Published online Jan 14, 2016. doi: 10.3748/wjg.v22.i2.471
Peer-review started: May 12, 2015
First decision: September 11, 2015
Revised: October 5, 2015
Accepted: November 9, 2015
Article in press: November 9, 2015
Published online: January 14, 2016
Processing time: 241 Days and 9.4 Hours
Advanced gastric cancer (AGC) is associated with a high mortality rate and, despite multiple new chemotherapy options, the survival rates of patients with AGC remains poor. After the discovery of targeted therapies, research has focused on the new treatment options for AGC. In the last two decades, many targeted molecules were developed against AGC. Currently, two targeted therapy molecules have been approved for patients with AGC. In 2010, trastuzumab was the first molecule shown to improve survival in patients with HER2-positive AGC as part of a first-line combination regimen. In 2014, ramucirumab was the second targeted molecule to improve survival rates and was suggested as treatment for patients with AGC who had progressed after first-line platinum plus fluoropyrimidine with or without anthracycline chemotherapy. Ramucirumab was the first targeted therapy acting as a single agent in patients with advanced gastroesophageal cancers. Although these two molecules were introduced into clinical use, many other promising molecules have been tested in phase I-II trials. It is obvious that in the near future many different targeted therapies will be in use for treatment of AGC. In this review, the current status of targeted therapies in the treatment of AGC and gastroesophageal junction tumors, including HER (2-3) inhibitors, epidermal growth factor receptor inhibitors, tyrosine kinase inhibitors, antiangiogenic agents, c-MET inhibitors, mammalian target of rapamycin inhibitors, agents against other molecular pathways fibroblast growth factor, Claudins, insulin-like growth factor, heat shock proteins, and immunotherapy, will be discussed.
Core tip: Trastuzumab was the first molecule shown to prolong both progression-free survival and overall survival in patients with advanced gastric cancer (AGC) when added to first-line chemotherapy in patients with AGC. In 2014, ramucirumab was approved as a single agent or in combination with paclitaxel for the treatment of patients with AGC. Many phase II-III clinical trials failed to showed activity of different targeted agents in patients with AGC. On the other hand, some molecules have shown promising activity in phase II trials and are expected to be in use in the coming years. Pertuzumab and c-Met pathway inhibitors have shown modest activity in a phase II trial. The results of two important ongoing phase III trials (JACOB and RILOMET-1) may change the recommendations for first-line treatment options in patients with AGC.
- Citation: Yazici O, Sendur MAN, Ozdemir N, Aksoy S. Targeted therapies in gastric cancer and future perspectives. World J Gastroenterol 2016; 22(2): 471-489
- URL: https://www.wjgnet.com/1007-9327/full/v22/i2/471.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i2.471
Gastric cancer is the second leading cause of cancer-related mortality worldwide, and its incidence is still increasing in developing countries[1]. Surgery is the curative treatment in the early stages of gastric cancer. However, the majority of patients with gastric cancer are diagnosed in the advanced stage in which curative treatment approaches are not possible[2]. In patients with advanced disease, chemotherapy improves the overall survival (OS) and progression-free survival (PFS) rates compared with best supportive care[3]. New chemotherapeutic agents have improved the response rates compared with previous chemotherapy regimens[4]. However, despite these new chemotherapy regimens, the 5-year survival rates of patients with advanced gastric cancer (AGC) are still only 20%-30%. Therefore, in recent years, research has focused on new treatment approaches for gastric cancer. In order to develop new molecules for gastric cancer treatment, it is very important to define the molecular pathways involved in the carcinogenesis of gastric cancer. A better understanding of molecular pathways that lead to cell growth, angiogenesis, and inhibition of apoptosis, will lead to new ideas for novel targeted therapies. In the last two decades, different molecules targeting different pathways were developed for the treatment of gastric cancer. In 2010, trastuzumab was the first molecule demonstrated to improve OS and PFS in patients with HER2-positive AGC[5]. Recently, ramucirumab was the second targeted molecule suggested for the treatment of AGC[6]. In addition to these two molecules, new targets and targeted therapies have been investigated. It is apparent that in the near future many different targeted therapies will be in use for the treatment of AGC. Accordingly, this review will focus on the current status of the available targeted therapies, and the therapies under investigation for the treatment of AGC.
Epidermal growth factor receptor (EGFR) is a cluster of receptors, including human EGFR (HER)-1, HER2, HER3 and HER4. EGFR is located on the outside of cellular membranes and is activated by binding of specific ligands. Stimulation of EGFR leads to cell proliferation and differentiation[7]. The ligand binding to the extracellular part of the receptor, homodimerization and heterodimerization occurs, resulting in phosphorylation of intracellular tyrosine kinase (TK), and phosphorylated TK activation of intracellular protein kinases[8]. All these activated intracellular pathways result in cell cycle progression, apoptosis, proliferation, angiogenesis, and metastasis[9]. EGFR overexpression was detected in 30%-50% of patients with gastric cancers, and was associated with poor prognosis and shorter OS[10]. Molecules which block different parts of the EGFR signaling cascade have been tested in clinical trials. These molecules can be divided into two groups, namely monoclonal antibodies that block EGFR, and TK inhibitors.
Anti-HER 2 monoclonal antibodies: HER2 is a transmembrane TK receptor with the potential to heterodimerize with HER1, HER3, or HER4. In HER2-positive tumors, the dimerization of HER2 with HER3 is an important process initiating oncogenic transformation[11]. Overexpression of HER2 is a marker of poor prognosis, and is associated with a high relapse rate in patients with breast cancer[12]. However, in gastric cancer patients, the prognostic value of amplified HER2 is controversial. A small study reported that in gastric cancer, HER2 overexpression or amplification significantly improved prognosis[13]. On the other hand, a systematic data analysis of the literature suggested a potential role for HER2 as a negative prognostic factor[14].
Trastuzumab is a monoclonal antibody against the HER2 extracellular domain. In the randomized phase III ToGA trial in patients with AGC, the addition of trastuzumab to first-line chemotherapy improved OS and PFS compared with chemotherapy alone[5]. In the ToGA trial, 3665 patients with AGC were screened for their HER2 amplification status using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). Of them, 594 had a +3 staining score on IHC or were FISH-positive (HER2:CEP17 ratio ≥ 2). These patients were randomly assigned to receive chemotherapy plus trastuzumab or chemotherapy alone. The patients received capecitabine combined with cisplatin or cisplatin combined with fluorouracil as a chemotherapy regimen. The proportion of HER2 positivity was 22.1%. Median OS was 13.8 mo (95%CI: 12-16) in patients receiving trastuzumab and chemotherapy compared to 11.1 mo (95%CI: 10-13) in patients receiving chemotherapy alone, and the difference was statistically significant (HR = 0.74; 95%CI: 0.60-0.91, P = 0.0046). In a post hoc analysis of the ToGA trial, the OS of patients with high HER2 expression (IHC2+ and FISH positive or IHC3+; n = 446) who received trastuzumab was 16·0 mo (95%CI: 15-19) compared with 11.8 mo (95%CI: 10-13) in patients receiving chemotherapy alone (HR = 0.65; 95%CI: 0.51-0.83, P = 0.036). Median PFS was also significantly improved in the trastuzumab plus chemotherapy arm compared with chemotherapy alone (median PFS: 6.7 mo vs 5.5 mo, HR = 0.71; 95%CI: 0.59-0.85, P = 0.0002). All grades of adverse events and serious adverse events (grade 3 or 4) were similar between the two groups. It was previously noted that trastuzumab might cause significant cardiac toxicity. However, in this trial, cardiac toxicity was rare and rates of cardiac events were similar between the trastuzumab plus chemotherapy and chemotherapy alone groups [17 (6%) vs 18 (6%)]. After the impressive results of the ToGA trial, trastuzumab in combination with cisplatin and a fluoropyrimidine (fluorouracil or capecitabine) was suggested as category 1 first-line therapy in patients with HER2 overexpressed AGC (National Comprehensive Cancer Network, European Society of Medical Oncology Guidelines). In 2010, the Food and Drug Administration, and European Medicine Agency approved trastuzumab in combination with chemotherapy for use in HER2-overexpressed AGC patients.
In a study presented at the American Society of Clinical Oncology (ASCO) Meeting 2013, trastzumab-naive patients with AGC were treated with trastzumab in combination with paxlitaxel. Forty six patients were enrolled and received paclitaxel (80 mg/m2 on days 1, 8, and, 15 q4w) plus trastuzumab (8 mg/kg initial dose, followed by 6 mg/kg, every 3 wk). The overall response rate (ORR) was 37.2% (95%CI: 23.0%-53.3%). Median PFS was 5.2 mo (95%CI: 3.9-6.6). The combination of trastuzumab with paxlitaxel as second-line therapy showed efficacy in AGC patients[15].
In the phase II NEOHX study, perioperative chemotherapy treatment with trastuzumab in combination with capecitabine and oxaliplatin was evaluated in patients with HER2-positive resectable gastric cancer. This combination regimen was administered as 3 cycles in the preoperative and postoperative period. Thirty six patients were enrolled. Three patiens had a pathological complete response (8.3%; 95%CI: 2%-22%). The disease-free survival at 18 and 24 mo was 71% (95%CI: 53%-83%) and 60%, respectively. Perioperative trastuzumab plus capecitabin/oxaliplatin showed promising efficacy[16] (Tables 1 and 2).
| Target | Agents | Name of trial and setting | Phase | Number of patients | PFS (mo) | P value | OS (mo) | P value | Results |
| HER-2 | Fluoropyrimide/cisplain + Trastuzumab vs Fluoropyrimide/cisplain | ToGA, First line | III | 594 | 6.7 vs 5·5 | < 0.001 | 13.8 vs 11.1 | 0.004 | PFS and OS was improved |
| HER-2 | Lapatinib plus once-per-week paclitaxel vs paclitaxel | TYTAN, Second line | III | 261 | 5.4 vs 4.4 | 0.850 | 11.0 vs 8.9 | 0.104 | No effect on PFS and OS |
| HER-2 | Cap/Ox plus lapatinib or Cap/Ox plus placebo | LOGiC, First line | III | 545 | 6.0 vs 5.4 | 0.100 | 12.2 vs 10.5 | 0.350 | No effect on PFS and OS |
| EGFR | Cisplatin and capecitabine + cetuximab vs cisplatin and capecitabine | EXPAND, First line | III | 904 | 4.4 vs 5.6 | 0.320 | 9·4 vs 10.7 | 0.950 | No effect on PFS and OS |
| EGFR | mEOC plus panitumumab vs EOC | REAL, First line | III | 553 | 6.0 vs 7.4 | 0.068 | 8·8 vs 11.3 | 0.013 | No effect on PFS and OS |
| VEGF | Bevacizumab plus cisp/cape/fluorouracil vs plc plus cisplatin/capecitabine/fluorouracil | AVAGAST, First line | III | 774 | 6.7 vs 5.3 | 0.003 | 12.1 vs 10.1 | 0.100 | PFS was improved, No effect on OS |
| VEGF | Ramucirumab vs placebo | REGARD, Second line | III | 355 | 2.1 vs 1.3 | < 0.001 | 5.2 vs 3.8 | 0.047 | PFS and OS was improved |
| VEGF | Ramucirumab plus paclitaxel vs placebo plus paclitaxel | RAINBOW, Second line | III | 665 | 4.4 vs 2.9 | < 0.001 | 9.6 vs 7·4 | 0.017 | PFS and OS was improved |
| VEGF | Apatinib 850 mg vs apatinib 425 vs placebo | NN, Third line | II | 144 | 3.67 vs 3.20 vs 1.40 | < 0.001 | 4.83 vs 4.27 vs 2.50 | < 0.001 | PFS and OS was improved |
| MET | Rilotumumab 15 mg/kg per rilotumumab 7.5 mg/kg, or placebo plus ECX | NN, First line | II | 121 | 5.1 vs 6.8 vs 4.2 | 0.016 | 9.7 vs 11.1 vs 8.9 | 0.100 | PFS and OS was improved |
| m-TOR | Everolimus 10 mg vs placebo | GRANITE, ≥ Second line | III | 656 | 1.7 vs 1.4 | 0.780 | 5.4 vs 4.3 | 0.124 | No effect on PFS and OS |
| Target | Agents | Name of Trial and Setting | Phase | Number of patients | PFS (mo) | OS (mo) | Results |
| HER-2 | Lapatinib | S0413, First line | II | 47 | 1.9 | 4.8 | Positive |
| HER-2 | Lapatinib plus ECF or ECX vs placebo plus ECF or ECX | EORTC 40071, First line | II | 28 | 7.1 vs 5.9 | 13.8 vs 10.1 | Negative1 |
| HER-2 | MK-2206 (AKT inhibitor) | S1005, Second line | II | 70 | 1.8 | 5.1 | Negative |
| Pan-HER | Saracatinib (Src inhibitor) | NN, ≥ Second line | II | 21 | 1.8 | 7.8 | Negative |
| Pan-HER | Dacomitinib | NN, ≥ Second line | II | 27 | 2.1 | 7.1 | Positive |
| EGFR | Cetuximab plus mFOLFOX6 | NN, First line | II | 40 | 5.5 | 9.9 | Positive |
| EGFR | Cetuximab plus FOLFIRI | NN, First line | II | 49 | 9.0 | 16.5 | Positive |
| EGFR | Panitumumab with dose dense DCF | NN, First line | II | 52 | 4.8 | 9.4 | Positive |
| VEGF | Bevacizumab plus irinotecan and cisplatin | NN, First line | II | 47 | 8.3 | 12.3 | Positive |
| VEGF | Bevacizumab plus docetaxel/oxaliplatin | NN, First line | II | 38 | 6.6 | 11.1 | Positive |
| m-TOR | Everolimus 10 mg | NN, ≥ Second line | II | 53 | 2.7 | 10.1 | Positive |
In 2011, Begnami and colleagues explored the gene and protein expression of the HER family in 221 patients with GC and analyzed the correlation between clinicopathological parameters. This report showed that HER2 and HER3 overexpression was associated with poor prognosis[17]. In a HER2-positive human gastric cancer xenograft Mouse models, pertuzumab in combination with trastuzumab was showed significant anti-tumor activity compared to each mono therapy[18]. Pertuzumab is a recombinant humanized monoclonal antibody that binds to the extracellular dimerization domain of HER2 and prevents heterodimerization of HER2 with the EGFR, HER3, and HER4[19]. In a phase III trial, it was demonstrated that pertuzumab in combination with trastuzumab improved OS in patients with advanced breast cancer[20]. In a phase II trial, pertuzumab and trastuzumab in combination with chemotherapy produced a partial response in 85% after 6 cycles of therapy in patients with AGC[21]. At the ASCO Meeting, the schema of ongoing randomized phase III trial of pertuzumab and trastzuzumab in combination with chemotherapy as first-line therapy in patients with advanced gastric or gastroesophageal junction cancer was presented. In this study, HER2 amplified AGC or advanced gastroesophageal cancer patients were randomized 1:1 to arm A: pertuzumab + trastuzumab + cisplatin + fluoropyrimidine or arm B: placebo + trastuzumab + cisplatin + fluoropyrimidine. The primary end point of this study is OS, and 780 patients will be enrolled from 33 countries. The results of this study are awaited by clinicians[22].
In gastric cell lines, binding of trastuzumab emtansine (T-DM1) to the cell surface HER2 was significantly increased by pertuzumab. Concomitant administration of peruzumab with T-DM-1 blocks HER3 and downstream TK pathways. The combination of two anti-HER enhanced their activity and this may be a promising anti-tumor combination against HER2-positive gastric cancer[23].
T-DM1 is a HER2 targeted antibody drug conjugate, composed of trastuzumab conjugated with a potent cytotoxic agent DM1 (derivative of maytansine). T-DM1 was designed to increase the efficacy of trastuzumab. T-DM1 binds to the extracellular domain of HER2 and is internalized into the tumor cell, then the antimicrotubule agent emtasine is relaesed[24]. The efficacy of T-DM1 was demonstrated in HER2-positive metastatic breast cancer patients[25]. In Her2-positive xenograft mouse models, T-DM1 in combination with pertuzumab showed significant antitumor activity compared with single agent therapies[26].
In a phase I trial, TD-1 in combination with capecitabine was tested in patients with advanced gastric or breast cancer. Of the six patients who received capecitabine 750 mg/m2 and T-DM1 3.6 mg/kg every 3 wk, four had parital and one had stable disease[27].
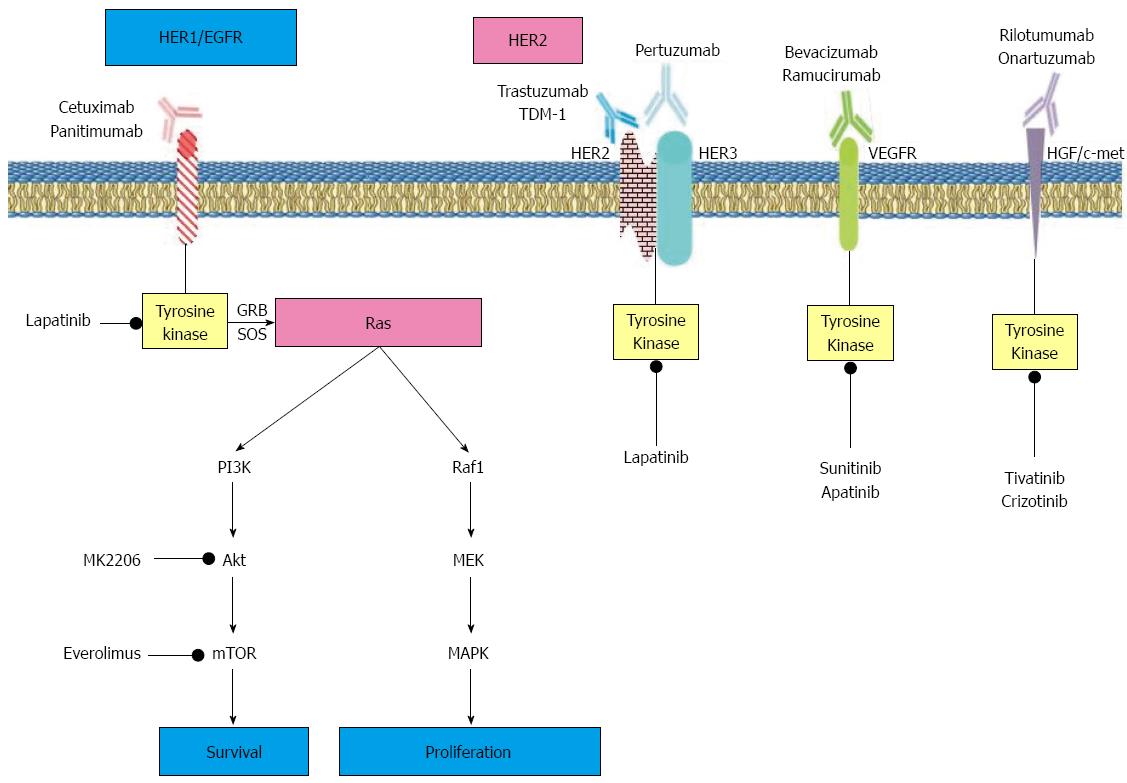
An ongoing phase II/III study (NCT01641939) will evaluate the efficacy of T-DM1 in patients with HER2-overexpressed AGC. In this study, T-DM1 will be compared with taxane chemotherapy alone. The patients will be randomized into three different groups as arm a: 3.6 mg/kg T-DM1 every 3 wk; arm b: 2.4 mg/kg T-DM1 every week; and arm c: standard taxane therapy (paclitaxel 80 mg/m2 per week or docetaxel 75 mg/m2 q3wk). The primary outcomes of the study are effective dose of T-DM1, and OS. The planned final data collection date is December 2016[28] (Figure 1).
Anti-HER3 monoclonal antibody: HER 3 is normally expressed in various tissues including gastrointestinal, urinary, respiratory, and reproductive tracts[29]. In tumor tissues the overexpression of HER3 is frequently accompanied by overexpression of EGFR and/or HER2[30]. HER3 has clinical significance in patients with gastric cancer. In a preclinical study evaluating the HER3 status of surgical specimens of 191 patients with gastric cancer, HER 3 positivity was associated with poor OS (P = 0.035)[17].
In HER2-positive tumors, Her2 hetorodimerizes with HER3 and promotes tumor progression[11]. In gastric cancer, HER3 overexpression was associated with poor prognosis and unfavorable survival[31]. In a phase I trial, LJM716 (fully human anti-HER3 monoclonal antibody) in combination with trastuzumab was evaluated in patients with HER2-positive metastatic breast or gastric cancer who had progressed on previous HER2-directed therapy. Of 30 evaluable patients, a partial response and stable disease was obtained in 2 and 12 (40%) patients with advanced breast or gastric cancer. The most frequent treatment-related adverse events (all grades) were diarrhea (91%), nausea (29%), fatigue (23%), and chills (20%). LJM716 is the first anti-HER3 monoclonal antibody reported to demonstrate clinical efficacy in combination with trastuzumab in trastuzumab-resistant patients[32].
MM-111 is a bispecific antibody targeting HER3, and forms a trimeric complex with HER2 and HER3. In a phase 1 trial, MM-111 in combination with five different chemotherapy regimens was tested in 86 patients with advanced cancer (11 bladder, 46 breast, 15 gastroesophageal, 14 other cancers). A partial response and stable disease were observed in 19 and 28 patients, respectively[33]. In an ongoing randomized phase II trial in patients with advanced gastric or esophageal cancer, MM-111 combined with paxlitaxel and trastuzumab is being evaluated as second-line therapy[34].
Anti-EGFR/HER1 monoclonal antibodies: Cetuximab is a chimeric monoclonal recombinant antibody binding to the extracellular domain of EGFR/HER1[35]. In a phase II trial, cetuximab was combined with the mFOLFOX6 regimen as first-line therapy in patients with metastatic gastric cancer. Patients received cetuximab 400 mg/m2 at week 1 and 250 mg/m2 weekly thereafter until disease progression and biweekly mFOLFOX6 (oxaliplatin 100 mg/m2, leucoverin 100 mg/m2, bolus fluorouracil followed by 46-h infusion of 2400 mg/m2) regimen. In this study, response rate was 50.0% (95%CI: 34.1%-65.9%) and median OS was 9.9 mo[36]. In another phase II trial, cetuximab was combined with irinotecan/leucoverin and fluorouracil as first-line treatment in patients with AGC. The response rate was 46% and disease control rate was 79%. The authors reported that the median PFS was 9.0 mo (95%CI: 7.1-15.6) and OS was 16.5 mo (95%CI: 11.7-30.1)[37]. In 2010, another phase II trial from Germany evaluated the efficacy of weekly cetuximab in combination with oxaliplatin 50 mg/m2, 5-FU 2000 mg/m2, and folinic acid 200 mg/m2 on days 1, 8, 15, and 22, in patients with metastatic gastric cancer. In this trial, median PFS was 7.6 mo (95%CI: 5.0-10.1 mo) and median OS was 9.5 mo (95%CI: 7.9-11.1 mo) with a reported response rate of 65% (95%CI: 50%-79%)[38]. One more phase II trial tested the efficacy of cetuximab in combination with XELOX in patients with AGC. Similar to other studies, the response rate was 52.3% and median PFS and OS were 6.5 mo (95%CI: 4.9-8.4) and 11.8 mo (95%CI: 6.7-16.8), respectively[39]. In a randomized phase III trial, cetuximab was added to cisplatin and capecitabine and compared with chemotherapy alone in patients with AGC. The primary endpoint of the study was PFS. The median PFS of 455 patients receiving cetuximab plus chemotherapy was 4.4 mo (95%CI: 4.2-5.5) compared with 5.6 mo (5.1-5.7) in 449 patients who were given chemotherapy alone (HR = 1.09; 95%CI: 0.92-1.29, P = 0.32). In the cetuximab plus chemotherapy and chemotherapy alone groups, OS was 9.4 mo and 10.7 mo, respectively (P = 0.95). In this randomized phase III trial, the addition of cetuximab to chemotherapy showed no additional benefit[40].
Panitumumab is a humanized, immunoglobulin G2 monoclonal antibody against the EGFR/HER1 extracellular domain[41]. In phase II trials, blocking the EGFR1 pathway resulted in high efficacy and improved PFS and OS[42]. Therefore, following the dose finding study of panitumumab in patients with AGC, the phase III REAL trial was conducted.
In a phase III trial, the efficacy of adding panitumumab to combined chemotherapy was tested in patients with advanced gastroesophageal carcinomas. In this trial, 553 patients were randomly assigned to receive standard dose EOC (epirubicin 50 mg/m2, oxaliplatin 130 mg/m2, and capecitabine 1250 mg/m2) or modified-dose EOC plus panitumumab (mEOC + P; epirubicin 50 mg/m2 and oxaliplatin 100 mg/m2 on day 1, capecitabine 1000 mg/m2 per day on days 1-21, and panitumumab 9 mg/kg on day 1). The primary endpoint of the study was OS. The median OS was 11·3 mo (95%CI: 9.6-13.0) in patients who received standard dose EOC compared with 8·8 mo (7.7-9.8) in patients who received panitumumab plus mEOC (HR = 1.37; 95%CI: 1.07-1.76, P = 0.013). In the standard dose EOC group, median PFS was 7.4 mo (6.3-8.5) compared with 6.0 mo (95%CI: 5.5-6.5) in the mEOC + P group (HR = 1.22; 95%CI: 0.98-1.52, P = 0.068). In the panitumumab plus EOC group, grade 3-4 adverse events such as diarrhea, mucositis, rash, and hypomagnesaemia were more frequent than in the EOC group. This trial concluded that adding panitumumab to chemotherapy in patients with advanced gasrtoesophageal cancer did not increase OS and was not recommended for clinical use[43]. In a recent phase II trial, panitumumab in combination with dose dense docetaxel/cisplatin/fluorouracil chemotherapy regimen was given as first-line treatment to 52 HER2-negative patients with advanced gastric or gastroesophageal junction tumors. Complete, partial, and stable responses was observed in 3, 29, and 10 patients, respectively. Progression occurred in eight patients. The overall response rate was 62% (95%CI: 48-75). Median PFS and OS were 4.8 mo (95%CI: 4.1-6.9) and 9.4 mo (95%CI: 7.4-11.6), respectively. Panitumumab in combination with dose dense docetaxel/cisplatin/fluorouracil (DCF) showed a high efficacy rate. This combination was suggested to be evaluated in a neoadjuvant setting[44] (Figure 1).
In an EGFR-positive cell line, inhibition of TK domains produced a good antiproliferative effect[45]. Following studies which showed improved OS after blocking HER2 in patients with AGC, researchers targeted the intracellular TK domains of these receptors. The first molecule evaluated in patients with gastric cancer was lapatinib. It is a small dual TK inhibitor blocking both EGFR/HER1 and HER2 TK domains by binding to the adenosine triphosphate (ATP) binding site of the receptor’s intracellular domain[46]. Lapatinib showed moderate activity in combination with capecitabine in patients with HER2-positive metastatic breast cancer[47]. In a phase II trial, lapatinib as a single agent showed modest activity in patients with AGC, with median OS of 4.8 mo (3.2-7.4)[48]. Then, the phase III TYTAN trial was conducted. In this trial, 261 patients identified as HER2-positive by FISH were randomized to receive lapatinib 1500 mg once daily plus once weekly paclitaxel 80 mg/m2 or paclitaxel alone as second-line treatment. The primary endpoint of the study was OS. Secondary endpoints were PFS, time to progression (TTP), ORR, time to response, response duration, and safety. In the lapatinib plus paclitaxel group, median OS was 11 mo (95%CI: 9.5-14.5) compared with 8.9 mo (95%CI: 7.4-11.1) in the paclitaxel alone group (HR = 0.84; 95%CI: 0.64-1.11, P = 0.10). In the lapatinib plus paclitaxel group, median PFS was 5.4 mo (95%CI: 3.9-5.7) compared with 4.4 mo (95%CI: 3.7-5.6) in the paclitaxel alone group (HR = 0.85; 95%CI: 0.63-1.13, P = 0.2441). The ORR was higher in patients receiving lapatinib plus paclitaxel compared with patients receiving paclitaxel alone (OR = 3.85, P < 0.001). Similar rates of adverse events were detected in both treatment arms. Lapatinib plus paclitaxel showed activity in the second-line setting treatment of advanced stage gastric cancer but it did not improve OS[49].
In a phase III trial, the efficacy of lapatinib in combination with a capecitabine/oxaliplatin (CapeOx) chemotherapy regimen in patients with HER2-positive AGC was evaluated. A total of 545 patients were randomized to CapeOx every 3 wk (oxaliplatin 130 mg/m2 day 1; capecitabine 850 mg/m2 BID days 1-14), and daily laptinib (1250 mg) (CapeOx + Lapatinib) or placebo (CapeOx + Placebo). The primary endpoint was OS. In the lapatinib plus chemotherapy and chemotherapy alone groups, median OS was 12.2 and 10.5 mo, respectively (HR = 0.91; 95%CI: 0.73-1.12, P = 0.35). In the lapatinib and chemotherapy alone group, the objective response rate was 53% and 40%, respectively. In subgroup analyses, improvement in OS was detected in Asian patients (HR = 0.68) and those under 60 years (HR = 0.69). Skin toxicity and diarrhea were significantly higher in patients receiving lapatinib compared with chemotherapy alone. The addition of lapatinib to chemotherapy in patients with AGC did not improve OS. It has clinical activity in some specified subpopulations[50].
In 2015, lapatinib in combination with epirubicin, cisplatin, and fluorouracil (ECF)/capecitabine as first-line therapy in metastatic gastric cancer was evaluated. Twenty eight patients were enrolled. The median PFS was 7.1 mo with lapatinib vs 5.9 mo with chemotherapy alone (HR = 0.94; 95%CI: 0.41-2.14). In patients with AGC, lapatinib did not show activity in combination with ECF[51] (Tables 1 and 2).
Activated HER2 forms heterodimers with HER3 and HER4, and the signals produced by the heterodimers activate intracellular TK pathways leading to tumor progression. Dacomitinib (PF00299804) is an irreversible pan-HER inhibitor in vitro and in in vivo models of gastric cancer. In preclinical studies, dacomitinib induced apoptosis in gastric cell lines[52]. In a phase II trial including patients with HER2-positive AGC, after failure of at least one prior chemotherapy regimen, the efficacy of dacomitinib was investigated. A total of 27 patients were enrolled. Dacomitinib was administered orally once daily (45 mg/d) continuously for 21 d every 4 wk. The median PFS was 2.1 mo (95%CI: 2.3-3.4). The median OS was 7.1 mo (95%CI: 4.4-9.8). A PR was observed in two patients and stable disease in nine patients. The objective response rate was 7.4% (95%CI: 0%-17.5%) and disease control rate was 40.7% (95%CI: 21.9%-59.6%). This study concluded that dacomitinib is effective and safe in patients with HER2-positive gastric tumors[53].
In HER2-positive malignancies, AKT (protein kinase B), which is a serine-threonine kinase, play a pivotal role during the transduction of activated HER2 signals, resulting in tumor cell survival and progression of tumors[54]. In patients with HER2-positive malignancies, AKT inhibitors may have potential therapeutic effects. In a phase Ib study, 17 patients (11 breast, 3 gastric, 1 esophageal cancer) with HER2-positive solid malignancies were enrolled. The AKT inhibitor MK2206 in combination with weekly paclitaxel 80 mg/m2 and trastuzumab 2 mg/kg was tested. Fourteen patients were evaluable for tumor response and two patients had a complete response, seven had a partial response, and four were stable. The most common grade 3/4 side effects were neutropenia (6 patients), febrile neutropenia (1 patient), peripheral neuropathy (1 patient), and depression (1 patient)[55]. Afterwards a phase II trial of MK2206 was performed in patients with metastatic gastric or gastroesophageal junction tumors as second-line therapy. Seventy patients were evaluated. The median PFS and OS were 1.8 mo (95%CI: 1.7-1.8 mo) and 5.1 mo (95%CI: 3.7-9.4 mo), respectively. The response rate was 1%. MK2206 has no beneficial activity in patients with advanced gastroesophageal cancers[56].
Src is a TK protein belonging to a family of non-receptor protein TKs (SFK). The activity of Src protein kinases are increased in epithelial human cancers[57]. The mitogenic signaling of activated EGFR works in a synergistic manner with Src TK, and activated EGFR ligand requires functional Src family kinases to transmit mitogenic responses[58]. Src is involved in EGFR pathways triggering cell proliferation, adhesion, invasion, migration, metastasis, and tumorigenesis[59]. It has been reported that the expression and/or activation of the Src family of protein kinases is increased in gastric cell lines[60]. In a study by Green et al[61], saracatinib (AZD0530), which is a small potent orally administered molecule inhibiting Src kinase activity by binding to ATP binding sites of Src kinases, inhibited tumor progression in a murine model of bladder cancer. In a phase II trial, 21 patients with locally advanced or metastatic gastric or gastroesophageal junction tumors received saracatinib 175 mg/d in a 28-d cycle until progression. In this study, patients received a median of two cycles (range, 1-10) of therapy. No objective response was seen in 17 evaluable patients. Three patients had stable disease and 13 progressive disease. Median OS was 7.8 mo (95%CI: 3.9-12.2 mo) and PFS was 1.8 mo (95%CI: 1.5-1.9 mo). Most serious side effects of saracatinib treatment were fatigue (2 patients), hypoxia (2), anemia (3), and lymphopenia (2). In this phase II trial, single agent saracatinib had no effect in first-line therapy of patients with advanced gastroesophageal cancer[62] (Figure 1)
Angiogenesis is one of the important steps of tumor growth, metastasis and progression[63]. Vascular endothelial growth factor (VEGF) is the key regulator of angiogenesis. The VEGF family has six members and the most widely defined is VEGF-A. In one study, specimens of 124 gastric cancers were evaluated, and an increased rate of hepatic metastasis, lymph node metastasis, and poor prognosis was associated with an increased number of microvessels in these specimens[64]. Maehara et al[65] reported that VEGF overexpression was associated with metastasis and poor prognosis. Based on these data, angiogenesis inhibitor molecules were evaluated in patients with AGC.
Bevacizumab is the humanized monoclonal antibody targeting VEGF-A. Experimental studies showed that bevacizumab blocked all isoforms of VEGF-A and VEGF-A-dependent angiogenesis[66]. In many different solid organ cancer cell line studies, bevacizumab inhibited tumor growth as a single agent, and also in combination with doxorubicin, topotecan, paclitaxel, docetaxel, or radiotherapy resulting in additive or synergistic effects[67]. In 2006, a phase II study of bevcizumab in combination with chemotherapy in the treatment of advanced gastroesophageal carcinoma was reported[68]. In this study, 47 patients with advanced gastroesophageal cancer were treated with bevacizumab 15 mg/kg on day 1, irinotecan 65 mg/m2, and cisplatin 30 mg/m2 on days 1 and 8, every 21 d. The primary endpoint was improvement in PFS compared with historical values. Median PFS and OS were 8.3 mo (95%CI: 5.5-9.9) and 12.3 mo (95%CI: 11.3-17.2), respectively. The chemotherapy toxicity did not increase, but bevacizumab-related toxicities were detected (grade 3 hypertension: 28%, gastric perforation: 6%, myocardial infarction: 2%). The study concluded that the addition of bevacizumab to chemotherapy improved the response rate, PFS, and OS by 75% compared with historical controls[68]. In a another phase II trial, previously untreated patients with advanced gastroesophageal disease received bevacizumab 7.5 mg/kg in combination with docetaxel (70 mg/m2) and oxaliplatin (75 mg/m2) on day 1 of each cycle. Thirty eight patients were included in this study. Complete, partial, and stable responses were detected in 5%, 37%, and 37% of patients, respectively. Median PFS and OS were 6.6 mo and 11.1 mo. The most common serious side effect was neutropenia detected in 34% of patients. The most important bevacizumab-related side effect was gastrointestinal perforation reported in 8% of patients. The addition of bevacizumab to docetaxel and oxaliplatin had promising activity[69]. The response rate of combination therapy of bevacizumab with modified docetaxel/cisplatin and fluorouracil chemotherapy was 67% (95%CI: 50%-81%). A total of 44 patients were enrolled in this study. The median PFS was 12 mo (95%CI: 8.8-18.2). The median OS was 16.8 mo (95%CI: 12.1-26.1) and 2-year survival was 37%. In this phase II trial, bevacizumab in combination with mDCF showed marked efficacy in patients with advanced gastroesophageal cancer[70]. Bevacizumab (15 mg/kg) was also evaluated in combination with capecitabine 850 mg/m2 BID on days 1-14, and oxaliplatin 130 mg/m2 on day 1 of a 21-d cycle, in patients with advanced gastroesophageal cancer. The median PFS and OS were 7.2 mo and 10.8 mo, respectively. Response rate was 51.4%. The regimen was well tolerated with favorable activity in advanced stage gastroesophageal cancer[71]. Following these phase II trials, a phase III trial of bevacizumab in combination with cisplatin and fluoropyrimidines was conducted in patients with AGC. Patients received bevacizumab 7.5 mg/kg or placebo followed by cisplatin 80 mg/m2 on day 1 plus capecitabine 1000 mg/m2 twice daily for 14 d or fluorouracil infusion, every 3 wk. The patients received cisplatin for 6 cycles and fluoropyrimidines and bevacizumab until progression. The primary endpoint was OS. A total of 774 patients were enrolled in the study and randomized to bevacizumab plus chemotherapy or chemotherapy alone. The median OS was 12.1 and 10.1, respectively (HR = 0.87; 95%CI: 0.73-1.03, P = 0.10). In the bevacizumab group, the median PFS was 6.7 mo compared with 5.3 mo in the chemotherapy alone group (HR = 0.80; 95%CI: 0.68-0.93, P = 0.0037). The response rate was significantly improved in the bevacizumab group compared with the chemotherapy alone group (46.0% vs 37.4%; P = 0.0315). In the bevacizumab plus chemotherapy and chemotherapy alone groups, the most common serious side effects were neutropenia (35% vs 37%), anemia (10% vs 14%), and decreased appetite (8% vs 11%). In first-line treatment of advanced stage gastric cancer, bevacizumab in combination with chemotherapy improved PFS and response rate compared with chemotherapy alone. However, this trial was insufficient to meet its primary endpoint[72]. In another phase III trial, the authors hypothesized that geographic differences might have affected the results of the AVAGAST trial and they performed the same study in Chinese patients. A total of 202 patients were randomized to receive bevacizumab plus cisplatin-capecitabine regimen or capecitabine-cisplatin combination alone. The median OS and PFS were similar in the two treatment groups[73].
In the literature, it was reported that the VEGF-A, which is the main moderator of angiogenesis act on VEGF receptor-2 (VEGFR2), is essential for tumor angiogenesis[74]. Ramucirumab is a humanized Ig G1 monoclonal antibody directed against to extracellular VEGF binding domain of VEGFR2 and blocks receptor activation of VEGFR2[75]. In a phase I trial of ramucirumab, 37 patients with solid organ malignancies were treated with 2 to 16 mg/kg of ramucirumab. Fifteen of 37 patients (40%) had either a partial response or stable disease. Among the evaluable patients, tumor perfusion and vascularity decreased in 69%. In this phase I trial, a wide range of ramucirumab doses showed antitumor activity and antiangiogenic effects[76]. This study showed that ramucirumab may be potential therapy for cancers in which VEGFR2 plays a crucial role in tumor progression. Afterwards, a phase III randomized double blind trial of ramucirumab was conducted in patients with advanced gastroesophageal cancer as second-line therapy at 119 centers in 29 countries. The patients with advanced gastroesophageal cancer who progressed after first-line platinum-containing or fluoropyrimidine-containing chemotherapy, were included in the study. A total of 355 patients were randomized 2:1 to receive ramucirumab (8 mg/kg, iv) plus best supportive care (n = 238) or placebo plus best supportive care (n = 117) every 2 wk until progression or unacceptable toxicity or death. The primary endpoint of the REGARD study was OS. In the ramucirumab group, the median OS was 5.2 mo (IQR: 2.3-9.9) compared with 3.8 mo (1.7-7.1) in those receiving placebo (HR = 0.776; 95%CI: 0.603-0.998, P = 0.047). For ramucirumab and placebo groups, the median PFS was 2.1 and 1.3 mo (HR = 0.483; 95%CI: 0.376-0.620, P < 0.001). The objective response rate in ramucirumab and placebo groups was 3.4% and 2.6%, respectively. Hypertension occurred more often in the ramucirumab group compared with the placebo group (16% vs 8%) and other adverse event rates were similar between the groups. Ramucirumab was the first targeted therapy acting as a single agent in patients with advanced gastroesophageal cancers[77]. The RAINBOW study is another phase III randomized, placebo-controlled, double-blind study conducted in 170 centers in 27 countries. In this study 665 patients with advanced gastroesophageal adenocarcinoma were randomized in a 1:1 ratio to receive ramucirumab 8 mg/kg (n = 330) or placebo (n = 335) intravenously biweekly plus weekly paclitaxel 80 mg/m2 intravenously in a 28-d cycle. The study enrolled patients who had locally advanced gastric or gastroesophageal junction adenocarcinoma, and determined the objective radiological or clinical disease progression during or within 4 mo of the last dose of first-line platinum and fluoropyrimidine doublet with or without anthracycline. In patients receiving ramucirumab plus paclitaxel, OS was significantly higher compared to patients receiving paclitaxel plus placebo (median OS: 9.6 mo, 95%CI: 8.5-10.8 vs 7.4 mo, 95%CI: 6.3-8.4; HR = 0.807; 95%CI: 0.678-0.962, P = 0.017). The median PFS in the groups receiving ramucirumab plus paclitaxel and paclitaxel plus placebo was 4.4 mo (95%CI: 4.2-5.3) and 2.9 mo (95%CI: 2.8-3.0), respectively (HR = 0.635; 95%CI: 0.536-0.752, P < 0.0001). In the ramucirumab group, the median duration of treatment was 18 wk (IQR: 10.0-31.1) compared with 12 wk (6.4-20.0) in those receiving paclitaxel with placebo. The incidence of grade 3 or higher side effects was higher in the ramucirumab plus paclitaxel group compared with the paclitaxel plus placebo group. These side effects were neutropenia (41% vs 19%), leucopenia (17% vs 7%), hypertension (14% vs 2%), abdominal pain (6% vs 3%), and fatigue (12% vs 5%). In patients with advanced stage gastroesophageal cancer who failed first-line therapy, the median PFS and OS was significantly increased with addition of ramucirumab to paclitaxel compared with paclitaxel alone[6]. In April 2014, the FDA approved ramucirumab as a single agent for the treatment of patients with advanced gastroesophageal adenocarcinoma and disease progression during or after prior treatment with fluoropyrimidine- or platinum-containing chemotherapy. Seven months later in November 2014, based on the results of the RAINBOW study, the FDA approved ramucirumab in combination with paclitaxel for advanced gastric or gastroesophageal cancer patients who progressed after first-line platinum plus fluoropyrimidine with or without an anthracycline chemotherapy (Tables 1 and 2).
Sunitinib is a small orally potent multi-TK inhibitor which blocks the TK domains of VEGF and, platelet derived growth factor receptor (PDGFR)[78]. In a phase I study, sunitinib in combination with capecitabine/oxaliplatin had an acceptable safety profile in patients with AGC. In this phase I study, the median PFS of sunitinitib in combination with capecitabine/oxaliplatin therapy was 7.6 mo (95%CI: 5.0-8.4)[79]. In 2011, sunitinib was evaluated as a second-line single agent therapy for patients with advanced gastric cancer who had received prior chemotherapy. A total of 78 patients were enrolled in this phase II trial, and patients received sunitinib 50 mg/d (4 wk on treatment, followed by 2 wk off treatment). A partial and stable response was detected in 2 (2.6%) and 25 (32.1%) patients, respectively. Median PFS and OS was 2.3 mo (95%CI: 1.6-2.6) and 6.8 mo (95%CI: 4.4-9.6), respectively. The most important grade 3 or more serious side effects were thrombocytopenia and neutropenia which were reported in 34.6% and 29.4% of patients, respectively[80]. This study showed that sunitinib has an insufficient effect as a single agent for patients with AGC. In an another open-label phase II trial, sunitinib in combination with docetaxel (sunitinib 37.5 mg and docetaxel 60 mg/m2) was compared with single agent docetaxel (60 mg/m2, every 3 wk) in patients with AGC as second-line therapy. A total of 107 patients were enrolled and randomized to a combination of sunitinib and docetaxel or single agent docetaxel. The median PFS was 3.9 mo (95%CI: 2.9-4.9) and 2.6 mo (95%CI: 1.8-3.5), respectively, and the difference was not significant (P = 0.206). However, the objective response rate was significantly higher in the combination arm (1.1% vs 14.3%, P = 0.002). The addition of sunitinib to docetaxel as second-line therapy in patients with AGC did not improve PFS, but increased the objective response rate[81].
Apatinib is an oral, highly potent TK inhibitor targeting VEGFR2. In a phase II trial, patients with metastatic gastric cancer who did not respond to two lines of chemotherapy regimens were randomized to receive placebo (group A), apatinib 850 mg once daily (group B), or apatinib 425 mg BID (group C). A total of 144 patients were enrolled and randomized to three groups. The OS was 2.50 mo (95%CI: 1.87-3.70), 4.83 mo (95%CI: 4.03-5.97), and 4.27 mo (95%CI: 3.83-4.77), respectively. PFS was 1.40 mo (95%CI: 1.20-1.83), 3.67 mo (95%CI: 2.17-6.80), and 3.20 mo (95%CI: 2.37-4.53), respectively. The differences between OS and PFS between the apatinib and placebo group were statiscally significant (P < 0.001). In the apatinib groups the most common serious adverse events were hand-foot syndrome and hypertension. In this phase II trial, apatinib showed activity in patients with AGC who had progressed after two lines of chemotherapy[82] (Figure 1).
c-Met is a protein TK encoded by proto oncogene Met. c-Met protein TK is activated by its ligand hepatocyte growth factor receptor (HGFR). c-Met is overexpressed and mutated in a variety of malignancies[83]. During wound healing and embryonic development c-Met activity is crucial. However, multiple mechanisms such as HGF stimulation, gene amplification or mutation, and cross-talk with other receptors could overactivate c-Met kinase. c-Met is activated via its natural ligand HGFR. Activation of c-Met results in angiogenesis, proliferation, migration, invasion, and metastasis of tumors. c-Met aberrant expression was found in gastric carcinoma cell lines[84]. In one study, 43 gastric carcinoma patients, were evaluated for expression of c-Met and HGF genes and mutations in the kinase domain of the Met gene. Met and HGF protein were expressed in 29 (67%) and 22 (51%) of these patients, respectively[85]. Gene amplification was detected in 10.2% of patients with gastric carcinoma and was associated with depth of tumor invasion and lymph node metastasis[86]. In a meta-analysis, the prognostic significance of c-Met amplification and expression was evaluated in 2258 patients with gastric cancer. It was demonstrated that c-Met amplification had an unfavorable impact on OS of patients with gastric cancer (HR = 2.57; 95%CI: 1.97-3.35)[87]. Activated Met sends signals through RAS-mitogen activated protein kinase (RAS-MAPK) and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K-Akt) pathways to initiate cellular processes including motility, survival, proliferation, morphogenesis, and angiogenesis[88]. A recent retrospective study showed Met overexpression in 4%-63% of gastric tumor tissues[89]. On the other hand, Met gene amplification was demonstrated in up to 5% of treatment-naive patients with gastric cancer[90]. Two types of c-Met inhibitors are described. These are monoclonal antibodies and small molecules inhibiting the enzymatic TK activity of cMet.
Rilotumumab (AMG 102) is a fully human monoclonal IgG2 antibody that binds HGF, prevents its binding to the Met TK receptor, and prevents activation of MET TK and downsignaling. Rilotumumab was evaluated in a phase 1 trial in patients with refractory solid tumors. Total 40 patients were enrolled into six dose escalation groups (0.5, 1, 3, 5, 10, or 20 mg/kg AMG 102 i.v. every 2 wk). Twenty three patients were evaluable for the treatment response and 16 had a best response of stable disease with PFS ranging from 7.9-40 wk. AMG 102-related side effects were fatigue (13%), constipation (8%), nausea (8%), vomiting (5%), anorexia (5%), myalgia (5%), and hypertension (5%)[91]. In a phase 1b and II study, rilotumumab in combination with epirubicin, cisplatin, and capecitabine (ECX) was tested as first-line therapy in patients with advanced gastric or esophagogastric junction cancer. In the phase 1b part of the study, a safe dose of rilotumumab in combination with ECX was identified. In the phase II part of the study, 121 patients were randomly assigned to receive rilotumumab 15 mg/kg plus ECX (n = 40); rilotumumab 7.5 mg/kg plus ECX (n = 42) or placebo plus ECX (n = 39). PFS was 5.1 mo (95%CI: 2.9-7.0), 6.8 mo (4.5-7.5) and 4.2 mo (2.9-4.9), respectively. The HR for the PFS in the rilotumumab 15 mg/kg and 7.5 mg/kg groups compared with the placebo group was 0.69 (80%CI: 0.49-0.97; P = 0.164) and 0.53 (80%CI: 0.38-0.73; P = 0.009), respectively. Adverse events detected more frequently compared with placebo group were neutropenia (in combined rilotumumab group: 54% vs placebo: 33%), anemia (40% vs 28%); thrombocytopenia (11% vs none), peripheral edema (27% vs 8%), and venous thromboembolism (20% vs 13%). Serious adverse events were more common in the rilotumumab group compared with the placebo group (neutropenia 44% vs 28% and venous thromboembolism 20% vs 10%). This phase II trial showed that addition of rilotumumab to ECX had greater activity compared with ECX alone and had a tolerable safety profile[92]. In a biomarker study of this phase II study, tumor and plasma samples were evaluated for Met protein levels and gene copy numbers to identify any subgroup of patients who might benefit from rilotumumab. Tumor samples were available for 62 patients in the rilotumumab 15 mg/kg and 7.5 mg/kg groups and 28 patients in the placebo group. In both rilotumumab groups, if more than 50% of tumors stained positive for Met this subgroup was defined as Met-high. The median OS for the Met-high group was 11.1 mo (80%CI: 9.2-13.3) compared with 5.7 mo (80%CI: 4.5-10.4) in the placebo group (HR = 0.29; 95%CI: 0.11-0.76, P = 0.012). On the other hand, in the rilotumumab group, the OS of Met-low patients (tumor cells < 50% positive) compared with the placebo group was not significantly different (HR = 1.84; 95%CI: 0.78-4.34). In the chemotherapy alone group, the Met-high subpopulation had a poor prognosis and shorter OS compared with the Met-low subpopulation (HR = 3.22; 95%CI: 1.08-9.63). In this study, the gene copy number of Met and plasma levels of soluble Met protein were not associated with PFS or OS. In patients receiving rilotumumab plus ECX, high staining rate (> 50%) of Met protein by immunohistochemistry was the predictor of response to rilotumumab therapy. In the ECX alone group, the subpopulation of patients with high (> 50%) Met staining had a poor prognosis[93].
In the RILOMET-1 study, planned 450 patients with advanced c-met positive gastric or GEJ cancer will be randomized to receive either ECX (epirubicin 50 mg/m2 and 60 mg/m2 on day 1, and oral capecitabine 625 mg/m2 twice daily on days 1-21) plus rilotumumab 15 mg/kg or placebo repeated every 3 wk. This study is ongoing, but not recruiting participants. It is estimated to complete the analyzes in March 2017[94].
An ongoing randomized phase III trial of rilotumumab in combination with cisplatin and capecitabine as first-line therapy for patients with advanced MET-positive gastric or gastroesophageal junction tumors plans to include 450 patients in the study population[95].
Onartuzumab is a monoclonal antibody which binds to the Met receptor and prevents the binding of HGF and activation of Met TKs[96]. In a phase 1 study, onartuzumab alone and in combination with bevacizumab was well tolerated. In this phase 1 study, one patient with solitary hepatic metastasis of gastric cancer showed a complete response following four cycles of treatment with single-agent onartuzumab (20 mg/kg)[97].
In an ongoing phase III trail, onartuzumab in combination with mFOLFOX6 regimen in patients with HER2-negative and Met-positive AGC as first-line therapy was evaluated. Patients were randomized to receive either mFOLFOX plus onartuzumab or placebo. mFOLFOX was planned to be administered for up to 12 cycles; onartuzumab and placebo was planned to be administered up to progression[98].
Tivantinib is a small molecule orally administered and a potent inhibitor of c-Met kinase. Tivantinib was tested in a phase I study in patients with refractory solid organ malignancies. A partial or stable response was achieved in 3 patients (3.8%) and 40 patients (50.6%), respectively. Grade 3 or more toxicity related to tivantinib was detected in two patients. In a phase 1 study, tivantinib 360 mg BID was well tolerated in patients with refractory advanced solid tumors[99]. In a phase II trial, tivantinib monotherapy was evaluated in Asian patients with AGC who were previously treated (1 or 2 lines of therapy). Tivantinib 360 mg orally BID was administered to 30 patients. No objective response was detected and the disease control rate was 36.7% (11/30 patients). Median PFS was 43 d (95%CI: 29.0-92.0). Serious side effects were observed in 13 patients (43.3%). In 4 (13.3%) patients c-Met gene amplification (≥ 5 copies/cell) was observed. In this phase II trial, tivantinib monotherapy showed modest activity in previously treated Asian patients with AGC[100].
Crizitonib is a small molecule orally administered and targets both c-Met and anaplastic lymphoma kinase TKs. In an expanded phase 1 cohort study, 489 patients with advanced gastroesophageal cancers were analyzed for Met, EGFR, and HER2 amplification status. In this study, four patients with Met-amplified tumors were treated with crizitonib (5%; n = 4 of 80), and two had tumor shrinkage (-30% and -16%); the PFS of the two patients was 3.7 and 3.5 mo. This study concluded that c-Met-amplified gastroesophageal cancers were highly aggressive and responsive to crizitonib therapy[101] (Figure 1).
Mammalian target of rapamycin (mTOR) is closely related to MAPK, and is a key regulator of cellular growth, proliferation, and angiogenesis[102]. Mutation of gene locations of proteins related to these pathways resulting in aberrant activation of mTOR pathway[103]. The mTOR pathway is activated in patients with gastric cancer, and could be a possible target for therapy[104]. The proteins involved downstream in the mTOR pathway, including eIF4E and 4E binding proteins, were shown to be increased in gastric cancer cell lines[103]. Everolimus (RAD001) is an oral inhibitor of the mTOR pathway, reducing phosphorylation of 4E-BP1 protein and production of proangiogenic factors such as hypoxia-induced factor alpha and VEGF in gastric tumor cell lines[105]. In gastric cancer xenograft models, everolimus significantly inhibited tumor growth[106]. In a phase I study of everolimus in patients with solid malignancies, nine patients were included. One patient with gastric cancer receiving 10 mg RAD001 daily showed a marked response to therapy[107]. In another phase I trial, everolimus was evaluated in combination with capecitabine in patients with AGC. After a median follow-up of 5.6 mo (range, 2.3-8.1), median PFS was 1.8 mo (95%CI: 0.8-2.8). The sum of the widest tumor diameters was decreased in 28.7% of the patients. Dose-limiting serious toxicities were infection, mucositis, hyperglycemia, and hyponatremia. The suggested dose of capecitabine in combination with everolimus was 650 mg/m2 BID and 5 mg BID, respectively[108]. In a phase II trial of everolimus (2 mg/daily × 5 mg/daily, 1-21 d) with capecitabine (2 mg/daily × 650 mg/daily, 1-14 d), 57 patients were enrolled and 43 patients were evaluable for response. Five achieved a partial response, 18 showed stable disease, and the disease control rate was 48.9% (95%CI: 34.6%-63.2%). The median PFS and OS were 11.0 wk (95%CI: 5.7-16.3) and 21.0 wk (95%CI: 14.3-27.7), respectively. Serious nausea, diarrhea, and stomatitis occurred in 2, 3, and 3 patients, respectively. In pretreated gastric cancer patients the combination of capecitabine with everolimus was shown to be effective[109].
In a phase II trial of everolimus in 53 patients with AGC who were previously treated with chemotherapy, the primary endpoint was disease control rate. Patients received 10 mg everolimus daily orally until disease progression or study discontinuation. None of the patients had a complete or partial response. However, tumor size was decreased in 45% of patients. The disease control rate was 56.0% (95%CI: 41.3%-70.0%). The median PFS and OS were 2.7 mo (95%CI: 1.6-3.0) and 10.1 mo (95%CI: 6.5-12.1), respectively. Common serious toxic effects of everolimus were anemia, hyponatremia, increased gamma-glutamyl transferase, and lymphopenia. Mild pneumonitis was detected in 15.1% of patients. In this phase II trial, single agent everolimus had favorable efficacy in patients with AGC[110]. Following the encouraging results of everolimus in phase II trials, the double blind, randomized phase III Granite-1 study was conducted in patients with AGC who had progressed after 1 or 2 lines of therapy. Patients were randomly assigned to receive everolimus 10 mg/daily or placebo with best supportive care in a 2:1 ratio. The primary endpoint of study was OS. The study population included 656 patients. In the everolimus group, the median OS was 5.4 mo compared with 4.3 mo in those receiving placebo (HR = 0.90; 95%CI: 0.75-1.08, P = 0.124). In the everolimus and placebo groups, median PFS was 1.7 mo and 1.4 mo, respectively (HR = 0.66, 95%CI: 0.56-0.78, P < 0.01). The most frequently observed adverse events were anemia, decreased appetite, and fatigue. In patients with AGC who progressed after first- or second-line chemotherapy, single agent everolimus did not improve OS[111] (Figure 1).
In preclinical gastric cancer cell studies, it was demonstrated that fibroblast growth factor receptor (FGFR)2 amplification was associated with increased tumour cell proliferation and survival. In gastric cancer xenograft models, amplified FGFR2 was associated with high tumor grade (P = 0.034)[112]. In a multicenter international trial, FGFR2 FISH was performed on tumor samples of 961 patients with gastric cancer. The FGFR2 positivity and association between clinicopathological features were analyzed. The FISH positivity rate of patients from the United Kingdom, China, and South Korea were 7.4%, 4.6%, and 4.2%, respectively (P > 0.05). FGFR2 amplification was associated with lymph node metastases (P < 0.0001) and poor OS (P = 0.007). This study showed that in patients with gastric cancer, FGFR2 amplification is associated with poor prognosis[113]. FGFR2 may be a novel target for treatment of patients with AGC.
Tetraspanins belong to a family of cell surface-associated proteins which play a major role in the immune system, cell survival, proliferation, adhesion, migration, and tumor invasion[114]. CD9 a novel protein which is a member of tetraspanin family[115]. In a study, CD9 expression of tissues from 78 AGC patients was compared with normal tissues. It has been demonstrated that CD9 expression was more prominent than in normal tissues[116]. In gastric cell lines, anti-CD-9 monoclonal antibody significantly suppressed tumor growth, increased apoptosis, and inhibited angiogenesis[117].
Claudins are a family of proteins including 27 proteins playing a major role in selective permeability of cellular tight junctions[118]. Claudins may have a role in gastric carcinogenesis and progression of gastric tumors. One study demonstrated that the claudin-18 splice variant 2 is a suitable target for therapeutic antibody development against gastric cancer[119]. After that, claudiximab, a chimeric monoclonal antibody against claudin 18.2 was developed. In a phase II trial, patients with advanced gastroesophageal cancer and positive staining for claudin 18.2 by immunohistochemistry were enrolled. The patients received claudiximab until disease progression. A total of 31 patients were evaluated for tumor response. A partial response and stable disease were demonstrated in 4 and 8 patients (ORR: 13%, disease control rate: 39%). The most frequent grade 3 adverse events were vomiting (8.24%), nausea (4.12%), and hypersensitivity (1.3%)[120]. In a phase II trial, the benefit of addition of claudiximab to a chemotherapy regimen is being tested. Patients with claudin 18.2 positive adenocarcinoma of gastric cancer were randomized to receive claudiximab plus EOX (Epirubicin/oxaliplatin/capecitabine) or EOX alone as first-line therapy (NCT01630083).
Immunologic checkpoint blockade with antibodies against different T cell regulatory mechanisms, including cyctotoxic T lymphocyte antigen (CTLA4), the programmed cell death protein-1 (PD-1) or its ligand (PD-L1), is an effective method for reversing cancer immunosuppression and thereby activating anti-tumor immunity against several different cancer types[121,122]. CTLA4 was the first negative immune checkpoint molecule to be targeted by monoclonal antibodies. Following the activation of T cells, CTLA4 is upregulated on the activated T cells and blocks further stimulation by the co-stimulatory molecule CD28. Therefore, CTLA4 has a negative effect on activated T cells and blocks further activation[123]. In a previous study, in patients with melanoma antibody against CTLA4 showed T cell activation[124]. In a phase II trial, the efficacy of tremelimumab, which was a monoclonal antibody targeting CTLA4, was evaluated as second-line therapy in patients with advanced gastric and esophageal adenocarcinomas. Eighteen patients were enrolled to in the. Tremelimumab was given every 3 mo until symptomatic disease progression. Four patients had stable disease and one patient achieved a partial response after eight cycles of therapy. The response rate of tremelimumab was low in patients with advanced gastroesophageal carcinoma[125]. In a randomized, open-label, two-arm, phase II trial, ipilimumab maintenance will be tested in patients with advanced gastroesophagel cancer who did not have progressive disease following first-line fluoropyrimdine and platin chemotherapy. The study plans to randomize 114 patients to ipilimumab maintenance arm and best supportive care arms[126].
PD-1 protein, a T cell co-inhibitory receptor, and one of its ligands, PD-L1, play a pivotal role in the ability of tumor cells to evade the host's immune system. In a preclinical study, expression of PD-L1 was found in human gastric carcinoma specimens but not in normal or gastric adenoma tissues[127]. In 2012, a phase I trial of intravenous anti-PD-L1 antibody in patients with advanced cancers was reported. This phase I study also included seven patients with AGC. None of the patients with gastric cancer demonstrated a partial or complete response[121].
Nivolumab, a fully human IgG4 PD-1 blocking antibody in combination with ipilimumab or as a single agent is being tested in an ongoing phase I/II trial in patients with solid malignancies, including patients with gastric cancers[128]. In a phase III trial, nivolumab is also being evaluated in patients with advanced gastroesophageal cancers as second- or third-line therapy (NCT02267343).
Insulin-like growth factor I (IGF-1) promotes growth of gastric cancer cells[129]. IGF receptor type I (IGF-IR) expression is associated with poor OS in patients with AGC. In a study of 87 tumor specimens, 67 (77%) showed IGF-IR expression (defined as > 10% membranous staining). Multivariate survival analysis showed that IGF-IR-positivity (HR = 2.14; 95%CI: 1.20-3.82, P = 0.01) was a predictor of poor outcome[130].
In a phase I trial, ganitumab (AMG 479) was tested in 19 patients including three patients with AGC. The most common serious (grade ≥ 3) adverse events were neutropenia (21%), leukopenia (16%), and lymphopenia (11%). Stable disease was observed in seven patients[131].
Heat shock protein 90 (HSP90) is highly expressed in cancer tissues. In tumor samples of 322 patients with gastric cancer, 69.6% demonstrated positive expression of HSP90. HSP90 protein expression was significantly associated with depth of invasion (P < 0.001), lymph node metastasis (P < 0.001), and stage of disease (P < 0.001). This study reported that HSP90 expression is an independent prognostc indicator of poor PFS and OS in patients with gastric cancer[132]. A second generation HSP90 inhibitor increased apoptosis in gastric cell lines[133]. Single agent efficacy of an HSP inhibitor was demonstrated in a phase I trial[134]. Ganetespib is a novel triazolone heterocyclic inhibitor of HSP90 and is a biologically rational treatment strategy for advanced esophagogastric cancers. In a single arm phase II trial, 26 patients with advanced gastroesophageal cancer received ganetespib 200 mg/m2 i.v. on days 1, 8, and 15 of a 28-d cycle as second- or third-line therapy. The most common serious adverse events were; leucopenia (12%), fatigue (12%), diarrhea (8%), and elevated alkaline phosphatase (8%). A complete response was detected in one patient. The ORR was 4%. The median PFS and OS was 1.6 mo and 2.8 mo, respectively. The study was terminated early due to insufficient efficacy of single agent ganetespib[135].
Trastuzumab was the first molecule shown to prolong both PFS and OS in patients with AGC when added to first-line chemotherapy. In April 2014, the FDA approved ramucirumab as a single agent for the treatment of patients with advanced gastroesophageal adenocarcinoma with disease progression during or after prior treatment with fluoropyrimidine- or platinum-containing chemotherapy. In November 2014, based on results of the RAINBOW study, the FDA approve ramucirumab in combination with paclitaxel for advanced gastric or gastroesophageal cancer patients who progressed after first-line platinum plus fluoropyrimidine with or without an anthracycline. The two targeted agents are in clinical use for patients with AGC (Table 1). Many phase II-III clinical trials failed to showed an effect of different targeted agents for patients with AGC (Tables 1 and 2). On the other hand, some of the molecules have shown promising effects in phase II trials and are expected to be in use presently. The pertuzumab and c-Met pathway inhibitors showed modest effects in phase II trials. The results of two important ongoing phase III trials (JACOB and RILOMET-1) may change the suggested first-line treatment options in patients with AGC (Table 3).
| Target | Agents | Trial number | Setting | Phase | Primary end point | Status |
| HER-2 | Arm A: pertuzumab + trastuzumab + cisplatin + fluoropyrimidine vs Arm B: placebo + trastuzumab + cisplatin + fluoropyrimidine | NCT01774786 | First line | III | OS | Recruiting |
| HER-2 | Arm A: Docetaxel or paclitaxel | NCT01641939 | First line | II/III | Phase II: Dose of TDM-1 | Recruiting |
| Arm B: T-DM1 3.6 mg/kg every 3 wk | ||||||
| Arm C: T-DM1 2.4 mg/kg once a week | Phase III: OS | |||||
| HER-3 | Arm A: MM-111 + Paclitaxel + Trastuzumab | NCT01774851 | ≥ Second line | II | PFS | Active, not recruiting |
| Arm B: Paclitaxel + Trastuzumab | ||||||
| MET | Arm A: Rilotumumab 15 mg/kg plus ECX | NCT01697072 | First line | III | OS | Active, not recruiting |
| Arm B: Placebo plus ECX | ||||||
| MET | Arm A: onartuzumab plus mFOLFOX6 | NCT01662869 | First line | III | OS | Active, not recruiting |
| Aram B: placebo plus mFOLFOX6 | ||||||
| CTLA4 | Arm A: Ipilimumab | NCT01585987 | Maintanence | II | PFS | Active, not recruiting |
| Arm B: Best Supportive Care | ||||||
| PD-1 | Arm A: Nivolumab (ONO-4538) | NCT02267343 | ≥ Second line | III | OS | Recruiting |
| Arm B: Placebo |
P- Reviewer: Aoyagi K, Ju J S- Editor: Qi Y L- Editor: Cant MR E- Editor: Ma S
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | Verdecchia A, Corazziari I, Gatta G, Lisi D, Faivre J, Forman D. Explaining gastric cancer survival differences among European countries. Int J Cancer. 2004;109:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;CD004064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 350] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 4. | Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3205-3209. [PubMed] |
| 5. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5319] [Article Influence: 354.6] [Reference Citation Analysis (3)] |
| 6. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1767] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 7. | Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709-7712. [PubMed] |
| 8. | Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. 2005;1:2005.0010. [PubMed] |
| 9. | Barnham KJ, Torres AM, Alewood D, Alewood PF, Domagala T, Nice EC, Norton RS. Role of the 6-20 disulfide bridge in the structure and activity of epidermal growth factor. Protein Sci. 1998;7:1738-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Gamboa-Dominguez A, Dominguez-Fonseca C, Quintanilla-Martinez L, Reyes-Gutierrez E, Green D, Angeles-Angeles A, Busch R, Hermannstädter C, Nährig J, Becker KF. Epidermal growth factor receptor expression correlates with poor survival in gastric adenocarcinoma from Mexican patients: a multivariate analysis using a standardized immunohistochemical detection system. Mod Pathol. 2004;17:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Hsieh AC, Moasser MM. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer. 2007;97:453-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Toikkanen S, Helin H, Isola J, Joensuu H. Prognostic significance of HER-2 oncoprotein expression in breast cancer: a 30-year follow-up. J Clin Oncol. 1992;10:1044-1048. [PubMed] |
| 13. | Jain S, Filipe MI, Gullick WJ, Linehan J, Morris RW. c-erbB-2 proto-oncogene expression and its relationship to survival in gastric carcinoma: an immunohistochemical study on archival material. Int J Cancer. 1991;48:668-671. [PubMed] |
| 14. | Jørgensen JT, Hersom M. HER2 as a Prognostic Marker in Gastric Cancer - A Systematic Analysis of Data from the Literature. J Cancer. 2012;3:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 15. | Iwasa SNK, Miki A, Noshiro H, Tsuburaya A, Nishida Y, Miwa H, Masuishi T, Yoshida K, Kodera Y, Boku N. Multicenter, phase II study of trastuzumab and paclitaxel to treat HER2-positive, metastatic gastric cancer patients naive to trastuzumab (JFMC45-1102). J Clin Oncol. 2013;abstr TPS4150. |
| 16. | Rivera F FP, Alfonso PG, Gallego J, Limon ML, Alsina M, Gomez LL, Galan M, Falco F, Manzano JL, González E. NEOHX study: Perioperative treatment with trastuzumab in combination with capecitabine and oxaliplatin (XELOX-T) in patients with HER-2 resectable stomach or esophagogastric junction (EGJ) adenocarcinoma - 18 m DFS analysis. J Clin Oncol. 2015;abstr 107. |
| 17. | Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, da Costa WL, Soares FA. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol. 2011;29:3030-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Yamashita-Kashima Y, Iijima S, Yorozu K, Furugaki K, Kurasawa M, Ohta M, Fujimoto-Ouchi K. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res. 2011;17:5060-5070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Metzger-Filho O, Winer EP, Krop I. Pertuzumab: optimizing HER2 blockade. Clin Cancer Res. 2013;19:5552-5556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1303] [Cited by in RCA: 1513] [Article Influence: 151.3] [Reference Citation Analysis (0)] |
| 21. | Kawajiri H, Takashima T, Kashiwagi S, Noda S, Onoda N, Hirakawa K. Pertuzumab in combination with trastuzumab and docetaxel for HER2-positive metastatic breast cancer. Expert Rev Anticancer Ther. 2015;15:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Tabernero J, Shen L, Ohtsu A, Yu R, Eng-Wong J, Kang YK. Pertuzumab (P) with trastuzumab (T) and chemotherapy (CTX) in patients (pts) with HER2-positive metastatic gastric or gastroesophageal junction (GEJ) cancer: An international phase III study (JACOB) [abstract]. J Clin Oncol. 2013;suppl 31:TPS4150. |
| 23. | Yamashita-Kashima Y, Harada N, Fujimoto-Ouchi K. Potentiation of trastuzumab emtansine (T-DM1)-driven antitumor activity by pertuzumab in a HER2-positive gastric cancer model. J Clin Oncol. 2012;abstr e13502. |
| 24. | Barginear MF, John V, Budman DR. Trastuzumab-DM1: a clinical update of the novel antibody-drug conjugate for HER2-overexpressing breast cancer. Mol Med. 2012;18:1473-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Welslau M, Diéras V, Sohn JH, Hurvitz SA, Lalla D, Fang L, Althaus B, Guardino E, Miles D. Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2-positive locally advanced or metastatic breast cancer. Cancer. 2014;120:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Yamashita-Kashima Y, Shu S, Harada N, Fujimoto-Ouchi K. Enhanced antitumor activity of trastuzumab emtansine (T-DM1) in combination with pertuzumab in a HER2-positive gastric cancer model. Oncol Rep. 2013;30:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Gelmon KA, Sablin PM, Serpanchy R, Soberino J, Cortes J, Villa D, Zoubir M, Freudensprung U, Xu J, Colthorpe C. Trastuzumab emtansine (T-DM1) plus capecitabine (X) in patients with HER2-positive MBC: MO28230 TRAX-HER2 phase 1 results. J Clin Oncol. 2014;32:5s. |
| 28. | Available from: http://www.clinical trials.gov. |
| 29. | Prigent SA, Lemoine NR, Hughes CM, Plowman GD, Selden C, Gullick WJ. Expression of the c-erbB-3 protein in normal human adult and fetal tissues. Oncogene. 1992;7:1273-1278. [PubMed] |
| 30. | Ito Y, Takeda T, Sakon M, Tsujimoto M, Higashiyama S, Noda K, Miyoshi E, Monden M, Matsuura N. Expression and clinical significance of erb-B receptor family in hepatocellular carcinoma. Br J Cancer. 2001;84:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 31. | Wang L, Yuan H, Li Y, Han Y. The role of HER3 in gastric cancer. Biomed Pharmacother. 2014;68:809-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Im SA, Baselga J, Kong A, Martin P, Lin CC, Dees EC, Schellens JM, Braud FG, Delgado L, Zucchetto M. A phase 1 dose-escalation study of anti-HER3 monoclonal antibody LJM716 in combination with trastuzumab in patients with HER2-overexpressing metastatic breast or gastric cancer. J Clin Oncol. 2014;abstr 2519. |
| 33. | Richards DA, Garcia AA, Denlinger SC, Conkling PR, Edenfield WJ, Anthony PS, Hellerstedt BA, Raju RN, Becerra C, Harb WA. A phase 1 study of MM-111, a bispecific HER2/HER3 antibody fusion protein, combined with multiple treatment regimens in patients with advanced HER2-positive solid tumors. J Clin Oncol. 2014;abstr 651. |
| 34. | Denlinger CS, Bendell JC, Alsina M, Watkins D, Chao Y, Cubillo A, Kunz PL, Sun W, Baeksgaard L, Chen LT. Randomized open-label phase 2 study of MM-111 and paclitaxel (PTX) with trastuzumab (TRAS) in patients with HER2-expressing carcinomas of the distal esophagus, gastroesophageal (GE) junction, and stomach who have failed front-line metastatic or locally advanced therapy. J Clin Oncol. 2014;abstr TPS4148. |
| 35. | Vincenzi B, Zoccoli A, Pantano F, Venditti O, Galluzzo S. Cetuximab: from bench to bedside. Curr Cancer Drug Targets. 2010;10:80-95. [PubMed] |
| 36. | Han SW, Oh DY, Im SA, Park SR, Lee KW, Song HS, Lee NS, Lee KH, Choi IS, Lee MH. Phase II study and biomarker analysis of cetuximab combined with modified FOLFOX6 in advanced gastric cancer. Br J Cancer. 2009;100:298-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Moehler M, Mueller A, Trarbach T, Lordick F, Seufferlein T, Kubicka S, Geissler M, Schwarz S, Galle PR, Kanzler S. Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: a prospective multi-center biomarker-oriented phase II study. Ann Oncol. 2011;22:1358-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 38. | Lordick F, Luber B, Lorenzen S, Hegewisch-Becker S, Folprecht G, Wöll E, Decker T, Endlicher E, Röthling N, Schuster T. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br J Cancer. 2010;102:500-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Kim C, Lee JL, Ryu MH, Chang HM, Kim TW, Lim HY, Kang HJ, Park YS, Ryoo BY, Kang YK. A prospective phase II study of cetuximab in combination with XELOX (capecitabine and oxaliplatin) in patients with metastatic and/or recurrent advanced gastric cancer. Invest New Drugs. 2011;29:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 680] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 41. | Stremitzer S, Sebio A, Stintzing S, Lenz HJ. Panitumumab safety for treating colorectal cancer. Expert Opin Drug Saf. 2014;13:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Pinto C, Di Fabio F, Siena S, Cascinu S, Rojas Llimpe FL, Ceccarelli C, Mutri V, Giannetta L, Giaquinta S, Funaioli C. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study). Ann Oncol. 2007;18:510-517. [PubMed] |
| 43. | Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 581] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 44. | Tomasello G, Toppo L, Mattioli R, Negri F, Curti A, Ratti M, Poli R, Lazzarelli S, Gerevini F, Colombi C. First-line dose-dense chemotherapy with docetaxel, cisplatin, folinic acid and 5- fluorouracil (DCF) plus panitumumab (P) in patients with locally advanced or metastatic cancer of the stomach or gastroesophageal junction (GEJ): A phase II multicenter trial. J Clin Oncol. 2015;abstr 146. |
| 45. | Piontek M, Hengels KJ, Porschen R, Strohmeyer G. Antiproliferative effect of tyrosine kinase inhibitors in epidermal growth factor-stimulated growth of human gastric cancer cells. Anticancer Res. 1993;13:2119-2123. [PubMed] |
| 46. | Nolting M, Schneider-Merck T, Trepel M. Lapatinib. Recent Results Cancer Res. 2014;201:125-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, Chan S, Jagiello-Gruszfeld A, Kaufman B, Crown J. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 566] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 48. | Iqbal S, Goldman B, Fenoglio-Preiser CM, Lenz HJ, Zhang W, Danenberg KD, Shibata SI, Blanke CD. Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol. 2011;22:2610-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 49. | Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, Tsuji A, Omuro Y, Li J, Wang JW. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 489] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 50. | Hecht JR, Qin S, Chung HC, Xu JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero AF, Salman P. Lapatinib in combination with capecitabine plus oxaliplatin (CapeOx) in HER2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma (AC): The TRIO-013/LOGiC Trial. J Clin Oncol. 2013;abstr LBA4001. |
| 51. | Moehler HM, Mauer ME, Messina CG, John MJ, Lang I, Cutsem EV, Freire J, Lutz MP, Roth A. Lapatinib combined with ECF/x as first-line metastatic gastric cancer (GC) according to HER2 and EGFR status: A randomized placebo controlled phase II (EORTC 40071). J Clin Oncol. 2015;abstr 80. |
| 52. | Nam HJ, Ching KA, Kan J, Kim HP, Han SW, Im SA, Kim TY, Christensen JG, Oh DY, Bang YJ. Evaluation of the antitumor effects and mechanisms of PF00299804, a pan-HER inhibitor, alone or in combination with chemotherapy or targeted agents in gastric cancer. Mol Cancer Ther. 2012;11:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Oh DY, Cho JY, Kang WK, Rha SY, Bang YJ. A phase II open-label trial of dacomitinib monotherapy in patients with HER2-positive advanced gastric cancer after failure of at least one prior chemotherapy regimen. J Clin Oncol. 2012;abstr 54. |
| 54. | Cassinelli G, Zuco V, Gatti L, Lanzi C, Zaffaroni N, Colombo D, Perego P. Targeting the Akt kinase to modulate survival, invasiveness and drug resistance of cancer cells. Curr Med Chem. 2013;20:1923-1945. [PubMed] |
| 55. | Chien JA, Melisko ME, Moasser MM, Kelley RK, Korn M, Ko HA, Pantoja N, Reinert A, Grabowsky JA, Magbanua MJ. Phase Ib dose-escalation trial of the AKT inhibitor (AKTi) MK2206 in combination with paclitaxel (P) and trastuzumab (H) in patients (pts) with HER2-overexpressing (HER2) cancer. J Clin Oncol. 2013;abstr 2605. |
| 56. | Ramanathan RK, McDonough SL, Kennecke HF, Iqbal S, Baranda JC, Seery TE, Lim HJ, Hezel AF, Vaccaro GM, Blanke CD. Phase 2 study of MK-2206, an allosteric inhibitor of AKT, as second-line therapy for advanced gastric and gastroesophageal junction cancer: A SWOG cooperative group trial (S1005). Cancer. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 57. | Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636-5642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 659] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 58. | Roche S, Koegl M, Barone MV, Roussel MF, Courtneidge SA. DNA synthesis induced by some but not all growth factors requires Src family protein tyrosine kinases. Mol Cell Biol. 1995;15:1102-1109. [PubMed] |
| 59. | Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 2000] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 60. | Takeshima E, Hamaguchi M, Watanabe T, Akiyama S, Kataoka M, Ohnishi Y, Xiao HY, Nagai Y, Takagi H. Aberrant elevation of tyrosine-specific phosphorylation in human gastric cancer cells. Jpn J Cancer Res. 1991;82:1428-1435. [PubMed] |
| 61. | Green TP, Fennell M, Whittaker R, Curwen J, Jacobs V, Allen J, Logie A, Hargreaves J, Hickinson DM, Wilkinson RW. Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Mol Oncol. 2009;3:248-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 62. | Mackay HJ, Au HJ, McWhirter E, Alcindor T, Jarvi A, MacAlpine K, Wang L, Wright JJ, Oza AM. A phase II trial of the Src kinase inhibitor saracatinib (AZD0530) in patients with metastatic or locally advanced gastric or gastro esophageal junction (GEJ) adenocarcinoma: a trial of the PMH phase II consortium. Invest New Drugs. 2012;30:1158-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 962] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 64. | Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Maehara Y, Kabashima A, Koga T, Tokunaga E, Takeuchi H, Kakeji Y, Sugimachi K. Vascular invasion and potential for tumor angiogenesis and metastasis in gastric carcinoma. Surgery. 2000;128:408-416. [PubMed] |
| 66. | Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593-4599. [PubMed] |
| 67. | Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671-680. [PubMed] |
| 68. | Shah MA, Ramanathan RK, Ilson DH, Levnor A, D’Adamo D, O’Reilly E, Tse A, Trocola R, Schwartz L, Capanu M. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201-5206. [PubMed] |
| 69. | El-Rayes BF, Zalupski M, Bekai-Saab T, Heilbrun LK, Hammad N, Patel B, Urba S, Shields AF, Vaishampayan U, Dawson S. A phase II study of bevacizumab, oxaliplatin, and docetaxel in locally advanced and metastatic gastric and gastroesophageal junction cancers. Ann Oncol. 2010;21:1999-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Shah MA, Jhawer M, Ilson DH, Lefkowitz RA, Robinson E, Capanu M, Kelsen DP. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol. 2011;29:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 71. | Uronis HE, Bendell JC, Altomare I, Blobe GC, Hsu SD, Morse MA, Pang H, Zafar SY, Conkling P, Favaro J. A phase II study of capecitabine, oxaliplatin, and bevacizumab in the treatment of metastatic esophagogastric adenocarcinomas. Oncologist. 2013;18:271-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968-3976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 887] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 73. | Shen L, Li J, Xu J, Pan H, Dai G, Qin S, Wang L, Wang J, Yang Z, Shu Y. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer. 2015;18:168-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 74. | Bruns CJ, Liu W, Davis DW, Shaheen RM, McConkey DJ, Wilson MR, Bucana CD, Hicklin DJ, Ellis LM. Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000;89:488-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Aprile G, Rijavec E, Fontanella C, Rihawi K, Grossi F. Ramucirumab: preclinical research and clinical development. Onco Targets Ther. 2014;7:1997-2006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, Leong S, O’Bryant C, Chow LQ, Serkova NJ. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 441] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 77. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1573] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 78. | Zhong H, Bowen JP. Recent advances in small molecule inhibitors of VEGFR and EGFR signaling pathways. Curr Top Med Chem. 2011;11:1571-1590. [PubMed] |
| 79. | Lee KW, Park SR, Oh DY, Park YI, Khosravan R, Lin X, Lee SY, Roh EJ, Valota O, Lechuga MJ. Phase I study of sunitinib plus capecitabine/cisplatin or capecitabine/oxaliplatin in advanced gastric cancer. Invest New Drugs. 2013;31:1547-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Bang YJ, Kang YK, Kang WK, Boku N, Chung HC, Chen JS, Doi T, Sun Y, Shen L, Qin S. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2011;29:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 81. | Yi JH, Lee J, Lee J, Park SH, Park JO, Yim DS, Park YS, Lim HY, Kang WK. Randomised phase II trial of docetaxel and sunitinib in patients with metastatic gastric cancer who were previously treated with fluoropyrimidine and platinum. Br J Cancer. 2012;106:1469-1474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 82. | Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 416] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 83. | Maulik G, Shrikhande A, Kijima T, Ma PC, Morrison PT, Salgia R. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev. 2002;13:41-59. [PubMed] |
| 84. | Kuniyasu H, Yasui W, Yokozaki H, Kitadai Y, Tahara E. Aberrant expression of c-met mRNA in human gastric carcinomas. Int J Cancer. 1993;55:72-75. [PubMed] |
| 85. | Park WS, Oh RR, Kim YS, Park JY, Shin MS, Lee HK, Lee SH, Yoo NJ, Lee JY. Absence of mutations in the kinase domain of the Met gene and frequent expression of Met and HGF/SF protein in primary gastric carcinomas. APMIS. 2000;108:195-200. [PubMed] |
| 86. | Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirao T, Nakano H. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 87. | Peng Z, Zhu Y, Wang Q, Gao J, Li Y, Li Y, Ge S, Shen L. Prognostic significance of MET amplification and expression in gastric cancer: a systematic review with meta-analysis. PLoS One. 2014;9:e84502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 88. | Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1153] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 89. | Lee HE, Kim MA, Lee HS, Jung EJ, Yang HK, Lee BL, Bang YJ, Kim WH. MET in gastric carcinomas: comparison between protein expression and gene copy number and impact on clinical outcome. Br J Cancer. 2012;107:325-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 90. | Ha SY, Lee J, Kang SY, Do IG, Ahn S, Park JO, Kang WK, Choi MG, Sohn TS, Bae JM. MET overexpression assessed by new interpretation method predicts gene amplification and poor survival in advanced gastric carcinomas. Mod Pathol. 2013;26:1632-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 91. | Gordon MS, Sweeney CS, Mendelson DS, Eckhardt SG, Anderson A, Beaupre DM, Branstetter D, Burgess TL, Coxon A, Deng H. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res. 2010;16:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 92. | Iveson T, Donehower RC, Davidenko I, Tjulandin S, Deptala A, Harrison M, Nirni S, Lakshmaiah K, Thomas A, Jiang Y. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15:1007-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 93. | Oliner KS, Anderson A, Lan Y, Iveson T, Donehower R, Jiang Y, Dubey S, Loh E. Evaluation of MET pathway biomarkers in a phase II study of rilotumumab (R, AMG 102) or placebo (P) in combination with epirubicin, cisplatin, and capecitabine (ECX) in patients (pts) with locally advanced or metastatic gastric (G) or esophagogastric junction (EGJ) cancer. J Clin Oncol. 2012;abstr 4005. |
| 94. | Cunningham D, Davidenko I, Ilson DH, Murad A, Tebbutt N, Jiang Y, Loh E, Dubey S; RILOMET-1 investigators. RILOMET-1: An international phase III multicenter, randomized, double-blind, placebo-controlled trial of rilotumumab plus epirubicin, cisplatin, and capecitabine (ECX) as first-line therapy in patients with advanced MET-positive gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. J Clin Oncol. 2013;abstr TPS4153. |
| 95. | Doi T, Muro K, Jiang Y, Jain RK, Lizambri R. A phase 3, multicenter, randomized, double-blind, placebo-controlled study of rilotumumab in combination with cisplatin and capecitabine (CX) as first-line therapy for Asian patients (pts) with advanced MET-positive gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The RILOMET-2 trial. J Clin Oncol. 2015;abstr TPS226. |
| 96. | Merchant M, Ma X, Maun HR, Zheng Z, Peng J, Romero M, Huang A, Yang NY, Nishimura M, Greve J. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc Natl Acad Sci USA. 2013;110:E2987-E2996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 97. | Salgia R, Patel P, Bothos J, Yu W, Eppler S, Hegde P, Bai S, Kaur S, Nijem I, Catenacci DV. Phase I dose-escalation study of onartuzumab as a single agent and in combination with bevacizumab in patients with advanced solid malignancies. Clin Cancer Res. 2014;20:1666-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 98. | Cunningham D, Tabernero J, Shah MA, Lordick F, Hack SP. MetGastric: A randomized phase III study of onartuzumab (MetMAb) in combination with mFOLFOX6 in patients with metastatic HER2-negative and MET-positive adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2013;abstr TPS4155. |
| 99. | Rosen LS, Senzer N, Mekhail T, Ganapathi R, Chai F, Savage RE, Waghorne C, Abbadessa G, Schwartz B, Dreicer R. A phase I dose-escalation study of Tivantinib (ARQ 197) in adult patients with metastatic solid tumors. Clin Cancer Res. 2011;17:7754-7764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 100. | Kang YK, Muro K, Ryu MH, Yasui H, Nishina T, Ryoo BY, Kamiya Y, Akinaga S, Boku N. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs. 2014;32:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 101. | Lennerz JK, Kwak EL, Ackerman A, Michael M, Fox SB, Bergethon K, Lauwers GY, Christensen JG, Wilner KD, Haber DA. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29:4803-4810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 372] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 102. | Polivka J, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142:164-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 613] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 103. | Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1040] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 104. | Tapia O, Riquelme I, Leal P, Sandoval A, Aedo S, Weber H, Letelier P, Bellolio E, Villaseca M, Garcia P. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014;465:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 105. | Martín ME, Pérez MI, Redondo C, Alvarez MI, Salinas M, Fando JL. 4E binding protein 1 expression is inversely correlated to the progression of gastrointestinal cancers. Int J Biochem Cell Biol. 2000;32:633-642. [PubMed] |
| 106. | Cejka D, Preusser M, Woehrer A, Sieghart W, Strommer S, Werzowa J, Fuereder T, Wacheck V. Everolimus (RAD001) and anti-angiogenic cyclophosphamide show long-term control of gastric cancer growth in vivo. Cancer Biol Ther. 2008;7:1377-1385. [PubMed] |
| 107. | Okamoto I, Doi T, Ohtsu A, Miyazaki M, Tsuya A, Kurei K, Kobayashi K, Nakagawa K. Phase I clinical and pharmacokinetic study of RAD001 (everolimus) administered daily to Japanese patients with advanced solid tumors. Jpn J Clin Oncol. 2010;40:17-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 108. | Lim T, Lee J, Lee DJ, Lee HY, Han B, Baek KK, Ahn HK, Lee SJ, Park SH, Park JO. Phase I trial of capecitabine plus everolimus (RAD001) in patients with previously treated metastatic gastric cancer. Cancer Chemother Pharmacol. 2011;68:255-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 109. | Lee SJ, Lee J, Lee J, Park SH, Park JO, Park YS, Lim HY, Kim KM, Do IG, Jung SH. Phase II trial of capecitabine and everolimus (RAD001) combination in refractory gastric cancer patients. Invest New Drugs. 2013;31:1580-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 110. | Doi T, Muro K, Boku N, Yamada Y, Nishina T, Takiuchi H, Komatsu Y, Hamamoto Y, Ohno N, Fujita Y. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol. 2010;28:1904-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 111. | Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935-3943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 112. | Das K, Gunasegaran B, Tan IB, Deng N, Lim KH, Tan P. Mutually exclusive FGFR2, HER2, and KRAS gene amplifications in gastric cancer revealed by multicolour FISH. Cancer Lett. 2014;353:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 113. | Su X, Zhan P, Gavine PR, Morgan S, Womack C, Ni X, Shen D, Bang YJ, Im SA, Ho Kim W. FGFR2 amplification has prognostic significance in gastric cancer: results from a large international multicentre study. Br J Cancer. 2014;110:967-975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 114. | Beckwith KA, Byrd JC, Muthusamy N. Tetraspanins as therapeutic targets in hematological malignancy: a concise review. Front Physiol. 2015;6:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 115. | Murayama Y, Oritani K, Tsutsui S. Novel CD9-targeted therapies in gastric cancer. World J Gastroenterol. 2015;21:3206-3213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 116. | Hori H, Yano S, Koufuji K, Takeda J, Shirouzu K. CD9 expression in gastric cancer and its significance. J Surg Res. 2004;117:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 117. | Nakamoto T, Murayama Y, Oritani K, Boucheix C, Rubinstein E, Nishida M, Katsube F, Watabe K, Kiso S, Tsutsui S. A novel therapeutic strategy with anti-CD9 antibody in gastric cancers. J Gastroenterol. 2009;44:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 118. | Iravani O, Tay BW, Chua PJ, Yip GW, Bay BH. Claudins and gastric carcinogenesis. Exp Biol Med (Maywood). 2013;238:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, Huber C, Türeci O. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14:7624-7634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 299] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 120. | Schuler M, Lordick F, Krilova A, Helbig U, Schulze-Bergkamen H, Thuss-Patience P, Wichert G, Schulmann K, Trarbach T, Bauer S. Safety, tolerability, and efficacy of the first-in-class antibody IMAB362 targeting claudin 18.2 in patients with metastatic gastroesophageal adenocarcinomas. J Clin Oncol. 2013;abstr 4080. |
| 121. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6283] [Article Influence: 483.3] [Reference Citation Analysis (0)] |
| 122. | Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3192] [Cited by in RCA: 3321] [Article Influence: 276.8] [Reference Citation Analysis (0)] |
| 123. | Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C, O’Day SJ, Hoos A, Humphrey R, Berman DM. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci. 2013;1291:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 124. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11779] [Article Influence: 785.3] [Reference Citation Analysis (0)] |
| 125. | Ralph C, Elkord E, Burt DJ, O’Dwyer JF, Austin EB, Stern PL, Hawkins RE, Thistlethwaite FC. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res. 2010;16:1662-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 126. | Moehler MH, Tan IB, Balogh A, Sanchez TK, Bang YJ. Sequential ipilimumab (Ipi) versus best supportive care (BSC) following first-line chemotherapy (Ctx) in patients (pts) with unresectable locally advanced or metastatic gastric or gastro-esophageal junction (GEJ) cancer: A randomized, open-label, two-arm, phase II trial (CA184-162) of immunotherapy as a maintenance concept. J Clin Oncol. 2013;abstr TPS4151. |
| 127. | Sun J, Xu K, Wu C, Wang Y, Hu Y, Zhu Y, Chen Y, Shi Q, Yu G, Zhang X. PD-L1 expression analysis in gastric carcinoma tissue and blocking of tumor-associated PD-L1 signaling by two functional monoclonal antibodies. Tissue Antigens. 2007;69:19-27. [PubMed] |
| 128. | Callahan MK, Chan E, Morse M, Pillai RN, Bono P, Jaeger D, Evans TR, Chau I, Calvo E, Le D. Phase I/II, open-label study of nivolumab (anti-PD-1; BMS-936558, ONO-4538) as monotherapy or combined with ipilimumab in advanced or metastatic solid tumors. J Clin Oncol. 2014;32 suppl:abstr TPS3114. |
| 129. | Li S, Lei X, Zhang J, Yang H, Liu J, Xu C. Insulin-like growth factor 1 promotes growth of gastric cancer by inhibiting foxo1 nuclear retention. Tumour Biol. 2015;36:4519-4523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Matsubara J, Yamada Y, Hirashima Y, Takahari D, Okita NT, Kato K, Hamaguchi T, Shirao K, Shimada Y, Shimoda T. Impact of insulin-like growth factor type 1 receptor, epidermal growth factor receptor, and HER2 expressions on outcomes of patients with gastric cancer. Clin Cancer Res. 2008;14:3022-3029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 131. | Murakami H, Doi T, Yamamoto N, Watanabe J, Boku N, Fuse N, Yoshino T, Ohtsu A, Otani S, Shibayama K. Phase 1 study of ganitumab (AMG 479), a fully human monoclonal antibody against the insulin-like growth factor receptor type I (IGF1R), in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2012;70:407-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 132. | Wang J, Cui S, Zhang X, Wu Y, Tang H. High expression of heat shock protein 90 is associated with tumor aggressiveness and poor prognosis in patients with advanced gastric cancer. PLoS One. 2013;8:e62876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 133. | Liu H, Lu J, Hua Y, Zhang P, Liang Z, Ruan L, Lian C, Shi H, Chen K, Tu Z. Targeting heat-shock protein 90 with ganetespib for molecularly targeted therapy of gastric cancer. Cell Death Dis. 2015;6:e1595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 134. | Mahadevan D, Kurtin SE, Cleary JM, Gandhi L, Lyons JF, Lock V, Lewis S, Shapiro G. First-in-human phase I study: Results of a second-generation non-ansamycin heat shock protein 90 (HSP90) inhibitor AT13387 in refractory solid tumors. J Clin Oncol. 2012;abstr 3028. |
| 135. | Kwak EL, Abrams TA, Carpenter A, Wolpin BM, Wadlow RC, Allen J, Heist RS, McCleary NJ, Chan JA, Goessling W. A phase II clinical trial of ganetespib (STA-9090) in previously treated patients with advanced esophagogastric cancers. J Clin Oncol. 2013;abstr 4090. |