Published online Apr 28, 2016. doi: 10.3748/wjg.v22.i16.4091
Peer-review started: December 17, 2015
First decision: January 13, 2016
Revised: March 9, 2016
Accepted: March 18, 2016
Article in press: March 18, 2016
Published online: April 28, 2016
Processing time: 125 Days and 5.5 Hours
AIM: To establish a mouse model of alcohol-driven hepatocellular carcinoma (HCC) that develops in livers with alcoholic liver disease (ALD).
METHODS: Adult C57BL/6 male mice received multiple doses of chemical carcinogen diethyl nitrosamine (DEN) followed by 7 wk of 4% Lieber-DeCarli diet. Serum alanine aminotransferase (ALT), alpha fetoprotein (AFP) and liver Cyp2e1 were assessed. Expression of F4/80, CD68 for macrophages and Ly6G, MPO, E-selectin for neutrophils was measured. Macrophage polarization was determined by IL-1β/iNOS (M1) and Arg-1/IL-10/CD163/CD206 (M2) expression. Liver steatosis and fibrosis were measured by oil-red-O and Sirius red staining respectively. HCC development was monitored by magnetic resonance imaging, confirmed by histology. Cellular proliferation was assessed by proliferating cell nuclear antigen (PCNA).
RESULTS: Alcohol-DEN mice showed higher ALTs than pair fed-DEN mice throughout the alcohol feeding without weight gain. Alcohol feeding resulted in increased ALT, liver steatosis and inflammation compared to pair-fed controls. Alcohol-DEN mice had reduced steatosis and increased fibrosis indicating advanced liver disease. Molecular characterization showed highest levels of both neutrophil and macrophage markers in alcohol-DEN livers. Importantly, M2 macrophages were predominantly higher in alcohol-DEN livers. Magnetic resonance imaging revealed increased numbers of intrahepatic cysts and liver histology confirmed the presence of early HCC in alcohol-DEN mice compared to all other groups. This correlated with increased serum alpha-fetoprotein, a marker of HCC, in alcohol-DEN mice. PCNA immunostaining revealed significantly increased hepatocyte proliferation in livers from alcohol-DEN compared to pair fed-DEN or alcohol-fed mice.
CONCLUSION: We describe a new 12-wk HCC model in adult mice that develops in livers with alcoholic hepatitis and defines ALD as co-factor in HCC.
Core tip: Chronic alcohol consumption leads to broad spectrum of disorders from steatosis to steatohepatitis to cirrhosis and hepatocellular cancer (HCC). Currently there are no animal models of HCC that evaluate effect of alcoholic liver disease (ALD) on HCC acceleration. We describe a new 12-wk HCC model in adult mice that develops in livers with alcoholic hepatitis and defines ALD as co-factor in HCC. Our model involves sequential step-wise progression of ALD to HCC and highlights the role of alcohol as a tumor promoting agent. Importantly, our model shows inflammation, cellular regeneration, and fibrosis, all the features of human HCC pathogenesis.
- Citation: Ambade A, Satishchandran A, Gyongyosi B, Lowe P, Szabo G. Adult mouse model of early hepatocellular carcinoma promoted by alcoholic liver disease. World J Gastroenterol 2016; 22(16): 4091-4108
- URL: https://www.wjgnet.com/1007-9327/full/v22/i16/4091.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i16.4091
Hepatocellular carcinoma (HCC) is the most common liver cancer, and worldwide it represents the fifth most common primary cancer diagnosed in patients[1]. HCC is also the third leading cause of cancer related mortality globally. In the United States, the incidence of HCC has tripled over the last two decades[2]. Unlike many other cancers with known associated risk factors, the underlying molecular pathophysiology for HCC is still not completely known. Most commonly, the incidence of HCC is linked to known risk factors including hepatitis B and C, aflatoxin and chronic alcohol drinking[3]. About 40% of individuals who chronically consume alcohol develop fatty liver, the first pathological condition that, with continued alcohol use, can advance to alcoholic hepatitis and cirrhosis[4]. In humans, alcohol is a known causal agent in the development of HCC[1]. Most HCC patients have a history of chronic liver disease and cirrhosis and cirrhosis remains the single common precursor to HCC development[5].
Increasing evidence suggests that chronic tissue inflammation promotes tumor development[6]. In alcoholic hepatitis recruitment of macrophages and neutrophils to the liver and activation of resident Kupffer cells results in high levels of pro-inflammatory cytokines (TNFα, IL-1β, MCP-1, IL-6) both in the liver and systemic circulation[7]. Because alcoholic liver disease (ALD) and alcoholic hepatitis are characterized by significant liver inflammation, here we hypothesized that the alcoholic liver environment may expedite HCC development.
Hepatocarcinogenesis is a multistep, multistage process that involves genetic and epigenetic alterations that ultimately lead to malignant transformation of hepatocytes[8]. Several animal models have been reported that mimic different steps leading to HCC. These models have helped to identify molecular mechanisms that underlay the development of HCC including genetically modified mouse models, transgenic models expressing viral genes, transgenic mice over-expressing oncogenes and transgenic models over-expressing growth factors[9,10]. Although valuable in facilitating detailed investigation of molecular pathways, the genetically modified animal models have been shown to vary significantly in their pattern of HCC development[10]. Of the chemically induced HCC animal models, the N-nitrosodiethylamine (DEN) based models are the most widely used and accepted. DEN acts as alkylating agent for DNA bases which initiates the formation of neoplasms[11].
Chronic alcohol consumption causes morbidity and leads to broad spectrum of disorders from steatosis to steatohepatitis to cirrhosis and hepatocellular cancer[12]. Currently there are no animal models of HCC that evaluates the effect of ALD on HCC acceleration. In this study, we administered 6 doses of DEN to 4 wk old adult C57bl/6 male mice followed by 7 wk of Lieber DeCarli alcohol diet feeding. We report up-regulation of liver inflammatory cell infiltration and fibrotic markers in mice receiving alcohol + DEN. Further, alcohol + DEN mice showed characteristic appearance of intrahepatic cysts and hepatic neoplastic foci at the end of the alcohol feeding compared to mice exposed to alcohol alone and DEN alone, respectively, supporting acceleration of HCC by ALD.
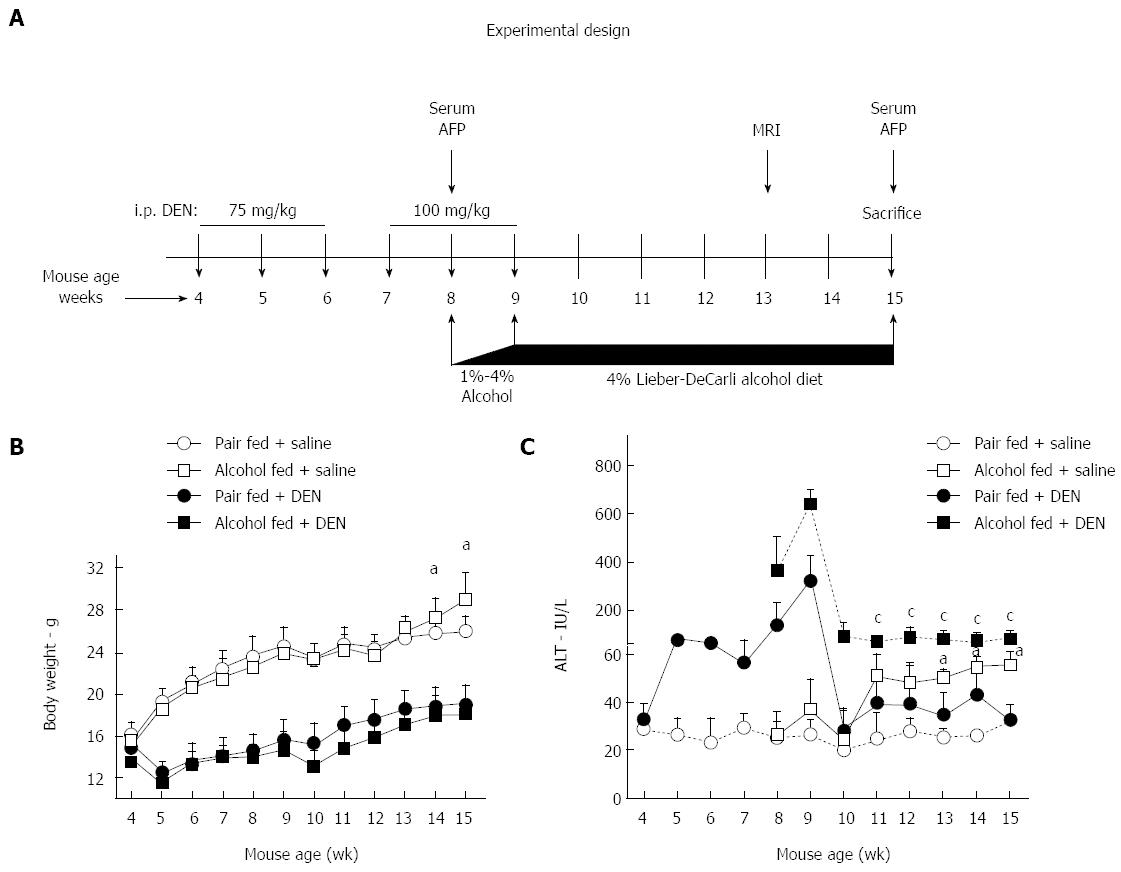
To establish a mouse model of hepatocellular cancer based on the Lieber-DeCarli alcohol diet, we injected 4 wk old C57bl/6 male mice with total 6 doses of DEN (Sigma, MO, United States) intraperitoneally. As shown in Figure 1A, a dose of 75 mg/kg DEN was administered weekly for the first 3 wk followed by a dose of 100 mg/kg DEN for next 3 wk. At week 8, the mice were randomly divided into alcohol and pair fed (control) groups. Age matched groups of mice without DEN were included in the study to understand the effect of chronic alcohol feeding. Depending on the experimental design, mice were fed 4% Lieber-Decarli alcohol diet or calorie matched control diet (Pair fed), (Bio-Serv, Flemington, NJ, United States) for 7 wk (Figure 1A). At sacrifice, blood and liver tissues were collected for further assays. The study protocol was approved by the Institutional Animal Use and Care Committee of the University of Massachusetts Medical School.
Magnetic resonance imaging (MRI) of liver was performed to monitor hyperplastic changes in liver. Images were obtained using 3T Philips Achieva whole-body MR scanner (Philips Medical Systems, Best, Netherlands) with a custom-made solenoid T/R coil with a diameter of 30 mm. The animals were anesthetized with 5% isoflurane mixed with carbogen (95% O2/5% CO2) and were maintained with 1% to 2% isofluorane. Coronal T2-weighted spin echo images were acquired with respiratory triggering to reduce the motion artifacts. The respiration rate was monitored with an optical probe (Model 1025T Monitoring and Gating System, SA Instruments Inc, Stony Brook, NY, United States). The output signal from the respiration monitor was used to trigger, in real time, the MR acquisition. As a consequence of the triggered acquisition, the TR value of around 2000 ms, corresponding to the respiration rate of around 30 bpm, was determined. Other imaging parameters were: echo time (TE) of 70 ms, flip angel of 90 degrees, TSE-factor of 8, number of average = 4, matrix size of 148 × 120, field of view of 30 × 25 mm2, slice thickness of 1 mm with no gap, acquisition time around 4 min for 22 slices. Hyperplastic nodules were distinguished from normal liver tissues on basis of differences in homogeneity and signal intensity. Pixel based nodule area quantitation was performed using ImageJ software.
Serum alanine aminotransferase (ALT) activity was determined using a kinetic method (D-TEK LLC, PA, United States). Serum AFP was assayed by ELISA (R&D systems, Minneapolis, MN, United States). Serum bilirubin was estimated using bilirubin assay kit (Abnova, Walnut, CA, United States) and the endotoxin levels were measured using LAL Chromogenic Endotoxin Quantitation Kit (Thermo Scientific, Rockford, IL, United States). Liver triglycerides were assayed in liver whole cell lysates using L-Type TriGlyceride M kit (Wako Diagnostics, Richmond, VA, United States).
Total RNA was extracted using the Direct-zol RNA MiniPrep according to the manufacturer’s instructions (Zymo Research, Irvin, CA, United States). RNA was quantified using Nanodrop 2000 (Thermo Scientific, Wilmington, DE). Complementary DNA (cDNA) synthesis was performed by reverse transcription of total RNA using the iScript Reverse Transcription Supermix (BIO-RAD, Hercules, CA, United States). Real-time quantitative PCR was performed using the CFX96 real-time detection system (Bio-Rad Laboratories, Hercules, CA, United States). Primers were synthesized by IDT, Inc. (Coralville, IA, United States). Accumulation of PCR products was detected by monitoring the increase in fluorescence of double-stranded DNA-binding dye SYBR Green during amplification. Relative gene expression was calculated by the comparative cycle threshold (Ct) method. The expression of target genes was normalized to the house-keeping gene, 18S rRNA, in each sample and the fold-change in the target gene expression between experimental groups was expressed as a ratio. Melt-curve analysis was used to confirm the authenticity of the PCR products.
Whole cell lysates, were prepared from mouse livers as described previously[13]. Proteins of interest were detected by immunoblotting with specific primary antibodies against: Cyp2e1 (ab1252 Millipore), β-actin-HRP (ab49900; Abcam). Respective horseradish peroxidase-labeled secondary antibodies were from Santacruz Biotechnology (Dallas, TX, United States). The specific immunoreactive bands of interest were detected by chemiluminescence (Bio-Rad, Hercules, CA, United States). The immunoreactive bands were quantified by densitometric analysis using the UVP System (Bio-Rad Laboratories, Hercules, CA, United States).
Sections of formalin-fixed, paraffin-embedded livers were stained with hematoxylin and eosin, or Sirius Red and assessed for histological features of carcinoma and fibrosis. To assess steatosis, Oil Red O staining was performed on cryofrozen liver tissue sections (OCT). Immunohistochemistry staining for PCNA (ab29; Abcam), MPO (ab9535; Abcam) was performed on formalin-fixed, paraffin-embedded livers according to the manufacturer’s instructions. Images were acquired on a Nikon microscope at magnification mentioned in figure legends. The quantitation of the Oil Red O, Sirius Red staining was performed using ImageJ software.
Statistical significance was determined using two-tailed t-test; ANOVA and Dunnett’s multiple comparison post-test were used to compare the means of multiple groups. Data are shown as mean ± SD and were considered statistically significant at P < 0.05. GraphPad Prism 6.02 (GraphPad Software Inc., La Jolla, CA, United States) was used for analysis.
ALD and alcoholic cirrhosis are major risk factors for HCC development in humans[14]. Here we hypothesized that the alcoholic liver environment expedites liver tumor development after repeated administration of a chemical carcinogen, DEN. In this study, male C57bl/6 mice received 6 weekly DEN injections, starting at age of 4 wk followed by 7 wk of chronic alcohol administration (Figure 1A). Weekly body weight monitoring showed that compared to pair-fed mice, alcohol-fed saline-injected mice gained weight significantly after 7 wk of alcohol feeding. Both DEN injected pair-fed and DEN-injected alcohol-fed mice showed attenuated weight gain during the entire course of experiment compared to mice without DEN injection (Figure 1B).
Serum ALT, a marker of hepatic injury, was significantly increased after the first DEN administration and remained elevated after repeated doses. In addition, co-administration of DEN and alcohol further increased ALT (weeks 8-9) that remained elevated in alcohol-fed mice. Introduction of alcohol feeding even in the saline injected group resulted in a significant and sustained increase in ALT compared to pair-fed control diet (Figure 1C). These results suggested that while DEN and alcohol, respectively induce liver injury, their combination results in synergistic hepatic injury leading to sustained high serum ALT levels.
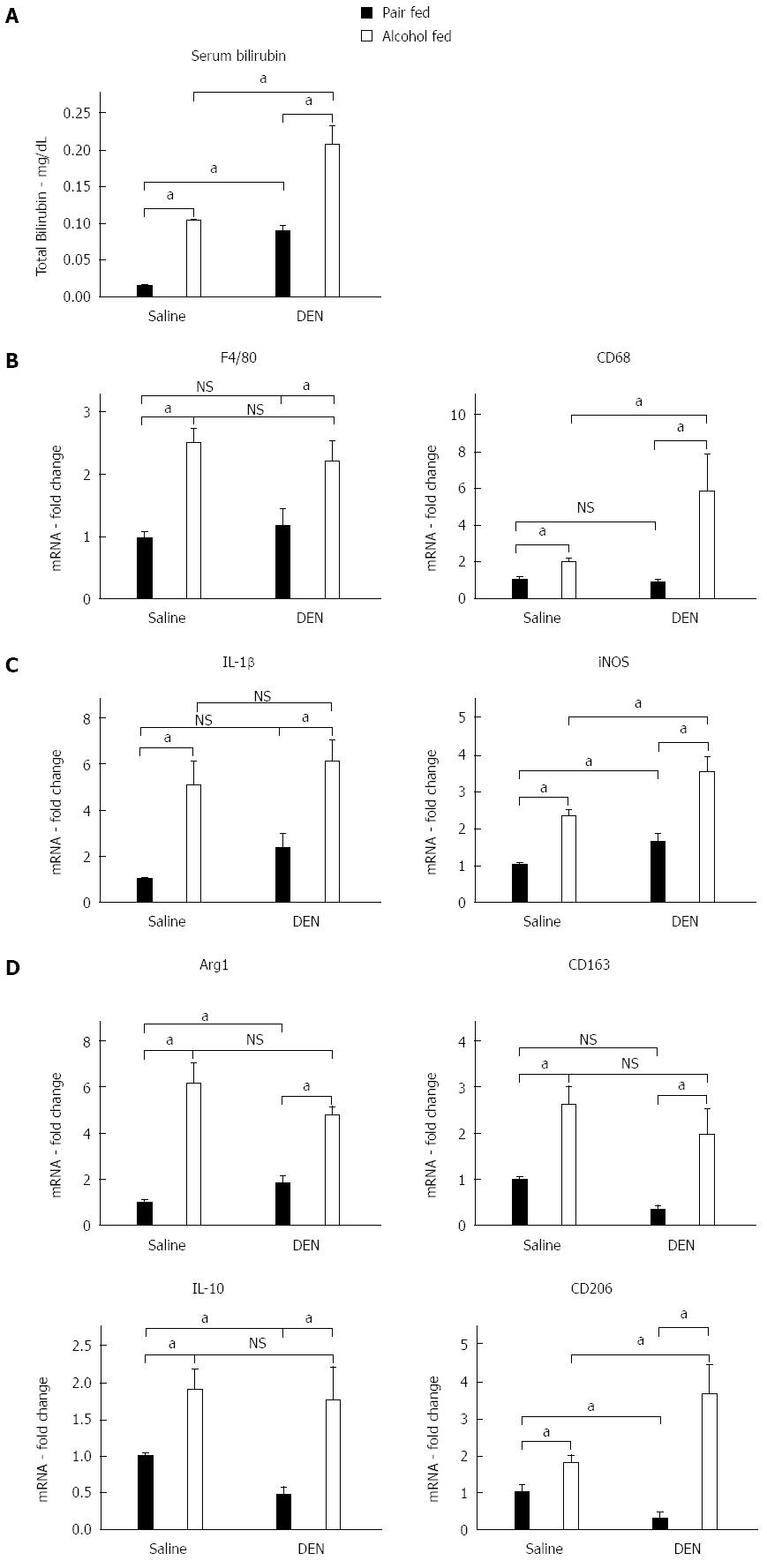
Previous reports showed that chronic alcohol feeding results in significant liver inflammation[15]. Human alcoholic hepatitis is characterized by liver injury, increased serum transaminases, increased bilirubin and inflammatory cell infiltration into the liver[16]. Thus, first, we assessed serum bilirubin which is increased in alcoholic hepatitis as well as in human HCC[17]. We found that similar to human patients with alcoholic hepatitis, serum bilirubin was increased in mice after chronic alcohol administration (Figure 2A). Serum bilirubin was also increased in mice that received DEN alone (Figure 2A). However, the greatest extent of serum bilirubin increase was found in alcohol-fed DEN-injected mice compared to any other experimental groups. This was indicative of advanced liver disease and raised the possibility of HCC (Figure 2A).
Chronic alcohol exposure also induces Kupffer cell activation and infiltration of macrophages into the liver and results in secretion of pro-inflammatory cytokines that drive inflammation in alcoholic hepatitis[18]. Kupffer cells and macrophages are the most abundant pro-inflammatory cell type in the liver and are known to contribute to ALDs[19]. Thus, next, we assessed the expression of the macrophage/Kupffer cell markers, F4/80, CD68 in the liver. The expression of the pan-macrophage marker, F4/80, was significantly elevated in all alcohol-fed mice with or without DEN. The macrophage activation marker, CD68, was up regulated to greater extent in alcohol-fed DEN-injected mice as compared to alcohol-fed saline-injected mice suggesting the presence of activated inflammatory macrophages in the liver (Figure 2B). Recent literature suggests that macrophages can be further classified into pro-inflammtory M1 or anti-inflammatory M2 macrophages[20]. These macrophage subtypes express unique markers[21]. We found a significant increase in the expression of M1 macrophage markers, IL-1β and iNOS, (Figure 2C) in livers of alcohol fed mice. Importantly, iNOS expression was significantly higher in alcohol fed-DEN injected mice compared to alcohol alone (Figure 2C). Livers from alcohol fed mice also showed higher expression of M2 macrophage markers, Arg1, CD163, IL-10 and CD206, compared to non-alcohol controls (Figure 2D) and CD206 the highest in alcohol fed-DEN injected mice (Figure 2D). These observations suggested increased macrophage recruitment to precancerous liver with mixed M1/M2 phenotype.
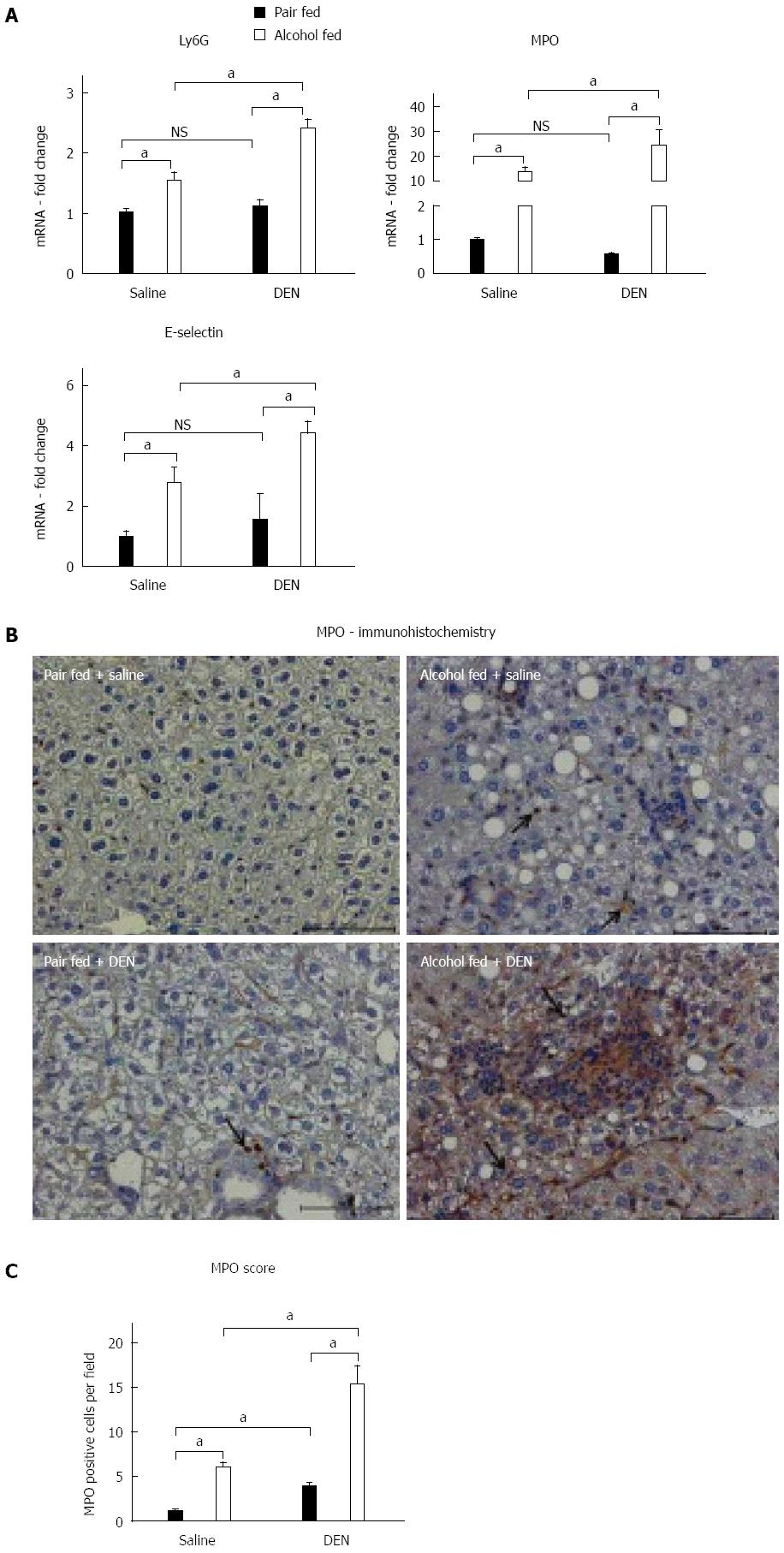
Neutrophils play a significant role in alcoholic hepatitis and also contribute to HCC[22]. Other studies have shown that alcohol alone upregulated expression of the characteristic neutrophil markers, Ly6G, MPO and E-selectin[23]. In our study, messenger RNA levels of these neutrophil markers were significantly up-regulated in alcohol-fed (Figure 3A) compared to pair-fed mice. The highest expression of Ly6G, MPO and E-selectin was found in alcohol-fed DEN-injected mice as compared to any other experimental groups (Figure 3A). DEN alone resulted in no change in neutrophil marker expression compared to saline injected controls (Figure 3A). We also confirmed the upregulation of MPO at the protein level using MPO immunostaining (Figure 3B) that showed significantly increased numbers of infiltrating neutrophils in alcohol-fed saline-injected mice compared to pair-fed controls (Figure 3B). Moreover, the highest levels of MPO staining, confirming neutrophil infiltration and activation, were present in the livers of DEN-injected alcohol-fed mice (Figure 3C). Taken together, our data showed a significant increase in key features of alcoholic hepatitis including increased serum bilirubin, a clinically relevant diagnostic marker, elevated macrophage, neutrophil after alcohol administration and an even greater elevation in all of these indices in the alcohol + DEN treated mice markers in our experimental model.
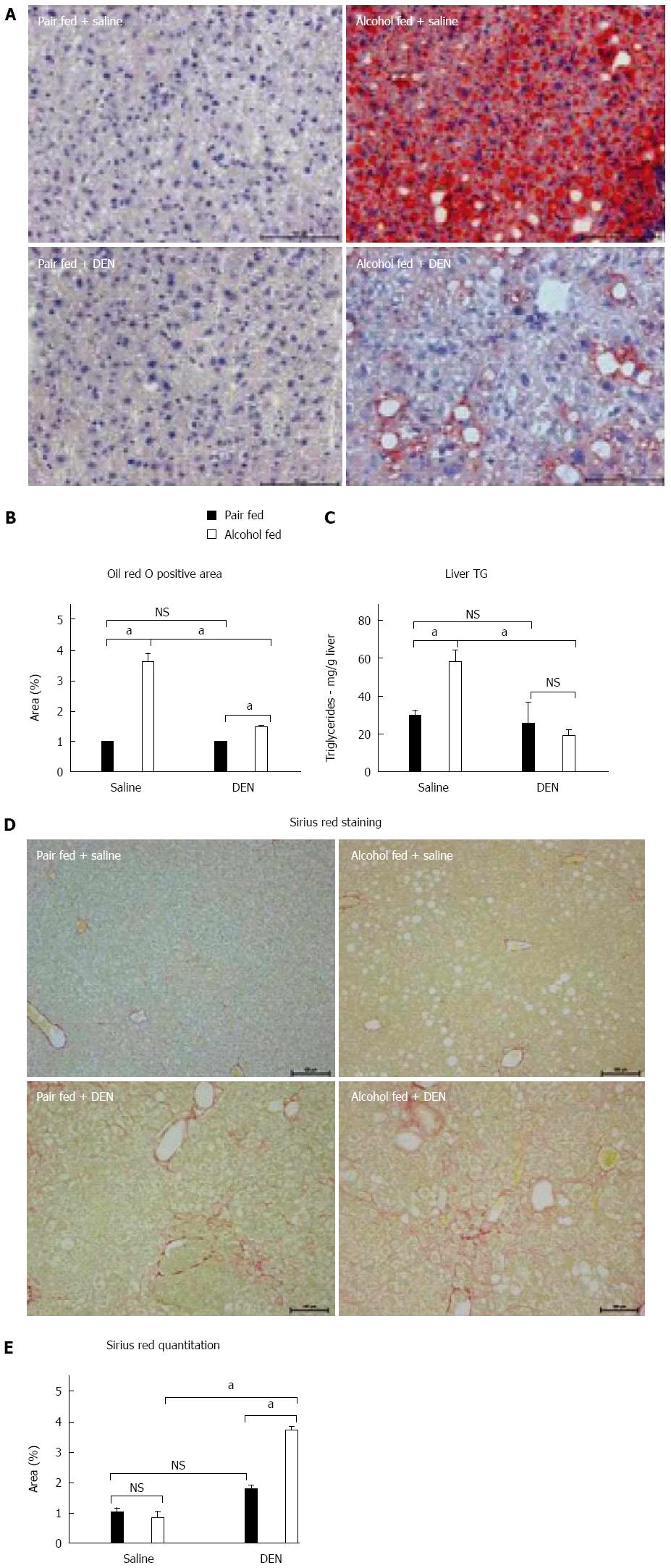
As we found sustained and increased liver injury and inflammation after DEN + alcohol administration, we next analyzed steatosis and fibrosis in the livers. There was a significant increase in steatosis in the livers of alcohol-fed mice compared to the pair-fed control as indicated by Oil-Red-O staining (Figure 4A). Interestingly, alcohol-induced liver steatosis was attenuated in DEN-injected mice compared to saline-injected alcohol-fed mice (Figure 4A). DEN alone did not induce steatosis (Figure 4A and B). Alcohol induced steatosis was further confirmed by measuring hepatic triglyceride content, which also showed reduction in alcohol-fed DEN-injected mice as compared to alcohol-fed saline-injected mice (Figure 4C). While steatosis is an early presentation of ALD, prolonged liver disease results in fibrosis that is considered as a sequential step towards HCC development[24]. Hence, we assessed the extent of fibrosis using Sirius Red staining. As shown in Figure 4D, alcohol-fed DEN-injected mice showed significantly increased fibrosis by Sirius Red staining indicating the presence of liver fibrosis compared to all other groups (Figure 4D). The increased fibrosis was quantified in Figure 4E. Thus, our experimental model of HCC with DEN + alcohol showed minimal steatosis and significant fibrosis indicative of advanced liver disease.
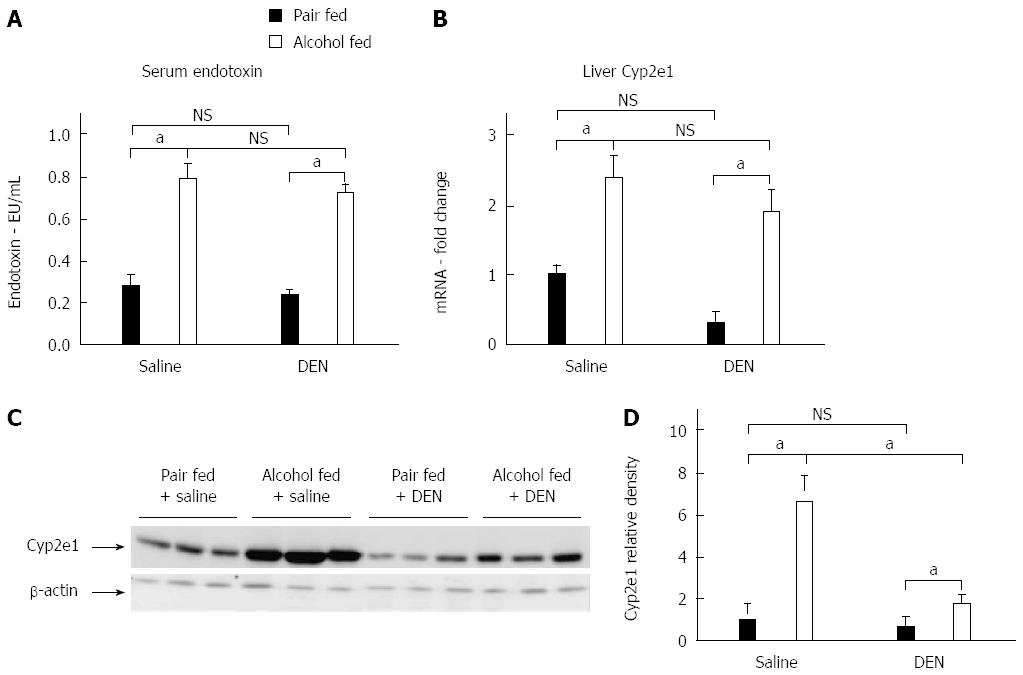
Chronic alcohol exposure leads to an increase in levels of circulating endotoxin, which can activate the immune cells in the liver causing inflammation[25]. TLR4 signaling has been shown to contribute to HCC[26]. We observed a significant increase in serum endotoxin levels after alcohol feeding while there was no significant difference between the endotoxin levels of alcohol-fed DEN injected mice and alcohol alone fed mice, at the time of sacrifice (Figure 5A). Cyp2e1, the enzyme that metabolizes alcohol in liver, was upregulated at both mRNA and liver protein levels in alcohol fed mice (Figure 5B and C) as compared to pair fed or DEN alone mice. Interestingly, the liver protein levels of Cyp2e1 in alcohol + DEN mice were significantly lower than alcohol alone fed mice (Figure 5D). This data supported the hypothesis of increased inflammation and Cyp2e1 activation after alcohol exposure in our experimental model.
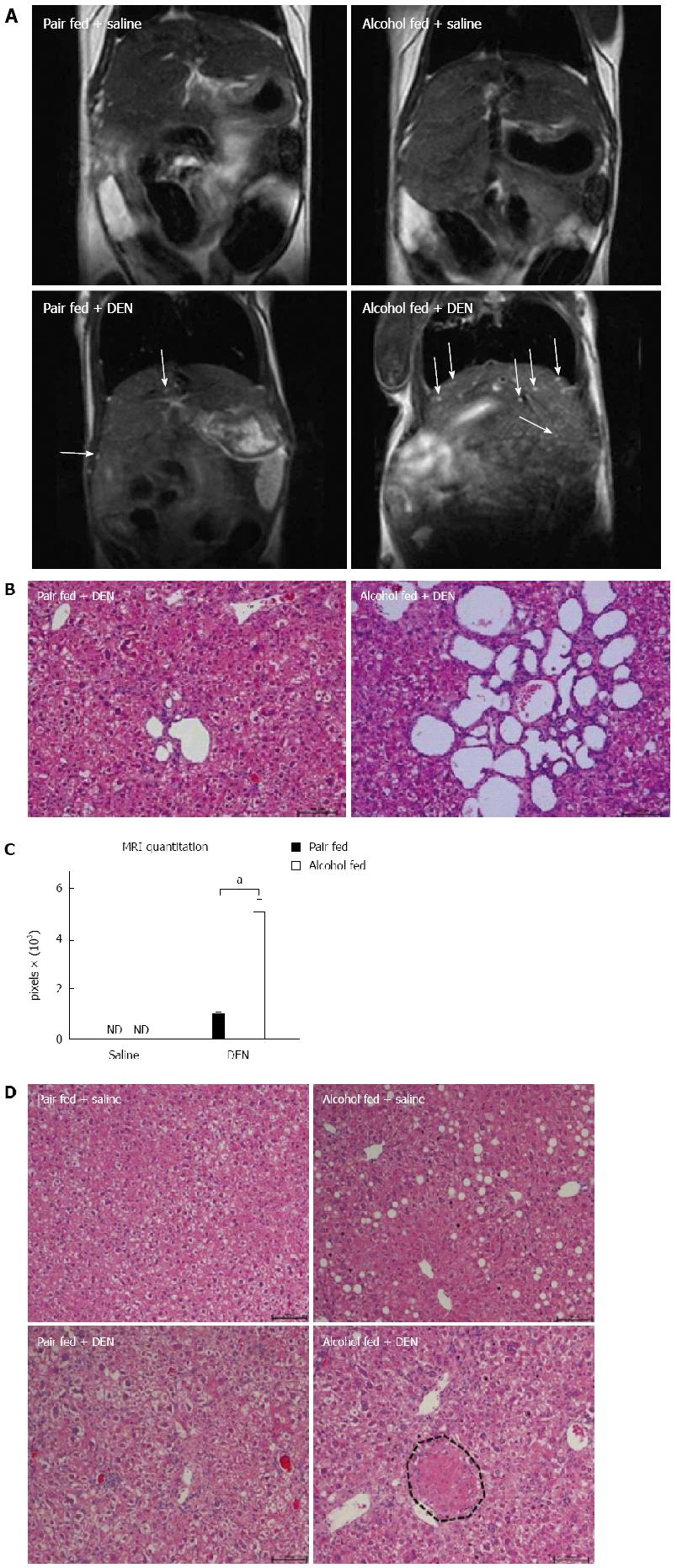
To ascertain the liver tumor and HCC development, we performed MRI on all experimental groups before sacrifice, at week 13 of age. The liver MRI revealed a few bright spots, characteristics of intrahepatic cysts, in DEN treated mice, however significantly higher numbers of hepatic cysts were present in the DEN + alcohol mice (Figure 6A). The increased numbers of intrahepatic cysts in DEN + alcohol mice were further confirmed by histology (Figure 6B). Livers of pair-fed or alcohol alone fed mice showed no lesions on MRI. The area of cysts from MRI data was quantified using ImageJ and shown in Figure 6C. In addition to hepatic cysts, histology evaluation revealed the presence of numerous liver nodules characterized by hepatic hyperplasia exclusively in alcohol-fed DEN-injected mice compared to all other experimental groups (Figure 6D). The pair-fed DEN-injected mice had very few intrahepatic cysts and no hepatic hyperplastic nodules. None of the alcohol fed or pair fed mice had cysts or hyperplastic nodules. These results indicated that alcoholic hepatitis expedited early liver tumor development.
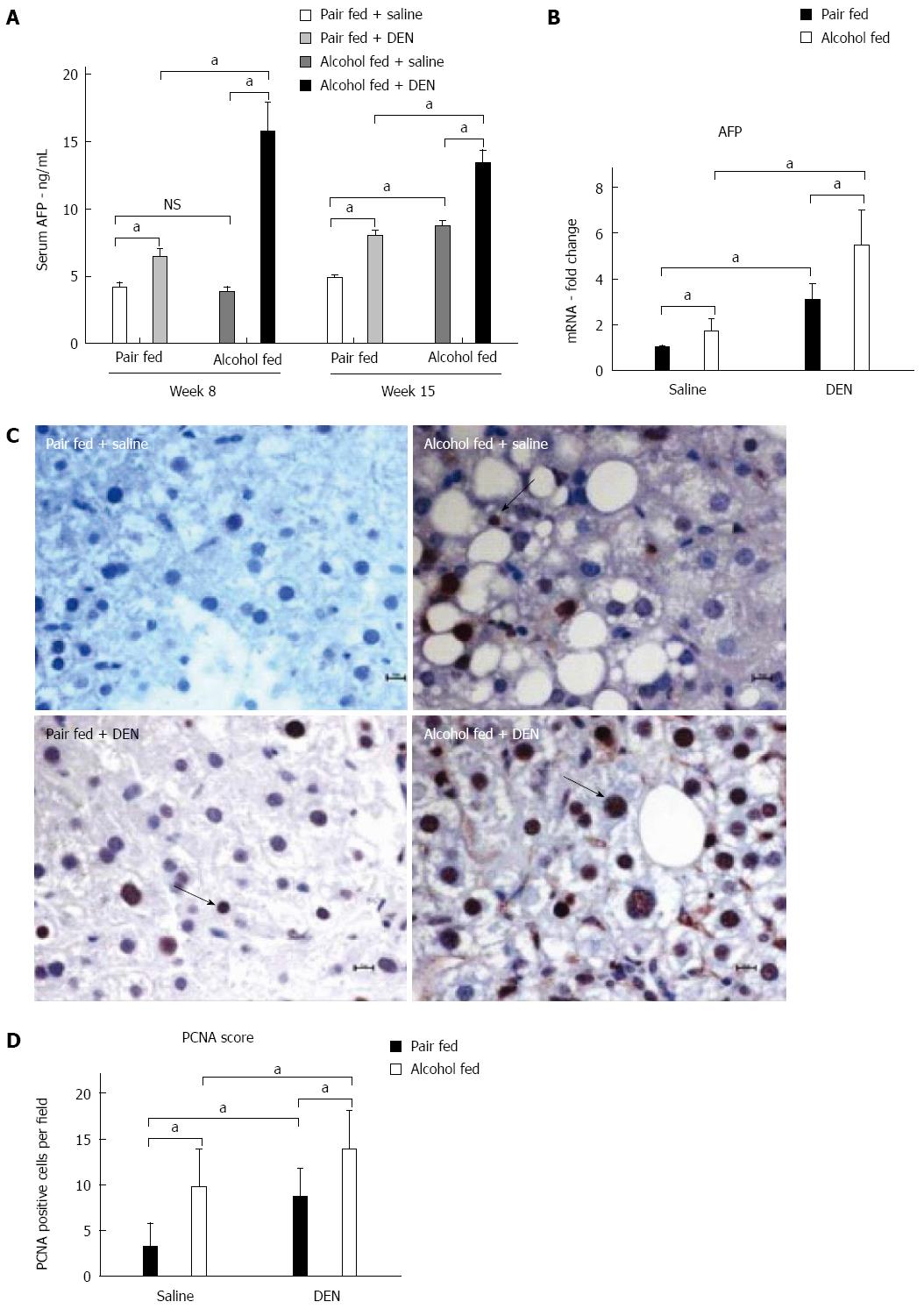
Next, we assessed the expression of characteristic HCC markers. The expression of AFP is directly associated with hepatocyte differentiation and HCC progression[27]. More importantly, AFP is the most commonly used noninvasive marker for HCC diagnosis[17]. We measured serum AFP levels at week 8 (at the beginning of alcohol feeding) and at week 15 (at the end of alcohol feeding). DEN alone increased serum AFP at both 8 and 15 wk while alcohol alone increased AFP only at 15 wk (Figure 7A). Importantly, there was a significant increase in serum AFP in alcohol + DEN mice compared to the DEN alone or alcohol alone groups not only at 8 but also at 15 wk, indicating early regeneration and potentially, HCC development (Figure 7A and B).
Chronic alcohol feeding is reported to induce hepatic proliferation[28]. Thus, to estimate cell proliferation, we performed PCNA staining on liver tissue sections (Figure 7C). Immunohistochemistry staining for PCNA showed significantly higher number of PCNA positive nuclei in alcohol-fed DEN-injected mice compared to all other groups (Figure 7C). Of note, both alcohol-fed saline-injected and pair-fed DEN-injected mice showed increased numbers of PCNA positive nuclei compared to pair-fed saline-injected controls (Figure 7D). Taken together, our experimental model showed hepatic hyperplasia confirmed on histology, significant up regulation in AFP and cell proliferation, providing evidence for the synergistic action of alcohol as a tumor promoting agent in DEN-induced early HCC.
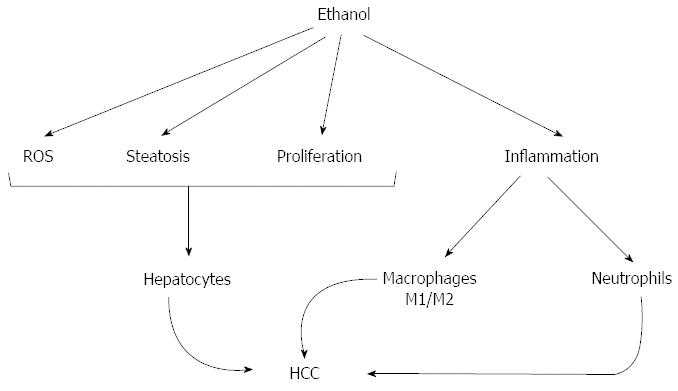
Chronic alcohol use leads to alcoholic hepatitis and cirrhosis[14]. Epidemiological data suggests that chronic heavy alcohol consumption and ALD is a significant risk factor towards the development of HCC[29]. In this study, we introduce a mouse model that demonstrates that chronic alcohol feeding resulting in alcoholic hepatitis in adult mice, expedites DEN (chemical) induced early liver tumor development. We found that features of human alcoholic hepatitis including inflammation, steatosis and fibrosis were all present in DEN-injected mice after alcohol feeding and resulted in present numbers of early HCC neoplastic foci (Figure 8). Our data indicate that increased neutrophil and macrophage activation is triggered in the alcoholic liver tissue microenvironment and may contribute to acceleration of HCC (Figure 8). In addition to steatohepatitis, liver fibrosis was also present in DEN + alcohol treated mice as indicated by Sirius Red staining. Further, our model showed increased numbers of intrahepatic cysts by the MRI that were confirmed as hepatobiliary cysts by histology and are features of early hepatobiliary cancer. Our model also showed significant induction in serum AFP, one of the well-established markers of HCC, upon alcohol feeding in the DEN-injected mice. Finally, our data showed enhanced cellular proliferation in response to chronic alcohol feeding which promoted progression to HCC.
DEN has been used since the 1960s to induce HCCs in laboratory animals, and is the most widely used chemical to induce liver cancer in mice[10]. DEN undergoes metabolic activation in hepatocytes by enzymes of the cytochrome P450 family and acts as a complete carcinogen, if injected in mice younger than 2 wk, when hepatocytes are still actively proliferating[30]. When administered to adult mice, tumor promotion is required and can be achieved by phenobarbital, carbon tetrachloride, partial hepatectomy. Our experimental model used 4 wk old male mice and provided evidence for the role of alcohol as tumor promoting agent in adult mice. In our study, male mice were chosen because of the higher prevalence of HCC in males compared to females in humans[3]. Irrespective of the promoting agent used, DEN based HCC models take at least 5 mo to show the first signs of tumor. Our model using alcohol as a tumor promoting agent demonstrated the first signs of early HCC in a much shorter time of 3 mo.
Although several carcinogen initiated HCC animal models have been described, none of them so far evaluated alcoholic hepatitis and ALD as a tumor promoting factor in wild-type mice[9]. A recent study performed in neonate mice combined chronic alcohol exposure in the drinking water with DEN injection and showed tumors at 48 wk[12]. However, alcohol administration in the drinking water does not cause ALD[31]. In our study, we employed the Lieber-DeCarli alcohol feeding model that results in features of human ALD including steatosis, inflammation and liver fibrosis[32]. By utilizing adult mice and the Lieber-DeCarli alcohol diet, our model shows acceleration of early hepatobiliary tumors after a chemical carcinogen exposure with some histopathological resemblance to human ALD.
Chemical toxins and viral agents are the two well known etiologies of hepatocellular cancer[1]. Irrespective of the underlying etiology, cellular inflammation is the common feature that has been shown to be present in human HCC patients as well as animal models[33]. It is also known that persistent inflammation in the absence of infection increases the risk and expedites the development of cancer. Inflammation in the liver is one of the hallmarks of alcoholic hepatitis[33] and it was prominent in our model with the highest level in alcohol-fed DEN treated mice. Activation and recruitment of neutrophils and macrophages to the liver is a well-known feature of alcoholic hepatitis[19,22], steatohepatitis and ALD[34]. In our experiments, we found increased expression and activation of neutrophils and macrophages in alcohol-fed mice and markers of inflammation were even higher in mice with liver tumors after DEN + alcohol feeding. Alcohol-induced sensitization of liver macrophages to portal endotoxin is a hallmark of ALD[35]. LPS recognition by TLR4 on macrophages and other cell types in the liver, activation of downstream signaling pathways culminating in activation of transcription factors such as NF-κB, AP-1 leads to increased inflammatory cytokine production in ALD. Recent literature suggests that macrophages can be further classified into pro-inflammatory M1 or anti-inflammatory M2 macrophages[20]. The M1 phenotype is characterized by the expression of high levels of proinflammatory cytokines, high production of reactive nitrogen and oxygen intermediates, promotion of Th1 response, and strong microbicidal and tumoricidal activity[20,36]. The alternatively activated or M2 macrophages are often referred to as tumor associated macrophages (TAMs)[20]. We observed a significant induction of both M1 (TNFα, iNOS) and M2 markers (Arg1, CD163, CD206) in our experimental model. The presence of M1 macrophages is consistent with the increased proinflammatory activation in ALD. This increase in M2 macrophage is likely suggestive of the presence of TAMs in the liver. TAMs represent the major inflammatory component of the stroma of liver cancer and can affect different aspects of the neoplastic transformation of the tissue. Many observations indicate that TAMs express several M2-associated pro-tumoural functions, including promotion of angiogenesis, matrix remodeling and suppression of adaptive immunity. The pro-tumoural role of TAMs in cancer is further supported by clinical studies that found a correlation between the high macrophage content of tumors and poor patient prognosis. TAMs represent a unique and distinct M2-skewed myeloid population and are now recognized as a potential target for anti-cancer therapy[37]. Therefore, we mixed M1/M2 MΦ presence with the increase in M2 macrophage markers observed in our model may be responsible for promoting tumor development in the DEN exposed alcohol-fed mice.
Alcohol is primarily metabolized in hepatocytes and its metabolites generate ROS, cause inflammation and steatosis. Long term alcohol exposure induces a state of chronic inflammation in the liver[38]. Both DEN and alcohol induce Cyp2E1, and the chronic alcoholic injury induced in our model synergized with the chemical injury to increase hepatocyte proliferation leading to appearance of early neoplastic foci found in our experimental model. In the context of cancer-related inflammation, key intracellular signal transducers have been identified both in preneoplastic cells as well as in inflammatory cells. Among these signaling pathways, sustained activation of transcription factors including NF-κB, p53, HIF-1α, β-catenin/Wnt, and Nrf2 in liver leads to chronic inflammation that is responsible for cancer progression[39]. Thus, the inflammation associated with alcoholic hepatitis may be a significant contributing factor to HCC development in alcohol fed DEN injected mice.
In addition to cellular inflammation, the alcohol fed DEN injected mice showed sustained higher ALT levels. Increased ALT levels are indicator of hepatocyte damage and can be used reliably as a marker of liver injury[40]. Initially in our experimental model, the ALT levels were significantly higher in all DEN injected mice and ALT returned to baseline after cessation of DEN administration. However, the alcohol fed mice showed sustained the higher ALT levels suggesting sustained liver injury. DEN acts as an alkylating agent for DNA bases which initiates the formation of neoplasms[11]. It has been proposed that the charged nucleophilic intermediates of DEN attack DNA bases thereby leading to mutations which initiate cellular transformation[11]. Chemical carcinogens including DEN exert their effect by generating ROS during their metabolism in the liver, which is responsible for DNA damage associated with increased risk of cancer development[39]. Importantly, DEN is metabolized in the liver by Cyp2E1, the same enzyme that metabolizes alcohol[41]. We also observed significantly higher TBARS in alcohol fed DEN injected mice, compared to alcohol alone mice (data not shown). Hence, it is possible that the hepatocytes from alcohol fed DEN injected mice are exposed to extremely higher ROS in addition to DNA damage which may expedite their transformation into early HCC observed in this group exclusively.
The expression of AFP is directly associated with hepatocyte differentiation[27]. Elevated levels of serum AFP are reported in alcoholic hepatitis patients[42]. However, as compared to alcoholic hepatitis patients, the AFP levels are much higher in human HCC patients[43]. Some reports show a clinical correlation between serum AFP and severity of liver disease[44]. Our data of serum AFP levels at week 8 and 15 clearly show the role of alcohol in stimulating AFP expression which can be correlated to advanced liver disease such as HCC. In addition to hepatocytes, the liver progenitor cells (LPC) also produce AFP during their cellular differentiation[45]. The LPCs play an important role in liver regeneration and homeostasis[46,47]. Increased serum AFP in our experimental model is also indicative of enhanced proliferation of LPCs which may be responding to chronic liver injury caused by alcohol feeding. This regenerative response by LPCs may also contribute to HCC development.
Chronic alcohol feeding is reported to induce Cyclin D1 protein that corresponds with enhanced hepatic proliferation[28]. More recently, Lanthier et al[48] reported that alcoholic hepatitis patients demonstrated a significant invasion of LPC and a higher number of proliferating hepatocytes compared to controls. This study on human alcoholic hepatitis patients suggests that hepatic cell proliferation plays a pivotal role in the prognosis of alcoholic hepatitis and HCC. A significantly higher number of PCNA positive cells in DEN + alcohol mice provide another evidence for the role of alcohol as inducer of hepatocyte and LPC proliferation.
Chronic liver injury such as long term alcohol exposure leads to steatohepatitis, hepatitis which can progress to fibrosis[24]. Hepatic fibrosis is an injury healing process in which excessive connective tissue accumulates in the liver[49]. The profibrotic factors like collagen are overproduced to assist the expansion of new hepatocytes. Hepatic fibrosis can progress to cirrhosis and in few cases to HCC[24]. In our experimental model of HCC, the alcohol fed DEN injected mice showed significantly higher fibrosis as compared to alcohol fed (alone) or DEN injected (alone) mice. The molecular pathways associated with fibrosis are very similar to liver regeneration, a cellular process in which mature terminally differentiated hepatocytes proliferate to replace the injured cells[50]. In adult mice, mature hepatocytes exist in a growth-arrested (G0) state which, after liver injury, leave their G0 state and enter the proliferative stages of the cell cycle during which they duplicate necessary components and synthesize new proliferation-specific molecules (G1 phase), replicate their DNA (S phase), and divide (M phase)[51]. It is conceivable that in our experimental model, hepatocytes with damaged DNA undergo multiple rounds of proliferation to complete the regeneration response. However, due to their damaged DNA, continue to proliferate leading to HCC. Other probable explanation could be that the hepatocytes with DEN induced DNA damage are exposed to increased amount of ROS and inflammation due to chronic alcohol exposure, which leads to their constant proliferation resulting in HCC development. These hypotheses can be the focus of future studies.
In conclusion, by combining multiple injections of DEN prior to chronic Lieber-DeCarli alcohol diet feeding, we developed a mouse model that displays the features of early HCC. Importantly, our model involves adult mice with alcoholic hepatitis, liver inflammation, cellular regeneration, and fibrosis, all the features associated with pathogenesis of human HCC. We believe that availability of such a model will provide unique opportunities to understand alcohol associated molecular and cellular mechanisms related to HCC.
The authors would like to thank members of the Szabo lab for their stimulating intellectual discussions and scientific input. The help provided by Shao-Kuan Zheng (Department of Radiology) in acquisition of MRI data is acknowledged. We thank Merin MacDonald and Candice Dufour for assistance with formatting the manuscript. The authors are grateful to services provided by DERC (histology and immunostaining) and CFAR (Primer Synthesis) core facilities at UMASS Medical School. CFAR core facility is supported by the University of Massachusetts Center for AIDS Research (P30 AI042845).
Hepatocellular carcinoma (HCC) is the most common liver cancer, and worldwide it represents the fifth most common primary cancer diagnosed in patients. Chronic alcohol consumption causes morbidity and leads to broad spectrum of disorders from steatosis to steatohepatitis to cirrhosis and hepatocellular cancer.
Several animal models have been reported that mimic different steps leading to HCC. Currently there are no animal models of HCC that evaluate the effect of alcoholic liver disease on HCC acceleration.
In this study, the authors administered 6 doses of diethyl nitrosamine (DEN) to 4 wk old adult C57bl/6 male mice followed by 7 wk of Lieber-DeCarli alcohol diet feeding. They found that features of human alcoholic hepatitis including inflammation, steatosis and fibrosis were all present in DEN-injected mice after alcohol feeding and resulted in higher numbers of early HCC neoplastic foci. The data indicate that increased neutrophil and macrophage activation is triggered in the alcoholic liver tissue microenvironment and may contribute to acceleration of HCC.
This combination of carcinogen pre-exposure and chronic alcohol consumption presents one of the most unique phenomenons of chronic alcohol leading to progression of HCC that occurs in humans. This availability of such a model will provide unique opportunities to understand alcohol associated molecular and cellular mechanisms related to HCC.
Lieber-DeCarli diet, a commercially available, carefully designed and well characterized rodent diet intended to mimic the clinical situation in which the various effects of alcohol occur in the setting of liver changes characterized by a fatty liver and inflammation.
This is an interesting manuscript devoted to increased DEN-induced HCC development by alcohol. An important aspect of this study is that mice were fed not ethanol in water, but ethanol in Lieber-DeCarli diet (pair-feeding), which by itself induces liver injury.
P- Reviewer: Osna NA, Takahashi T S- Editor: Gong ZM L- Editor: A E- Editor: Liu XM
| 1. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 2. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21360] [Article Influence: 2136.0] [Reference Citation Analysis (3)] |
| 3. | Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109:542-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 355] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 4. | Szabo G, Dolganiuc A. Hepatitis C core protein - the “core” of immune deception? J Hepatol. 2008;48:8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15 Suppl 4:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 356] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 6. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8165] [Article Influence: 544.3] [Reference Citation Analysis (0)] |
| 7. | Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12:387-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 462] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 8. | Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 416] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 9. | Li Y, Tang ZY, Hou JX. Hepatocellular carcinoma: insight from animal models. Nat Rev Gastroenterol Hepatol. 2012;9:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Bakiri L, Wagner EF. Mouse models for liver cancer. Mol Oncol. 2013;7:206-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 11. | Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 1996;71:57-81. [PubMed] |
| 12. | Brandon-Warner E, Walling TL, Schrum LW, McKillop IH. Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis. Alcohol Clin Exp Res. 2012;36:641-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Ambade A, Catalano D, Lim A, Kopoyan A, Shaffer SA, Mandrekar P. Inhibition of heat shock protein 90 alleviates steatosis and macrophage activation in murine alcoholic liver injury. J Hepatol. 2014;61:903-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | O’Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology. 2010;51:307-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 852] [Article Influence: 56.8] [Reference Citation Analysis (2)] |
| 15. | Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 16. | Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 17. | Drinane M, Shah VH. Alcoholic Hepatitis: Diagnosis and Prognosis. Clinical Liver Disease. 2013;2:80-83. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 545] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 19. | Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol. 2007;22 Suppl 1:S53-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3128] [Cited by in RCA: 3355] [Article Influence: 305.0] [Reference Citation Analysis (0)] |
| 21. | Lee J, French B, Morgan T, French SW. The liver is populated by a broad spectrum of markers for macrophages. In alcoholic hepatitis the macrophages are M1 and M2. Exp Mol Pathol. 2014;96:118-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Xu R, Huang H, Zhang Z, Wang FS. The role of neutrophils in the development of liver diseases. Cell Mol Immunol. 2014;11:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 23. | Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology. 2013;58:1814-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 24. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1493] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 25. | Wang Y, Liu Y, Kirpich I, Ma Z, Wang C, Zhang M, Suttles J, McClain C, Feng W. Lactobacillus rhamnosus GG reduces hepatic TNFα production and inflammation in chronic alcohol-induced liver injury. J Nutr Biochem. 2013;24:1609-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 26. | Machida K, Tsukamoto H, Mkrtchyan H, Duan L, Dynnyk A, Liu HM, Asahina K, Govindarajan S, Ray R, Ou JH. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci USA. 2009;106:1548-1553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Abelev GI, Eraiser TL. Cellular aspects of alpha-fetoprotein reexpression in tumors. Semin Cancer Biol. 1999;9:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Apte UM, McRee R, Ramaiah SK. Hepatocyte proliferation is the possible mechanism for the transient decrease in liver injury during steatosis stage of alcoholic liver disease. Toxicol Pathol. 2004;32:567-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20499] [Article Influence: 2049.9] [Reference Citation Analysis (20)] |
| 30. | Swann PF, Coe AM, Mace R. Ethanol and dimethylnitrosamine and diethylnitrosamine metabolism and disposition in the rat. Possible relevance to the influence of ethanol on human cancer incidence. Carcinogenesis. 1984;5:1337-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt TJ. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion--absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin Exp Res. 2007;31:1746-1758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 567] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 33. | Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 294] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 34. | Donohue TM. Alcohol-induced steatosis in liver cells. World J Gastroenterol. 2007;13:4974-4978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 35. | Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 392] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 36. | Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3696] [Cited by in RCA: 4718] [Article Influence: 362.9] [Reference Citation Analysis (1)] |
| 37. | Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1125] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 38. | Meadows GG, Zhang H. Effects of Alcohol on Tumor Growth, Metastasis, Immune Response, and Host Survival. Alcohol Res. 2015;37:311-322. [PubMed] |
| 39. | Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 919] [Cited by in RCA: 962] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 40. | Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC; Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 41. | Chowdhury G, Calcutt MW, Nagy LD, Guengerich FP. Oxidation of methyl and ethyl nitrosamines by cytochrome P450 2E1 and 2B1. Biochemistry. 2012;51:9995-10007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Mendenhall CL, Chedid A, French SW, Ray M, Roselle GA, Grossman CJ, Weesner RE, Gartside PS. Alpha-fetoprotein alterations in alcoholics with liver disease. V.A. Cooperative Study Groups. Alcohol Alcohol. 1991;26:527-534. [PubMed] |
| 43. | Gowda S, Desai PB, Hull VV, Math AA, Vernekar SN, Kulkarni SS. A review on laboratory liver function tests. Pan Afr Med J. 2009;3:17. [PubMed] |
| 44. | Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnù L. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 45. | Sell S, Leffert HL. Liver cancer stem cells. J Clin Oncol. 2008;26:2800-2805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 46. | Itoh T, Miyajima A. Liver regeneration by stem/progenitor cells. Hepatology. 2014;59:1617-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 47. | Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14:561-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 48. | Lanthier N, Rubbia-Brandt L, Lin-Marq N, Clément S, Frossard JL, Goossens N, Hadengue A, Spahr L. Hepatic cell proliferation plays a pivotal role in the prognosis of alcoholic hepatitis. J Hepatol. 2015;63:609-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361-368. [PubMed] |
| 50. | Marquardt JU, Seo D, Gómez-Quiroz LE, Uchida K, Gillen MC, Kitade M, Kaposi-Novak P, Conner EA, Factor VM, Thorgeirsson SS. Loss of c-Met accelerates development of liver fibrosis in response to CCl(4) exposure through deregulation of multiple molecular pathways. Biochim Biophys Acta. 2012;1822:942-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Diehl AM. Alcohol and liver regeneration. Clin Liver Dis. 1998;2:723-738. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |