhered. After 24 h, the cells were rinsed with cold PBS and stained with 2’,7’-dichlorofluorescin diacetate (H2DCFDA; Sigma-Aldrich, St. Louis, MO, United States) diluted in serum-free medium. After incubation for 30 min at 37 °C in the dark, the cells were washed with serum-free medium three times and resuspended in cold PBS. The DCF fluorescence was observed by a fluorescence microscope (BD Biosciences) and the fluorescence intensity was measured by FCM (BD Biosciences, San Jose, CA, United States). To investigate the role of ROS production in the cytotoxicity of QD-PDT, SW1990 cells were preincubated with 5 mmol/L N-acetylcysteine (NAC) (Sigma-Aldrich), a ROS scavenger, for 1 h before treatment. Cell viability was evaluated by the CCK-8 assay.
Real-time RT-PCR was performed using 7500/7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, United States) to analyze the apoptosis-related mRNA expression of QD-PDT treated SW1990 cells, such as Bax, Bcl-2 and caspase-3. The primers used in the real-time RT-PCR assay were Bax forward (5’-GGAAGAAGATGGGCTGAGG-3’); Bax reverse (5’-TGTCCCGAAGGAGGTTTATT-3’); Bcl-2 forward (5’-CCGGATCACCATCTGAAGAG-3’); Bcl-2 reverse (5’-AGGGCAAAGAAATGCAAGTG-3’); Caspase-3 forward (5’-AGATGGTTTGAGCCTGAGCA-3’); Caspase-3 reverse (5’-CAGTGCGTATGGAGAAATGG-3’); and GAPDH forward (5′-TGCACCACCAACTGCTTAG-3′); GAPDH reverse (5′-GGATGCAGGGATGATGTTC-3′).
Proteins were resolved by SDS-PAGE and blotted onto polyvinylidene difluoride membranes. Anti-Bcl-2 (dilution 1:1000; Cell Signaling Technology, Danvers, MA, United States;), anti-Bax (dilution 1:1000; Cell Signaling Technology), anti-caspase 3 (dilution 1:1000; Cell Signaling Technology), anti-cleaved caspase-3 (dilution 1:1000; Cell Signaling Technology) and anti-β-actin (dilution 1:1000; Cell Signaling Technology) antibodies were used to detect their corresponding proteins followed by anti-rabbit or anti-mouse IgG secondary antibodies (dilution 1:1000; Cell Signaling Technology). Image acquisition was performed with the ChemiDoc XRS+ system (Bio-Rad, Hercules, CA, United States). The optical densities of the protein bands were measured by GS710 Densitometer and analyzed with Quantity One image analysis software (Bio-Rad Laboratories).
All experiments were performed in triplicate. The results were expressed as mean ± SD and analyzed by the Student’s t test with SPSS version 13.0 (SPSS Inc., Chicago, IL, United States). Comparisons among multiple groups of data were analyzed by one-way analysis of variance. P < 0.05 was considered statistically significant.
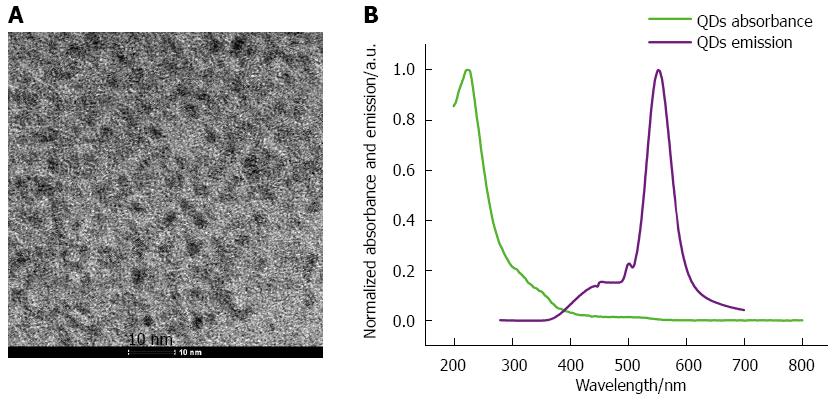
QDs were synthesized as previously described. The TEM results showed that QDs were spherical particles with an average size of 5 nm. The peaks of QDs in the UV-Vis analysis showed that the absorbance of QDs was the highest in the UV part of the spectrum, and decreased exponentially when approaching higher wavelengths. The photoluminescence spectra demonstrated that QDs have highest luminescence in the visible part of the spectrum, especially at 560 nm (Figure 1).
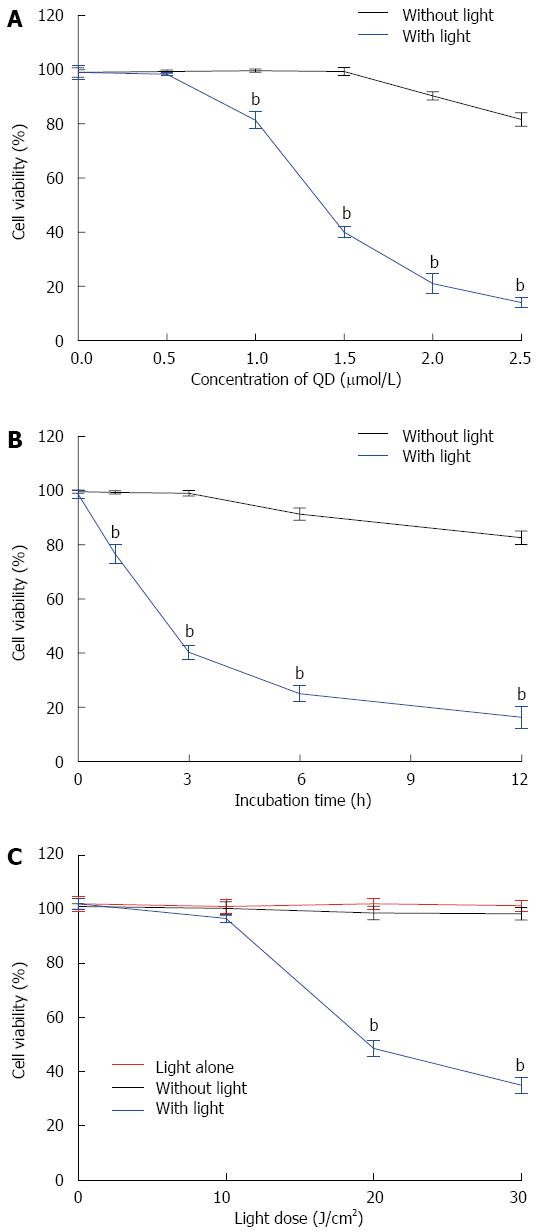
The CCK-8 assay was used to examine the viability of SW1990 cells after different treatments. Cell viability was decreased when the concentration of QDs increased (Figure 2A). Longer incubation time led to lower viability (Figure 2B). Cell viability showed a greater reduction with illumination (Figure 2C). QDs with illumination induced more cytotoxicity in SW1990 cells than QDs alone. More cell damage occurred when the light dose was higher. Illumination alone (10, 20 and 30 J/cm2) without QDs had limited effects on SW1990 cells (Figure 2C).
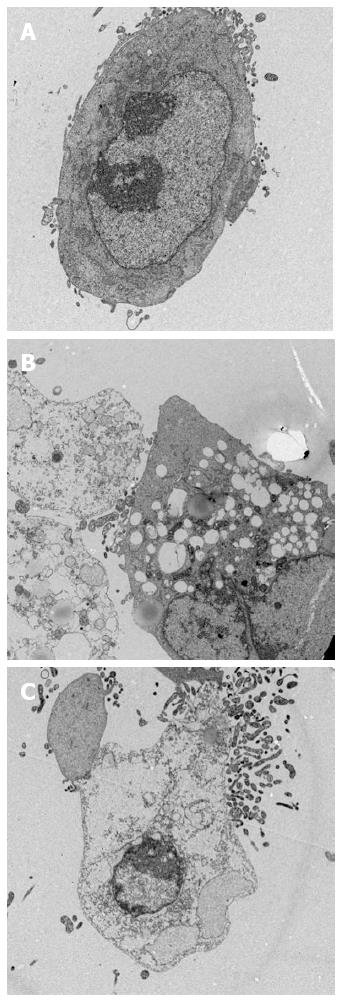
The QD-PDT-induced subcellular damage of SW1990 cells was detected by TEM (Figure 3). Under normal conditions, SW1990 cells had a round shape and well-structured mitochondria in the cytoplasm. The cell nucleus was round or class round in the middle of cytoplasm. Nevertheless, after treatment with QDs and illumination, SW1990 cells were significantly damaged. Vacuoles and irregularly sized mitochondria appeared. Organelle degeneration, and chromatin condensation and aggregation at the periphery of the nucleus were observed (Figure 3B and C). The main difference between treatment with 1.5 and 2 μmol/L was the percentage of apoptotic and dead cells, thus the latter induced more cell death.
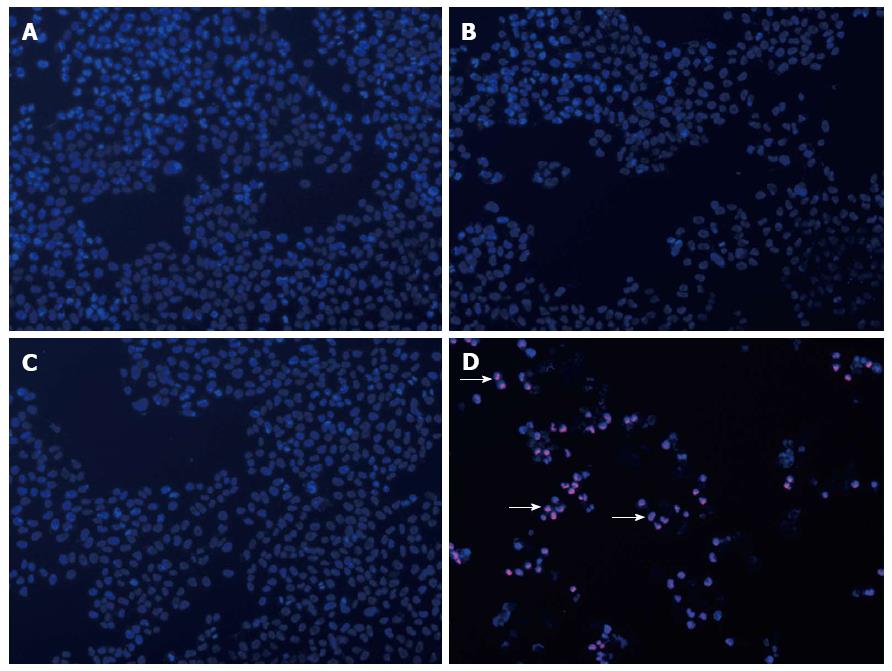
The percentage of apoptotic and necrotic cells was analyzed by fluorescence microscopy and FCM. SW1990 cells were stained with PI and Hoechst 33342. There were more apoptotic bodies in Group D and several cells were even stained with red fluorescence (Figure 4). FCM indicated that the percentage of apoptotic cells was higher in Group D, however, the percentage of necrotic cells remained at a low level (Figure 5).
ROS generation was determined by DCF fluorescence in SW1990 cells. The intracellular ROS content in SW1990 cells treated with light (20 J/cm2) was increased. However, ROS formation significantly increased in SW1990 cells with QDs (1.5 μmol/L, 3 h) and PDT (1.5 μmol/L, 3 h, 20 J/cm2), especially in cells with PDT (Figure 6A and B). These results indicated that illumination enhanced ROS generation in SW1990 cells treated with QDs.
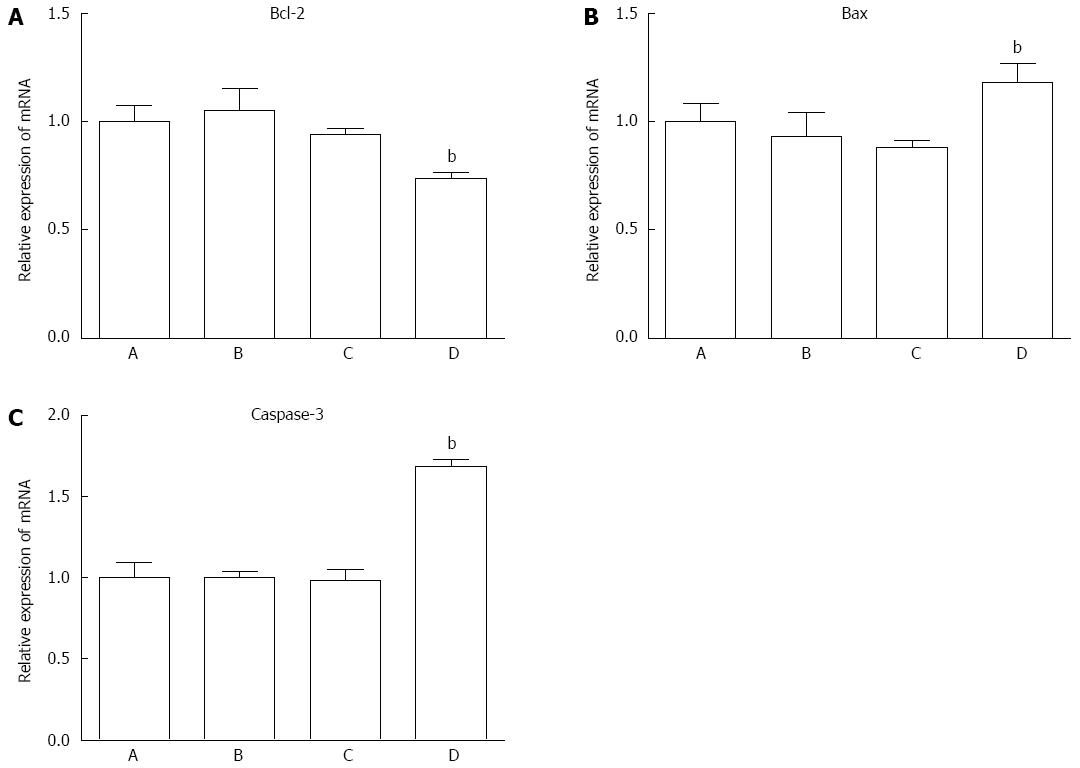
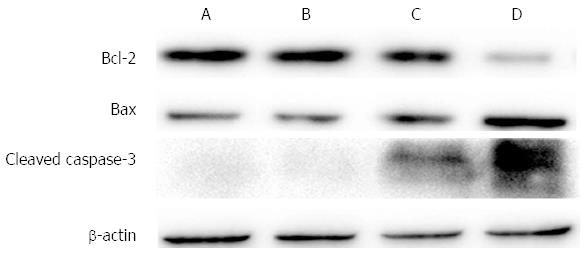
The mRNA expression level of Bax, Bcl-2 and caspase-3 was measured by RT-PCR. The expression level of each gene was normalized to GAPDH. The mRNA expression level of Bax and caspase-3 increased significantly as compared to control cells, while the level of Bcl-2 decreased (Figures 7 and 8). The protein expression level of these three genes was consistent with corresponding mRNA expression.
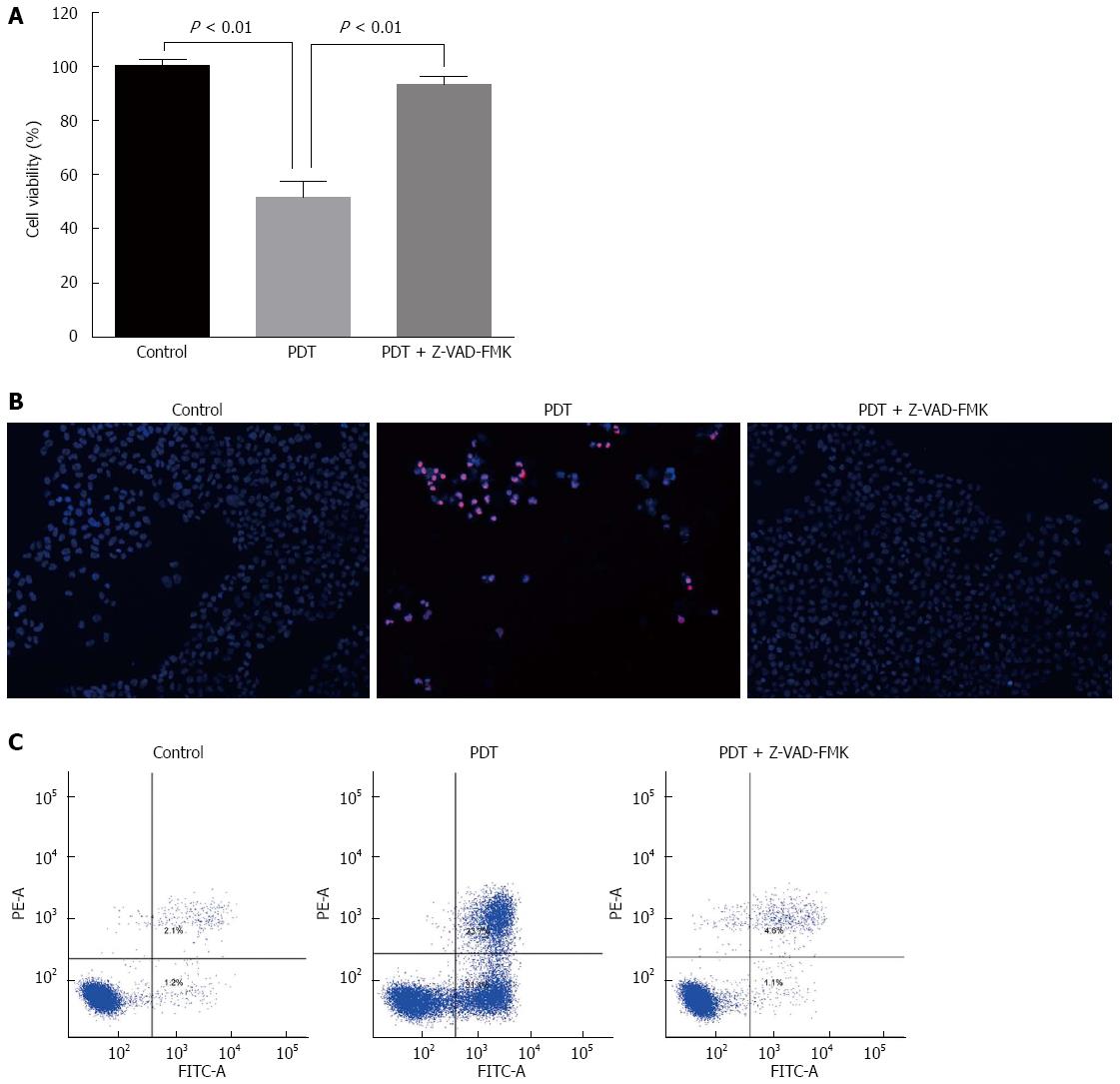
Pretreatment with an antioxidant (NAC), markedly restored cell viability of SW1990 cells after QD-PDT treatment (Figure 6C), which verified the role of ROS in QD-PDT-induced cytotoxicity. To demonstrate the role of apoptosis in QD-PDT, the pan-caspase inhibitor Z-VAD-FMK was added to the cell culture 1 h before treatment. Inhibition of caspase activation by Z-VAD-FMK abrogated QD-PDT-induced cell death (Figure 9).
PDT has been widely used clinically to treat a wide range of malignant cancers, such as esophageal and skin cancer. PDT consisted of two parts: administration of a PS and exposure to light to activate the agent[13,14]. In this study, we synthesized QDs with a CdSe core and ZnS shell and demonstrated the possible QD-induced PDT effects on pancreatic cancer cells.
Selection of an appropriate light wavelength was important. Blue light resulted in inefficient tissue penetration, unlike red and infrared radiation. The range of 600-1200 nm was considered the optical window for tissue penetration. However, only light < 800 nm could generate 1O2, and light > 800 nm could not provide sufficient energy to initiate photosensitization[15]. Thus, there is no ideal single light source for all PDT reactions, even with the same PS. In this study, we selected 365 nm as our illumination wavelength, which happened to be the appropriate excitation wavelength for CdSe/ZnS QDs.
It is reported that cadmium induces ROS generation and triggers apoptosis via a caspase-dependent pathway[16,17]. Recently, some studies have shown that CdSe-core QDs induce cell death by releasing free Cd2+ from the CdSe lattice, and this effect could be impeded by the addition of a coating such as ZnS[18]. Here, we synthesized water-soluble CdSe/ZnS QDs. A ZnS coating made the QDs more biocompatible with cells. However, it effectively reduced ROS generation. QDs are generally used as bioimaging probes for tracing and immunostaining cells[19,20]. In this study, QDs were used as photosensitizers in PDT of the pancreatic cancer cell line SW1990. QDs with ZnS coating showed less cytotoxicity in the dark, even when incubated with cells for 12 h at a concentration of 1.5 μmol/L. However, when irradiated by UV, the cytotoxicity of QDs was apparent. Cell viability was decreased when the concentration of QDs increased and incubation time with QDs and light dose increased, which was similar to other studies[21]. TEM, fluorescence microscopy, and FCM illustrated the ability of QDs to generate PDT.
During PDT, ROS generation increased, as reported by Waterhouse et al[22], who suggested that the mitochondrion was a vital organelle in programmed cell death and could be mediated by many regulatory factors of apoptosis. To determine whether ROS were increased in QD-induced PDT, we used a probe to detect intracellular ROS variation. Surprisingly, even when coated with ZnS, QDs still generated ROS after illumination, which was statistically significant compared with the control, light and QDs groups. Inhibition of ROS generation with NAC attenuated the cytotoxicity of QD-induced PDT of pancreatic cancer cells, thus ROS were important in this procedure.
To investigate the molecular mechanism of QD-induced PDT, we chose three representative proteins (Bcl-2, Bax and caspase-3) to identify their connection with QD-induced PDT. In this study, we observed apoptosis during QD-induced PDT. Apoptosis has been widely studied and is believed to be triggered by several signals, including a series of proteins[23,24]. The Bcl-2 family of proteins constitutes a central checkpoint[25]. Bax and Bcl-2 are two members of the Bcl-2 family and function as regulatory proteins[26,27]. In this study, we found that QDs increased Bax expression and decreased Bcl-2 expression at the mRNA and protein levels. Several studies have clearly defined Bax as a proapoptotic protein and Bcl-2 as an antiapoptotic protein[28]. In our study, Group D (cells with PDT) showed higher expression of Bax and lower expression of Bcl-2, which to some degree explained the greater apoptosis and necrosis in this group. These results were consistent with other studies[29,30]. Caspase-3 is a member of the cysteine-aspartic acid protease family. As an executioner, caspase-3 is practically inactive until it is cleaved by an initiator caspase when apoptotic signaling events occur. Caspase-3 can be activated in apoptotic cells through extrinsic or intrinsic pathways[31-34]. In this study, cleaved caspase-3 was observed after cells were treated with QDs and illumination. To confirm that apoptosis was involved in the QD-induced PDT effects on pancreatic cancer cells, Z-VAD-FMK was used to restore cell survival and indeed promote cell survival. These results indicated that Bcl-2, Bax and caspase-3 participated in the process of QD-PDT-induced apoptosis. Specifically, QD-PDT downregulated Bcl-2, upregulated Bax, and facilitated caspase-3 cleavage, thus promoting the killing of pancreatic cancer cells.
In summary, this study showed that QDs could be potential PSs for PDT to treat pancreatic cancer by inhibiting SW1990 cell proliferation and inducing apoptosis through ROS generation. QD-PDT may induce apoptosis through ROS-, caspase-3-mediated apoptotic pathways, with upregulation of apoptosis signaling molecules such as Bax and downregulation of Bcl-2. These findings provide a new application for PDT in pancreatic cancer. However, more preclinical and clinical trials should be undertaken before further clinical application.
Pancreatic cancer is one of the most malignant tumors and has a poor prognosis. Conventional treatments such as surgery, chemotherapy or radiotherapy are still ineffective. Thus, new therapies and drugs are required.
Photodynamic therapy (PDT) has been used as adjuvant therapy in a wide range of malignant cancers, such as esophageal and skin cancer, with good curative effects. In PDT, only cells in contact with the photosensitizer (PS), light and oxygen are affected, which make it superior to other adjuvant therapies.
Quantum dots (QDs) have large absorption spectra, narrow and symmetric emission bands, and a high molar extinction coefficient, which make them superior to conventional PSs in PDT. In this study, the authors synthesized QDs with a CdSe core and a ZnS shell and demonstrated the inhibitory effect of QD-induced PDT on pancreatic cancer cells. This effect may be due to ROS generation, caspase-3 cleavage and some apoptotic molecular regulation.
In the present study, QD-induced PDT showed cytotoxicity to pancreatic cancer cells. This reveals a new potential therapeutic strategy for pancreatic cancer.
PDT is an innovative treatment that utilizes a photosensitizing agent followed by light exposure to treat certain diseases.
Authors investigated photodynamic effect of CdSe/ZnS QDs on pancreatic SW1990 cancer cells, and concluded that it could be used as a photosensitizer inhibiting SW1990 cells proliferation and apoptotic protein expression regulation. The study is interesting, with convincing results and conclusions.
P- Reviewer: Lee HC, Sperti C S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer. Gut. 2013;62:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 2. | Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1764] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 3. | Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4767] [Cited by in RCA: 4710] [Article Influence: 214.1] [Reference Citation Analysis (0)] |
| 4. | Morris RL, Azizuddin K, Lam M, Berlin J, Nieminen AL, Kenney ME, Samia AC, Burda C, Oleinick NL. Fluorescence resonance energy transfer reveals a binding site of a photosensitizer for photodynamic therapy. Cancer Res. 2003;63:5194-5197. [PubMed] |
| 5. | Saczko J, Skrzypek W, Chwiłkowska A, Choromańska A, Poła A, Gamian A, Kulbacka J. Photo-oxidative action in cervix carcinoma cells induced by HPD - mediated photodynamic therapy. Exp Oncol. 2009;31:195-199. [PubMed] |
| 6. | Mohammadpour H, Majidzadeh-A K. Antitumor effect of conditioned media derived from murine MSCs and 5-aminolevulinic acid (5-ALA) mediated photodynamic therapy in breast cancer in vitro. Photodiagnosis Photodyn Ther. 2015;12:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Choi KH, Chung CW, Kim CH, Kim do H, Jeong YI, Kang DH. Effect of 5-aminolevulinic acid-encapsulate liposomes on photodynamic therapy in human cholangiocarcinoma cells. J Nanosci Nanotechnol. 2014;14:5628-5632. [PubMed] |
| 8. | Weaver J, Zakeri R, Aouadi S, Kohli P. Synthesis and characterization of quantum dot-polymer composites. J Mater Chem. 2009;19:3198-3206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | José-Yacamán M, Gutiérrez-Wing C, Santiago P, Ascencio JA, Camacho A. Synthesis and characterization of quantum dot superlattices. Microsc Microanal. 2002;8:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Liu J, Feng G, Liu R, Tomczak N, Ma L, Gurzadyan GG, Liu B. Bright quantum-dot-sized single-chain conjugated polyelectrolyte nanoparticles: synthesis, characterization and application for specific extracellular labeling and imaging. Small. 2014;10:3110-3118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Anas A, Akita H, Harashima H, Itoh T, Ishikawa M, Biju V. Photosensitized breakage and damage of DNA by CdSe-ZnS quantum dots. J Phys Chem B. 2008;112:10005-10011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Samia AC, Chen X, Burda C. Semiconductor quantum dots for photodynamic therapy. J Am Chem Soc. 2003;125:15736-15737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 492] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 13. | Chen J, Keltner L, Christophersen J, Zheng F, Krouse M, Singhal A, Wang SS. New technology for deep light distribution in tissue for phototherapy. Cancer J. 2002;8:154-163. [PubMed] |
| 14. | Kawczyk-Krupka A, Bugaj AM, Latos W, Zaremba K, Wawrzyniec K, Sieroń A. Photodynamic therapy in colorectal cancer treatment: the state of the art in clinical trials. Photodiagnosis Photodyn Ther. 2015;12:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Juzeniene A, Nielsen KP, Moan J. Biophysical aspects of photodynamic therapy. J Environ Pathol Toxicol Oncol. 2006;25:7-28. [PubMed] |
| 16. | Oh SH, Lim SC. A rapid and transient ROS generation by cadmium triggers apoptosis via caspase-dependent pathway in HepG2 cells and this is inhibited through N-acetylcysteine-mediated catalase upregulation. Toxicol Appl Pharmacol. 2006;212:212-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 17. | Hu KH, Li WX, Sun MY, Zhang SB, Fan CX, Wu Q, Zhu W, Xu X. Cadmium Induced Apoptosis in MG63 Cells by Increasing ROS, Activation of p38 MAPK and Inhibition of ERK 1/2 Pathways. Cell Physiol Biochem. 2015;36:642-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Ipe BI, Lehnig M, Niemeyer CM. On the generation of free radical species from quantum dots. Small. 2005;1:706-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Kairdolf BA, Smith AM, Stokes TH, Wang MD, Young AN, Nie S. Semiconductor quantum dots for bioimaging and biodiagnostic applications. Annu Rev Anal Chem (Palo Alto Calif). 2013;6:143-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 377] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 20. | Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6698] [Cited by in RCA: 4848] [Article Influence: 242.4] [Reference Citation Analysis (0)] |
| 21. | Ismail AF, Ali MM, Ismail LF. Photodynamic therapy mediated antiproliferative activity of some metal-doped ZnO nanoparticles in human liver adenocarcinoma HepG2 cells under UV irradiation. J Photochem Photobiol B. 2014;138:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Waterhouse NJ, Goldstein JC, Kluck RM, Newmeyer DD, Green DR. The (Holey) study of mitochondria in apoptosis. Methods Cell Biol. 2001;66:365-391. [PubMed] |
| 23. | Wang K. Molecular mechanisms of hepatic apoptosis regulated by nuclear factors. Cell Signal. 2015;27:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Flusberg DA, Sorger PK. Surviving apoptosis: life-death signaling in single cells. Trends Cell Biol. 2015;25:446-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Zhao J, Li X, Zou M, He J, Han Y, Wu D, Yang H, Wu J. miR-135a inhibition protects A549 cells from LPS-induced apoptosis by targeting Bcl-2. Biochem Biophys Res Commun. 2014;452:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Barrasa JI, Santiago-Gómez A, Olmo N, Lizarbe MA, Turnay J. Resistance to butyrate impairs bile acid-induced apoptosis in human colon adenocarcinoma cells via up-regulation of Bcl-2 and inactivation of Bax. Biochim Biophys Acta. 2012;1823:2201-2209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Ouyang YB, Lu Y, Yue S, Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012;12:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 28. | Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1103] [Cited by in RCA: 1117] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 29. | Srivastava M, Ahmad N, Gupta S, Mukhtar H. Involvement of Bcl-2 and Bax in photodynamic therapy-mediated apoptosis. Antisense Bcl-2 oligonucleotide sensitizes RIF 1 cells to photodynamic therapy apoptosis. J Biol Chem. 2001;276:15481-15488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Xue LY, Chiu SM, Oleinick NL. Photochemical destruction of the Bcl-2 oncoprotein during photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene. 2001;20:3420-3427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Allison RR, Moghissi K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin Endosc. 2013;46:24-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 432] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 32. | Park HJ, Kim YJ, Leem K, Park SJ, Seo JC, Kim HK, Chung JH. Coptis japonica root extract induces apoptosis through caspase3 activation in SNU-668 human gastric cancer cells. Phytother Res. 2005;19:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Yan F, He Q, Hu X, Li W, Wei K, Li L, Zhong Y, Ding X, Xiang S, Zhang J. Direct regulation of caspase-3 by the transcription factor AP-2α is involved in aspirin-induced apoptosis in MDA-MB-453 breast cancer cells. Mol Med Rep. 2013;7:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Yang LQ, Fang DC, Wang RQ, Yang SM. Effect of NF-kappaB, survivin, Bcl-2 and Caspase3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand. World J Gastroenterol. 2004;10:22-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |