Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3418
Peer-review started: August 25, 2015
First decision: September 9, 2015
Revised: October 17, 2015
Accepted: November 30, 2015
Article in press: December 1, 2015
Published online: March 28, 2016
Processing time: 214 Days and 15.4 Hours
AIM: To evaluate daclatasvir vs telaprevir, each combined with peginterferon alfa-2a/ribavirin (pegIFN/RBV), in treatment-naive hepatitis C virus (HCV) genotype (GT) 1-infected patients.
METHODS: In this phase 3, randomized, open-label, noninferiority study, 602 patients were randomly assigned (2:1) to daclatasvir vs telaprevir, stratified by IL28B rs12979860 host genotype (CC vs non-CC), cirrhosis status (compensated cirrhosis vs no cirrhosis), and HCV GT1 subtype (GT1a vs GT1b). Patients were selected by study inclusion criteria from a total of 793 enrolled patients. Patients received daclatasvir 60 mg once daily or telaprevir 750 mg 3 times daily plus pegIFN/RBV. Daclatasvir recipients received 24 wk of daclatasvir plus pegIFN/RBV; those without an extended rapid virologic response (eRVR; undetectable HCV-RNA at weeks 4 and 12) received an additional 24 wk of pegIFN/RBV. Telaprevir-treated patients received 12 wk of telaprevir plus pegIFN/RBV followed by 12 (with eRVR) or 36 (no eRVR) wk of pegIFN/RBV. The primary objective was to compare for noninferiority of sustained virologic response rates at posttreatment week 12 (SVR12) in GT1b-infected patients. Key secondary objectives were to demonstrate that the rates of anemia (hemoglobin < 10 g/dL) and rash-related events, through week 12, were lower with daclatasvir + pegIFN/RBV than with telaprevir + pegIFN/RBV among GT1b-infected patients. Resistance testing was performed using population-based sequencing of the NS5A region for all patients at baseline, and for patients with virologic failure or relapse and HCV-RNA ≥ 1000 IU/mL, to investigate any link between NS5A polymorphisms associated with daclatasvir resistance and virologic outcome.
RESULTS: Patient demographics and disease characteristics were generally balanced across treatment arms; however, there was a higher proportion of black/African Americans in the daclatasvir groups (6.0% and 8.2% in the GT1b and GT1a groups, respectively) than in the telaprevir groups (2.2% and 3.0%). Among GT1b-infected patients, daclatasvir plus pegIFN/RBV was noninferior to telaprevir plus pegIFN/RBV for SVR12 [85% (228/268) vs 81% (109/134); difference, 4.3% (95%CI: -3.3% to 11.9%)]. Anemia (hemoglobin < 10 g/dL) was significantly less frequent with daclatasvir than with telaprevir [difference, -29.1% (95%CI: -38.8% to -19.4%)]. Rash-related events were also less common with daclatasvir than with telaprevir, but the difference was not statistically significant. In GT1a-infected patients, SVR12 was 64.9% with daclatasvir and 69.7% with telaprevir. Among both daclatasvir and telaprevir treatment groups, across GT1b- or GT1a-infected patients, lower response rates were observed in patients with IL28B non-CC and cirrhosis - factors known to affect response to pegIFN/RBV. Consistent with these observations, a multivariate logistic regression analysis in GT1b-infected patients demonstrated that SVR12 was associated with IL28B host genotype (CC vs non-CC, P = 0.011) and cirrhosis status (absent vs present, P = 0.031). NS5A polymorphisms associated with daclatasvir resistance (at L28, R30, L31, or Y93) were observed in 17.3% of GT1b-infected patients at baseline; such variants did not appear to be absolute predictors of failure since 72.1% of these patients achieved SVR12 compared with 86.9% without these polymorphisms. Among GT1b-infected patients, treatment was completed by 85.4% (229/268) in the daclatasvir group, and by 85.1% (114/134) in the telaprevir group, and among GT1a-infected patients, by 67.2% (90/134) and 69.7% (46/66), respectively. Discontinuations (of all 3 agents) due to an AE were more frequent with telaprevir than with daclatasvir, whereas discontinuations due to lack of efficacy were more frequent with daclatasvir, due, in part, to differences in futility criteria.
CONCLUSION: Daclatasvir plus pegIFN/RBV demonstrated noninferiority to telaprevir plus pegIFN/RBV for SVR12 and was well-tolerated in treatment-naive GT1b-infected patients, supporting the use of daclatasvir with other direct-acting antivirals.
Core tip: This phase 3 study describes the first prospective comparison of an NS5A inhibitor and an NS3/4A protease inhibitor in peginterferon-based regimens. Combinations of peginterferon alfa-2a/ribavirin (pegIFN/RBV) with boceprevir or telaprevir were the standard-of-care for genotype (GT) 1-infected patients at the time of study design. In treatment-naive GT1b-infected patients, daclatasvir (NS5A inhibitor) plus pegIFN/RBV achieved a sustained virologic response at posttreatment week 12 (SVR12) of 85% and demonstrated noninferiority to telaprevir plus pegIFN/RBV showing 81% SVR12. Daclatasvir plus pegIFN/RBV was well-tolerated, with a superior safety profile for anemia compared with telaprevir plus pegIFN/RBV. These results support the ongoing investigation of daclatasvir in all-oral combinations in multiple patient populations.
- Citation: Jacobson I, Zeuzem S, Flisiak R, Knysz B, Lueth S, Zarebska-Michaluk D, Janczewska E, Ferenci P, Diago M, Zignego AL, Safadi R, Baruch Y, Abdurakhmanov D, Shafran S, Thabut D, Bruck R, Gadano A, Thompson AJ, Kopit J, McPhee F, Michener T, Hughes EA, Yin PD, Noviello S. Daclatasvir vs telaprevir plus peginterferon alfa/ribavirin for hepatitis C virus genotype 1. World J Gastroenterol 2016; 22(12): 3418-3431
- URL: https://www.wjgnet.com/1007-9327/full/v22/i12/3418.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i12.3418
Chronic infection with hepatitis C virus (HCV) affects 130-150 million people globally and is a major cause of cirrhosis and hepatocellular carcinoma[1]. Of the 7 HCV genotypes (GTs) identified[2], GT1 is the most prevalent worldwide, and accounts for 75% of all infections in the United States (US)[3]. GT1 can be classified into the two main subtypes GT1a and GT1b, of which GT1b is the most common worldwide, predominating in Europe, Japan, and China; in the US 36% of all GT1 infections are subtype 1b[4].
Peginterferon alfa plus ribavirin (pegIFN/RBV) has traditionally been used to treat HCV. However, this regimen achieves only limited sustained virologic response (SVR) rates of 40%-50%[5,6] and is associated with a high frequency of adverse events (AEs)[7]. Although pegIFN/RBV-based therapies continue to be the standard of care in some countries[8], HCV treatment has evolved toward direct-acting antiviral agents (DAAs) that target specific viral proteins[5,6], with the first all-oral combinations for GT1 recently approved in Japan, Europe, the US, and Canada[9-15].
Combinations of pegIFN/RBV with one of the NS3/4A protease inhibitors boceprevir or telaprevir were the first DAA-based regimens approved and at the time of study design, the standard-of-care for GT1-infected patients. With telaprevir plus pegIFN/RBV, SVR rates increased from < 50% with pegIFN/RBV alone to 72%-75% in GT1-infected, treatment-naive patients[16-18]. However, skin rash and anemia are frequent, and sometimes severe, adverse events (AEs) observed with telaprevir[16-19]. In patients treated with telaprevir plus pegIFN/RBV, rash has been reported in 35%-37% of patients (compared with 24% with pegIFN/RBV), necessitating premature discontinuation of telaprevir in 7% of patients, and anemia in 37%-42% (compared with 19% with pegIFN/RBV)[16-18]. Combinations of pegIFN/RBV with more recent DAAs, such as the NS5B inhibitor sofosbuvir or the NS3/4A protease inhibitor simeprevir, achieved SVR rates of 80%-90% and demonstrated a more favorable safety profile than telaprevir plus pegIFN/RBV[20-22]. Telaprevir plus pegIFN/RBV has been compared with simeprevir plus pegIFN/RBV in treatment-experienced GT1-infected patients[23]; however, for treatment-naive patients, no direct comparison of telaprevir vs a non-protease inhibitor DAA has been performed to date.
Daclatasvir is a potent, once-daily, pangenotypic NS5A inhibitor[24,25] that has been studied and shown to be well-tolerated in > 13000 patients. In phase 2 trials in treatment-naive patients infected with GT1-4, daclatasvir + pegIFN/RBV demonstrated greater efficacy than pegIFN/RBV alone[26,27]. In GT1-infected patients, daclatasvir plus pegIFN/RBV achieved SVR at posttreatment week 24 (SVR24) rates of 60% compared with 38% with pegIFN/RBV; response rates were consistently higher in patients with GT1b (77%) than in those with GT1a (55%)[27], a finding that has also been observed with other DAA + pegIFN/RBV combinations[16,21,28]. Daclatasvir-containing pegIFN-free regimens are approved for treatment of chronic HCV infection in a number of countries: daclatasvir plus asunaprevir (ASV, NS3 inhibitor) was approved as the first all-oral treatment for GT1 in Japan[9], and daclatasvir plus sofosbuvir (with or without ribavirin) is approved in Europe for GT1, 3, and 4[10], and in Canada for GT1, 2, and 3[29]. Daclatasvir is also approved in the US, indicated in combination with sofosbuvir for the treatment of chronic HCV GT3 infection[30].
This phase 3 COMMAND-3 study compared the safety and efficacy of daclatasvir, an NS5A inhibitor, with that of telaprevir, a protease inhibitor, each in combination with pegIFN/RBV, in treatment-naive patients with GT1 infection, with a focus on GT1b-infected patients.
This was a phase 3, randomized, open-label, noninferiority study in treatment-naive patients with GT1 infection (Study AI444-052; ClinicalTrials.gov number NCT01492426). Overall, 602 patients were randomly assigned (2:1) to daclatasvir vs telaprevir, stratified by IL28B rs12979860 host genotype (CC vs non-CC), cirrhosis status (compensated cirrhosis vs no cirrhosis), and HCV GT1 subtype (GT1a vs GT1b). Patients with compensated cirrhosis were capped at 25%. GT1a-infected patients were capped at 200 (33%); this cap was introduced during enrollment (May 2012) based on data from a previous daclatasvir phase 2 study indicating that daclatasvir was more effective in GT1b-infected than in GT1a-infected patients[27]. Patients were treated with daclatasvir 60 mg/d (n = 402) or telaprevir 750 mg 3 times/d (n = 200) in combination with pegIFN alfa-2a 180 μg once weekly and RBV [weight-based dosing of 1000 mg/d (< 75 kg) or 1200 mg/d (≥ 75 kg)]. Daclatasvir-treated patients with undetectable HCV-RNA at weeks 4 and 12 [extended rapid virologic response (eRVR)] had a planned treatment duration of 24 wk of daclatasvir plus pegIFN/RBV; those without eRVR received an additional 24 wk of pegIFN/RBV (total of 48 wk of therapy), provided they did not experience treatment futility. Telaprevir-treated patients received 12 wk of telaprevir plus pegIFN/RBV, followed by 12 (with eRVR) or 36 (without eRVR) weeks of pegIFN/RBV alone, provided they did not experience treatment futility. Patients in both groups were followed for 24 wk (without eRVR) or 48 wk (with eRVR) posttreatment.
In the daclatasvir group, treatment futility, which mandated discontinuation of all study drugs, was defined as: (1) virologic breakthrough [> 1-log10 increase in HCV-RNA over nadir or confirmed HCV-RNA ≥ lower limit of quantification (LLOQ) after confirmed undetectable HCV-RNA while on treatment beginning at week 2 of therapy]; (2) week 12 HCV-RNA > 1000 IU/mL; or (3) week 24 HCV-RNA ≥ LLOQ. In the telaprevir group, treatment futility was defined per prescribing information as week 4 or 12 HCV-RNA > 1000 IU/mL or week 24 HCV-RNA detectable confirmed; virologic breakthrough was not included in the telaprevir futility criteria, per the prescribing information[19]. Relapse was defined as undetectable HCV-RNA at the end of treatment (EOT) followed by confirmed HCV-RNA ≥ LLOQ at any follow-up visit.
The study included treatment-naive patients aged 18 years or older, with GT1a or GT1b infection, and HCV-RNA ≥ 10000 IU/mL at screening. Patients with no cirrhosis or compensated cirrhosis [by liver biopsy at any time, or by FibroScan™ (≥ 14.6 kPa) within 1 year of screening] were eligible for inclusion. No previous treatment of HCV with interferon-based regimens or DAAs was allowed. Other exclusion criteria included evidence of decompensated liver disease (including a history or presence of ascites, bleeding varices, or hepatic encephalopathy), evidence of a medical condition contributing to chronic liver disease other than HCV, documented or suspected hepatocellular carcinoma or other malignancies, co-infection with HIV or hepatitis B virus, alanine aminotransferase ≥ 5 × the upper limit of normal, hemoglobin < 12 g/dL (120 g/L) for women and < 13 g/dL (130 g/L) for men, platelet count < 90 × 109 cells/L, international normalized ratio ≥ 1.7, albumin < 3.5 g/dL (35 g/L), or any criterion that would exclude the patient from receiving pegIFN/RBV or telaprevir. Patients were randomized to a regimen within stratum via block randomization (block size of 6) and using an interactive voice response system prepared by the sponsor.
Primary and secondary objectives were amended during enrollment to focus on GT1b-infected patients based on the results of previous phase 2 data[27]. The primary objective was to demonstrate that daclatasvir plus pegIFN/RBV was noninferior to telaprevir plus pegIFN/RBV for SVR at posttreatment week 12 (HCV-RNA < LLOQ at posttreatment week 12) in GT1b-infected patients. The first two secondary objectives were to demonstrate that the rates of anemia (hemoglobin < 10 g/dL) and of rash-related events, through week 12, were lower with daclatasvir plus pegIFN/RBV than with telaprevir plus pegIFN/RBV, among GT1b-infected patients (see Supplementary Material and Methods for the definition of rash-related events). Additional secondary objectives included noninferiority comparisons between arms of undetectable HCV-RNA at week 4 [rapid virologic response (RVR)], week 12 [complete early virologic response (cEVR)], and weeks 4 and 12 (eRVR), and HCV-RNA < LLOQ at post-treatment week 24 (SVR24), in GT1b-infected patients. The final secondary objective was a noninferiority comparison between arms of SVR12 in GT1a-infected patients.
HCV-RNA was assayed using the Roche HCV COBAS® TaqMan® test v2.0 (LLOQ = 25 IU/mL; limit of detection approximately 10 IU/mL). HCV GT and subtype were determined by Versant HCV GT 2.0 assay (LIPA) and were analyzed by ICON Central Laboratories, Inc. IL28B genotype was determined by polymerase chain reaction amplification coupled with allelic discrimination. Resistance testing was performed using population-based sequencing of the NS5A region for all patients at baseline, and for patients with virologic failure or relapse and with amplifiable (HCV-RNA ≥ 1000 IU/mL) plasma samples. Safety monitoring was based on the incidences of AEs, serious AEs (SAEs), discontinuations due to AEs, laboratory abnormalities, vital signs, and physical examinations.
The statistical methods of this study were reviewed by the biometrics group at Bristol-Myers Squibb. A noninferiority margin of -12% was employed in this study. A 2-sided 95% confidence interval (CI) for the difference in rates, daclatasvir plus pegIFN/RBV minus telaprevir plus pegIFN/RBV, was used to test for noninferiority. To demonstrate noninferiority, the lower bound of the CI had to be > -0.12. A sample size of 400 GT1b-infected patients, randomized 2:1 to daclatasvir plus pegIFN/RBV vs telaprevir plus pegIFN/RBV, provided 91% power to show that the SVR12 rate of daclatasvir plus pegIFN/RBV was noninferior to that of telaprevir plus pegIFN/RBV at the 5% significance level, assuming SVR12 rates of 85% for both regimens[16].
Each secondary comparison was conducted at the 5% level and proceeded hierarchically according to the order of the objectives (see Objectives and assessments). Testing of an endpoint was performed only if the null hypothesis of the preceding endpoint was rejected. Safety comparisons, for anemia and rash-related events, were for superiority, i.e., to show that daclatasvir plus pegIFN/RBV was less toxic than telaprevir plus pegIFN/RBV. Secondary efficacy comparisons were for noninferiority (noninferiority margin -12%).
Efficacy analyses were restricted to all treated patients and were performed using a modified intent-to-treat (mITT) analysis (patients with missing HCV-RNA measurements were considered failures). For the primary endpoint, an analysis based on SVR documented on or after (if follow-up week 12 HCV-RNA was missing) posttreatment week 12 was also conducted. A stratum-adjusted, 2-sided, asymptotic 95%CI was used to compute the difference in SVR12 rates between arms[31]. The strata were those used in the randomization. Stratum-adjusted CIs were also used for testing differences between rates for secondary efficacy endpoints.
All authors had access to the study data and have reviewed and approved the final manuscript.
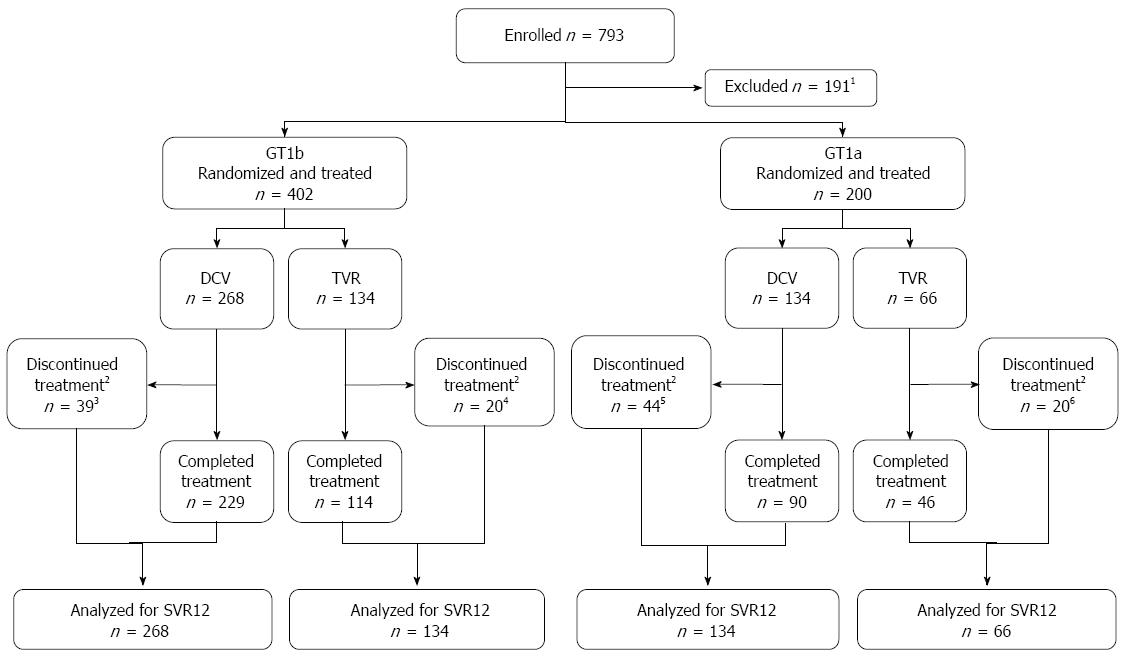
Overall, 793 patients were screened; of these, 402 GT1b-infected patients were randomized and treated with daclatasvir plus pegIFN/RBV (n = 268) or telaprevir plus pegIFN/RBV (n = 134), and 200 GT1a-infected patients were randomized and treated with daclatasvir plus pegIFN/RBV (n = 134) or telaprevir plus pegIFN/RBV (n = 66) (Figure 1). Among GT1b-infected patients, treatment was completed by 85.4% (229/268) in the daclatasvir group, and by 85.1% (114/134) in the telaprevir group, and among GT1a-infected patients, by 67.2% (90/134) and 69.7% (46/66), respectively. Discontinuations (of all three agents) due to an AE were more frequent with telaprevir than with daclatasvir, whereas discontinuations due to lack of efficacy were more frequent with daclatasvir. This was due, in part, to the different futility criteria in the two treatment groups. Posttreatment follow-up was initiated by 384 patients in the daclatasvir group and by 191 patients in the telaprevir group and was completed by 359 (93.5%) and 181 (94.8%), respectively.
Patient demographics and disease characteristics were generally balanced across treatment arms (Table 1); however, there was a higher proportion of black/African Americans in the daclatasvir groups (6.0%-8.2%) than in the telaprevir groups (2.2%-3.0%). Mean HCV-RNA levels ranged from 6.23-6.31 log10 IU/mL, 9.7%-13.6% of patients had cirrhosis, and there was a high proportion of patients with a non-CC IL28B genotype (68.7%-79.9%) across all groups.
| n (%) | GT1b | GT1a | ||
| DCV + pegIFN/RBV (n = 268) | TVR + pegIFN/RBV (n = 134) | DCV + pegIFN/RBV (n = 134) | TVR + pegIFN/RBV (n = 66) | |
| Age (yr), median (range) | 46.0 (18-71) | 48.0 (19-69) | 49.0 (19-67) | 51.5 (28-69) |
| Male | 159 (59.3) | 72 (53.7) | 98 (73.1) | 47 (71.2) |
| Race | ||||
| White | 243 (90.7) | 129 (96.3) | 120 (89.6) | 63 (95.5) |
| Black/African American | 16 (6.0) | 3 (2.2) | 11 (8.2) | 2 (3.0) |
| Asian | 6 (2.2) | 2 (1.5) | 1 (0.7) | 0 |
| Other | 3 (1.1) | 0 | 2 (1.5) | 1 (1.5) |
| HCV-RNA log10 (IU/mL), mean (SD) | 6.23 (0.701) | 6.23 (0.577) | 6.30 (0.637) | 6.31 (0.636) |
| HCV-RNA ≥ 800000 IU/mL | 196 (73.1) | 97 (72.4) | 104 (77.6) | 51 (77.3) |
| IL28B genotype | ||||
| CC | 53 (19.8) | 27 (20.1) | 42 (31.3) | 20 (30.3) |
| CT | 161 (60.1) | 86 (64.2) | 73 (54.5) | 37 (56.1) |
| TT | 53 (19.8) | 21 (15.7) | 19 (14.2) | 9 (13.6) |
| Not reported | 1 (0.4) | 0 | 0 | 0 |
| Cirrhosis | ||||
| Present | 26 (9.7) | 15 (11.2) | 16 (11.9) | 9 (13.6) |
In GT1b-infected patients, SVR12 rates were 85.1% (228/268) with daclatasvir plus pegIFN/RBV vs 81.3% (109/134) with telaprevir plus pegIFN/RBV (mITT; primary endpoint) (Table 2); the difference between treatment arms was 4.3% (95%CI: -3.3% to 11.9%), demonstrating noninferiority (lower bound of the 95%CI greater than -12%). Similar SVR12 rates in GT1b-infected patients were obtained when using the next available HCV-RNA value for patients with missing posttreatment week 12 measurements [daclatasvir plus pegIFN/RBV: 85.8% (230/268); telaprevir plus pegIFN/RBV: 82.1% (110/134)]. Response rates observed for the secondary efficacy endpoints (RVR, eRVR, cEVR, SVR24) in GT1b-infected patients appeared similar between treatment arms (Table 2); however, no formal comparisons could be made because the test for the difference in rash-related events between arms, which preceded the comparisons of secondary efficacy endpoints in the testing hierarchy, was not statistically significant (see Safety section). Among GT1b-infected patients with SVR12, three patients did not achieve SVR24 (one patient in each arm relapsed between posttreatment weeks 12 and 24, and one patient in the daclatasvir group had a missing posttreatment week 24 HCV-RNA measurement).
| Outcome, n/n (%) | DCV + pegIFN/RBV | TVR + pegIFN/RBV |
| Efficacy | ||
| SVR12 (mITT)12 | 228/268 (85.1) | 109/134 (81.3) |
| SVR12 on or after PT week 123 | 230/268 (85.8) | 110/134 (82.1) |
| RVR (HCV-RNA undetectable at week 4)14 | 207/268 (77.2) | 106/134 (79.1) |
| cEVR (HCV-RNA undetectable at week 12)14 | 243/268 (90.7) | 121/134 (90.3) |
| eRVR (HCV-RNA undetectable at weeks 4 and 12)14 | 201/268 (75.0) | 98/134 (73.1) |
| EOTR (HCV-RNA undetectable at EOT) | 244/268 (91.0) | 131/134 (97.8) |
| SVR2414 | 226/268 (84.3) | 108/134 (80.6) |
| Failures | ||
| Non-SVR12 | 40/268 (14.9) | 25/134 (18.7) |
| On-treatment failures | 21/268 (7.8) | 3/134 (2.2) |
| Virologic breakthrough | 11/268 (4.1) | NA5 |
| Treatment futility other than virologic breakthrough | 3/268 (1.1) | 0 |
| HCV-RNA detectable at EOT | 7/268 (2.6) | 3/134 (2.2) |
| Posttreatment relapse6 | 12/244 (4.9) | 20/131 (15.3) |
| HCV-RNA undetectable at EOT but missing PT week 12 data | 7/244 (2.9) | 2/131 (1.5) |
In GT1b-infected patients, observed SVR12 rates tended to be higher in the daclatasvir arm than in the telaprevir arm in subgroups based on demographic and disease status, such as age, sex, IL28B, cirrhosis, and baseline HCV-RNA level (Table 3). Of note, among cirrhotics, a higher proportion of patients receiving daclatasvir vs telaprevir achieved SVR12 (76.9% vs 66.7%). In the daclatasvir group, SVR12 appeared to be independent of age and sex. In both treatment groups, lower response rates were observed in patients with IL28B non-CC, baseline HCV-RNA ≥ 800000 IU/mL, or cirrhosis, baseline factors known to affect response to pegIFN/RBV[32]. Consistent with these observations, a multivariate logistic regression analysis in GT1b-infected patients demonstrated that SVR12 was associated with IL28B host genotype (CC vs non-CC, P = 0.011), baseline HCV-RNA (< 800000 IU/mL vs≥ 800000 IU/mL, P = 0.016), and cirrhosis status (absent vs present, P = 0.031). Virologic response was not associated with age, sex, race, or type of treatment (Supplementary Table 3).
| SVR121, n/n (%) | DCV + pegIFN/RBV(n = 268) | TVR + pegIFN/RBV (n = 134) |
| Age (yr) | ||
| < 65 | 218/256 (85.2) | 103/126 (81.7) |
| ≥ 65 | 10/12 (83.3) | 6/8 (75.0) |
| Sex | ||
| Male | 134/159 (84.3) | 61/72 (84.7) |
| Female | 94/109 (86.2) | 48/62 (77.4) |
| Race | ||
| White | 208/243 (85.6) | 105/129 (81.4) |
| Black/African American | 11/16 (68.8) | 2/3 (66.7) |
| Asian | 6/6 (100.0) | 2/2 (100.0) |
| Other | 3/3 (100.0) | 0 |
| Baseline HCV-RNA | ||
| < 800000 IU/mL | 66/72 (91.7) | 33/37 (89.2) |
| ≥ 800000 IU/mL | 162/196 (82.7) | 76/97 (78.4) |
| Cirrhosis | ||
| Absent | 208/242 (86.0) | 99/119 (83.2) |
| Present | 20/26 (76.9) | 10/15 (66.7) |
| IL28B genotype | ||
| CC | 51/53 (96.2) | 23/27 (85.2) |
| CT | 132/161 (82.0) | 69/86 (80.2) |
| TT | 44/53 (83.0) | 17/21 (81.0) |
Forty (14.9%) patients in the daclatasvir group and 25 (18.7%) patients in the telaprevir group did not achieve SVR12 (Table 2). Virologic breakthrough occurred in 11 (4.1%) patients in the daclatasvir group. Virologic breakthrough was not assessed as a futility criteria for the telaprevir group, per the telaprevir prescribing information[19]. Relapse (among patients with undetectable HCV-RNA at EOT) was reported in 12/244 (4.9%) patients in the daclatasvir group and 20/131 (15.3%) patients in the telaprevir group. Other posttreatment failures, occurring in 7/244 (2.9%) patients in the daclatasvir group and 2/131 (1.5%) patients in the telaprevir group, were due to undetectable HCV-RNA at EOT but missing posttreatment week 12 HCV-RNA.
At baseline, 249/268 GT1b-infected patients treated with daclatasvir plus pegIFN/RBV had available NS5A population-based sequencing data. In 43/249 (17.3%) of these patients, one or more of the NS5A polymorphisms L28M/V, R30H/Q, L31M, or Y93H were detected at baseline; of these, 72.1% (31 patients) achieved SVR12, of which 61.3% (19 patients) had a non-CC IL28B genotype. Of the remaining 12 patients who did not achieve SVR12, 11 had a non-CC IL28B genotype. Among patients without NS5A polymorphisms at baseline, 87% (179/206) achieved SVR12.
Among the 40 GT-1b patients in the daclatasvir group who did not achieve SVR12, 32 had evaluable samples at baseline and at the time of failure. In two patients, the same NS5A resistance-associated variants (RAVs) were detected at both baseline and failure: L31M-Y93H in one patient, and L28V-R30Q-L31M-Q62D in a second patient. Among the remaining 30 patients, the most common treatment-emergent NS5A RAVs were L31F/I/M/V (22 patients) and Y93H (21 patients); RAVs at L31 and Y93 emerged together in 18 patients. NS5A L31 and Y93 RAVs also emerged together in one daclatasvir-treated patient who achieved SVR12 but relapsed at posttreatment week 24. Of the eight non-SVR12 patients who were not tested at failure [due to undetectable HCV-RNA at last available visit (n = 5), missing HCV-RNA measurement (n = 2), or lost to follow-up while HCV-RNA was < 1000 IU/mL (n = 1)], seven had no NS5A polymorphisms at baseline, and one had no baseline NS5A sequence available.
In GT1a-infected patients, SVR12 rates were 64.9% in the daclatasvir group and 69.7% in the telaprevir group. Other outcomes and treatment failures in GT1a-infected patients are shown in Supplementary Table 1. Baseline NS5A sequences were available for 123 GT1a-infected patients treated with daclatasvir; the polymorphisms Q30R, L31M, and/or Y93N were detected in 6 (5%) patients, of whom 5 achieved SVR12. Among 34 GT1a-infected virologic failures with evaluable samples, the most common emergent NS5A RAVs at failure were Q30E/H/R (32 patients). In patients with emergent Q30 RAVs, L31 variants were also frequently detected, either as on-treatment emergent RAVs (19 patients) or pre-existing at baseline (one patient). In a daclatasvir-treated patient who relapsed after achieving SVR12, the NS5A RAV Q30E was detected at posttreatment week 24.
Safety, pooled across GT1 subtypes, is shown in Table 4. AEs leading to discontinuation of any study drug were more frequent with telaprevir (18.5%) vs daclatasvir (7.0%). In the daclatasvir group, the most common (≥ 1%) AEs leading to discontinuation of any study drug were psychiatric (1.5%), skin (1.5%), or hemolytic events (1.2%); in the telaprevir group the most common AEs were skin events (9.0%), hemolytic events (4.5%), general disorders (3.0%), gastrointestinal events (2.0%), psychiatric events (1.5%), infections and infestations (1.5%), nervous system disorders (1.5%), or renal events (1.0%). Serious AEs (SAEs) were reported in 6.5% of daclatasvir-treated patients and in 10.0% of telaprevir-treated patients. Drug-related SAEs were reported in 3.5% (n = 14) and 8.0% (n = 16) of treated patients in the daclatasvir and telaprevir arms, respectively, including one case of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome in each treatment group and five cases of anemia in the telaprevir group. The daclatasvir-treated patient with DRESS syndrome, a 62-year-old male with GT1b-infection, developed grade 3 rash after approximately 2.5 mo of therapy, followed by hyperthermia and hypereosinophilia (eosinophils 1.12 × 109 cells/L) leading to hospitalization. Topical corticosteroid (betamethasone) and trimeprazine resulted in rapid improvement and normalization of the eosinophil count (0.41 × 109 cells/L) within 10 d of discontinuing study therapy. The patient achieved SVR12. The five telaprevir-treated patients with a related SAE of anemia all had grade 4 anemia with hemoglobin levels of 54-72 g/L; all 5 patients received blood transfusions and 2 discontinued study therapy. The most common (> 25%) AEs were fatigue, headache, asthenia, and pruritus in the daclatasvir group, and anemia, fatigue, nausea, rash, pruritus, headache, and asthenia in the telaprevir group. Grade 3 or 4 laboratory abnormalities were comparable between treatment groups, except for grade 3 or 4 hemoglobin levels, which were more frequent with telaprevir (20.5%) than with daclatasvir (6.5%). Grade 3 or 4 bilirubin elevations occurred in 3% of patients in the telaprevir group compared with 1% in the daclatasvir group.
| Event, n (%) | DCV + pegIFN/RBV (n = 402) | TVR + pegIFN/RBV (n = 200)1 |
| Death | 1 (0.2)2 | 1 (0.5)2 |
| SAEs | 26 (6.5)2 | 20 (10.0)2 |
| AEs leading to discontinuation of any study drug | 28 (7.0)2 | 37 (18.5)2 |
| AEs leading to discontinuation of all 3 study drugs | 25 (6.2) | 25 (12.5) |
| AEs (grade 1-4) ≥ 20% | ||
| Fatigue | 140 (34.8) | 81 (40.5) |
| Headache | 137 (34.1) | 57 (28.5) |
| Asthenia | 109 (27.1) | 53 (26.5) |
| Pruritus | 107 (26.6) | 75 (37.5) |
| Anemia | 96 (23.9) | 99 (49.5) |
| Rash | 93 (23.1) | 69 (34.5) |
| Nausea | 88 (21.9) | 74 (37.0) |
| Neutropenia | 87 (21.6) | 27 (13.5) |
| Alopecia | 86 (21.4) | 32 (16.0) |
| Influenza-like illness | 85 (21.1) | 38 (19.0) |
| Dry skin | 84 (20.9) | 34 (17.0) |
| Pyrexia | 80 (19.9) | 42 (21.0) |
| Grade 3 or 4 emergent laboratory abnormalities | ||
| Hemoglobin | 26 (6.5) | 41 (20.5) |
| Absolute neutrophil count | 104 (25.9) | 41 (20.5) |
| Lymphocytes | 67 (16.7) | 44 (22.0) |
| Platelet count | 15 (3.7) | 8 (4.0) |
| ALT | 3 (0.7) | 4 (2.0) |
| AST | 7 (1.7) | 1 (0.5) |
| Total bilirubin | 4 (1.0) | 6 (3.0) |
| Serum creatinine increased | 1 (0.2) | 0 |
One death was reported in each treatment group, both of which occurred during off-treatment follow-up and were considered unrelated to therapy. In the daclatasvir group, the patient died during posttreatment week 4 from multiple fractures and a subdural hematoma related to a fall. In the telaprevir group, the patient, who had discontinued treatment at week 16 (due to bacteremia), died during posttreatment week 4 from sepsis secondary to cirrhosis due to hepatitis C.
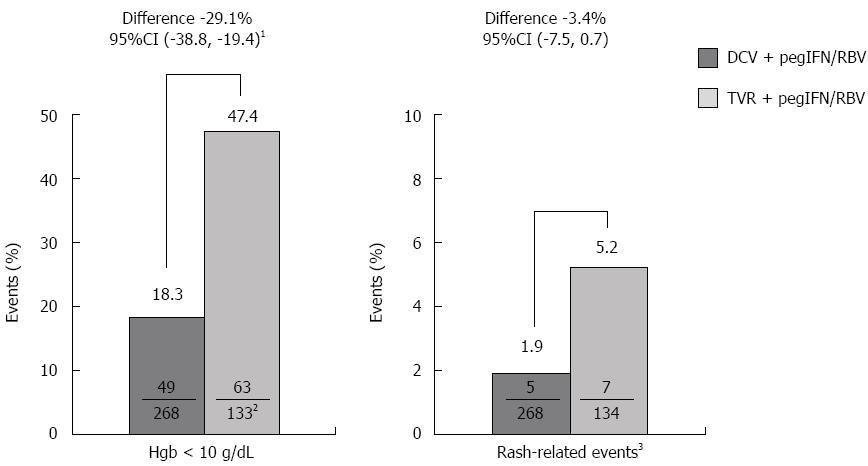
Grade 1-4 anemia was experienced by 23.9% of GT1-infected patients in the daclatasvir group, and 49.5% in the telaprevir group, including 3.2% (n = 13) and 13.5% (n = 27), respectively, with grade 3 or 4 anemia. Incidences for the secondary endpoint of hemoglobin < 10 g/dL in GT1b-infected patients through week 12 were significantly lower in the daclatasvir group than in the telaprevir group (daclatasvir: 18.3% vs telaprevir: 47.4%; difference, -29.1; 95%CI: -38.8% to -19.4%; Figure 2). Grade 1-4 rash [single Medical Dictionary for Regulatory Activities (MedDRA) term] was reported in 23.1% of GT1-infected patients in the daclatasvir group and in 34.5% in the telaprevir group, including 1.0% (n = 4) and 3.5% (n = 7), respectively, with grade 3 or 4 rash. Incidences for the secondary endpoint of rash-related events (composite MedDRA term) in GT1b-infected patients through week 12 were also lower with daclatasvir than with telaprevir, however the difference was not statistically significant given the low event rates in both arms (daclatasvir: 1.9%; telaprevir: 5.2%; difference, -3.4; 95%CI: -7.5% to 0.7%; Figure 2).
Safety was also assessed in subgroups of patients with and without cirrhosis at baseline (Supplementary Table 2). In both treatment arms, anemia, reduced platelet count, and elevated total bilirubin were more frequent in patients with than in those without cirrhosis. Among cirrhotic patients, those treated with telaprevir experienced a higher frequency of the AEs anemia (58.3% vs 33.3%), rash (41.7% vs 21.4%), fatigue (50.0% vs 38.1%), and nausea (37.5% vs 11.9%), and of SAEs (16.7% vs 7.1%); in contrast, neutropenia was more common with daclatasvir (26.2%) than with telaprevir (16.7%) but was not associated with an increase in infections (daclatasvir: 21.4%; telaprevir: 41.7%).
This study evaluated the efficacy and safety of daclatasvir plus pegIFN/RBV compared with telaprevir plus pegIFN/RBV in HCV GT1-infected patients and is the first head-to-head comparison of two different DAA classes - an NS5A inhibitor vs an NS3/4A protease inhibitor - in combination with pegIFN/RBV in treatment-naive patients. In treatment-experienced GT1-infected patients simeprevir (NS3/4A protease inhibitor) plus pegIFN/RBV has been compared with telaprevir plus pegIFN/RBV, with simeprevir plus pegIFN/RBV demonstrating noninferiority to telaprevir plus pegIFN/RBV for SVR12 (54% vs 55%) and a more favourable safety profile[23].
In the present study in treatment-naive, GT1b-infected patients, SVR12 rates achieved with daclatasvir plus pegIFN/RBV were noninferior to those observed with telaprevir plus pegIFN/RBV, and anemia was significantly less frequent among daclatasvir-treated patients than in telaprevir-treated patients. Rash-related events were also less common among patients receiving daclatasvir vs telaprevir. Daclatasvir plus pegIFN/RBV demonstrated high SVR12 rates in GT1b-infected patients across all subgroups of baseline factors known to affect response rates to pegIFN/RBV (cirrhosis, IL28B genotype, age, sex, baseline viral load). Importantly, in difficult-to-cure patients with cirrhosis, SVR12 rates were higher with daclatasvir plus pegIFN/RBV than with telaprevir plus pegIFN/RBV (76.9% vs 66.7%). On-treatment treatment futility was more common with daclatasvir than with telaprevir; however this may have been related to the different futility criteria between the two treatment groups. However, posttreatment relapse was more frequent with telaprevir than with daclatasvir (15% vs 5%).
Using population-based sequencing of the NS5A region, NS5A polymorphisms associated with daclatasvir resistance (L28, R30, L31, and/or Y93) were observed in 17% of GT1b-infected patients at baseline. Although such variants may be associated with virologic outcome to daclatasvir plus pegIFN/RBV, they do not appear to be absolute predictors of failure because 72% of GT1b-infected patients with these polymorphisms at baseline achieved SVR12 in this study. Furthermore, since most (11/12) patients with baseline NS5A polymorphisms who did not achieve SVR12 also had a non-CC IL28B genotype, it may be that IL28B genotype has a stronger association with virologic failure. A similar observation was reported in a previous daclatasvir plus pegIFN/RBV phase 2 study, in which only 2/10 GT1b-infected patients with L31 or Y93 variants failed to achieve a response, but 30/32 GT1b virologic failures were non-CC IL28B[27].
A relevant consideration is that the persistence of RAVs may influence further treatment options in an IFN-free context. In this study, patients were not monitored beyond post-treatment week 24, thus analysis of the persistence of NS5A RAVs was not undertaken. However, persistence beyond one year has been described previously in GT1b- and GT1a-infected patients[33,34]. Persistence of emergent NS5A RAVs has also been described from an interim analysis of a three-year follow-up study[35]. Detection of viral variants by direct sequencing limits the ability to detect low-frequency variants within a viral population; however, despite this limitation, the results of these analyses have been useful for describing relationships between RAVs and clinical outcomes.
In this study, response rates with daclatasvir plus pegIFN/RBV were lower and virologic failure was more frequent in patients infected with HCV GT1a than in patients infected with GT1b. Most likely, this is due to a lower resistance barrier of daclatasvir in GT1a-infected patients rather than to a higher potency in GT1b-infected patients, since the EC50 of daclatasvir was low in both GT1a-infected patients (20 pM) and GT1b-infected patients (4 pM)[24]. These lower response rates observed among GT1a-infected patients are consistent with previous studies of daclatasvir[27] and have also been observed with other DAAs, including telaprevir[16], boceprevir[28], and simeprevir[21].
Daclatasvir plus pegIFN/RBV was generally well-tolerated, with an overall safety profile similar to that of pegIFN/RBV alone, with no new safety or tolerability concerns attributable to daclatasvir. In the overall GT1-infected population, anemia events (grade 1-4 and grade 3 or 4) were less common in the daclatasvir group (24% and 3%, respectively) than in the telaprevir group (50% and 14%, respectively), and were also less frequently a reason for treatment discontinuation (daclatasvir: 0.5%; telaprevir: 4.0%). Among GT1b-infected patients, daclatasvir plus pegIFN/RBV demonstrated superiority over telaprevir plus pegIFN/RBV for the secondary endpoint of hemoglobin < 10 g/dL (18% vs 47%).
Grade 1-4 and grade 3 or 4 rash (single MedDRA term) were also less frequent in the overall GT1-infected population in patients receiving daclatasvir (23% and 1%, respectively) than in those receiving telaprevir (35% and 4%, respectively), as were discontinuations due to rash (daclatasvir: 0.2%; telaprevir: 4.5%). The grade 1-4 events of rash observed in the daclatasvir group are most likely related to pegIFN/RBV, because the observed rate (23%) is comparable to that historically reported with pegIFN/RBV alone[16,27], and no cases of rash have been reported with daclatasvir all-oral regimens, including daclatasvir plus sofosbuvir[36]. Rash-related events (composite MedDRA term) were evaluated as a secondary endpoint in this study in GT1b-infected patients, and a lower incidence was observed in those receiving daclatasvir than in those receiving telaprevir, but this difference was not statistically significant due to low event rates in both arms. The single case of DRESS syndrome observed with daclatasvir is the only case reported to date in the daclatasvir development program and real-word experience.
At the time this study was designed, telaprevir plus pegIFN/RBV was the standard of care for the treatment of HCV GT1 infection; however, other DAAs have now been approved, both in combination with pegIFN/RBV and as part of IFN-free regimens. Currently approved all-oral treatment options for GT1-infected patients include daclatasvir plus sofosbuvir ± RBV (approved for GT1, 3, and 4 in the EU[10]), which has been shown to achieve SVR12 rates of up to 98% in treatment-naive GT1-infected patients[36], and the dual combination daclatasvir plus asunaprevir (approved for GT1b in Japan[9]), which has been shown to achieve SVR12 rates of 90% in GT1b-infected treatment-naive patients[37]. Other approved all-oral regimens include the combinations of sofosbuvir + ledipasvir ± RBV[11,12] and simeprevir plus sofosbuvir ± RBV[13,14,38], which achieved SVR rates of up to 98% and 94%, respectively, in this patient population. Ombitasvir/paritaprevir/ritonavir plus dasabuvir, which was recently approved, provided SVR rates of 90% (without RBV) and 95%-97% (with RBV) in treatment-naive noncirrhotic patients infected with GT1a, and of 99% (without RBV) and 98%-100% (with RBV) in those infected with GT1b[36,39,40]; in GT1-infected patients with cirrhosis SVR12 was 96%[41]. Daclatasvir is also being evaluated as part of an all-oral, fixed-dose combination with asunaprevir and beclabuvir (formerly BMS-791325). In phase 3 studies, this regimen without RBV has provided SVR12 rates of 92% in GT1a- and 1b-infected treatment-naive patients without cirrhosis[42], and SVR12 rates of 93%-98% in GT1a- and 1b-infected patients with compensated cirrhosis, with or without RBV[43]; in these studies, SVR12 rates were lower in patients infected with GT1a than in those infected with GT1b when RBV was excluded from the regimen.
Although all-oral regimens have become the new standard of care in chronic hepatitis C management, pegIFN/RBV-based therapies will remain a potentially important treatment option in such settings as low-income countries or possibly for patients who have failed DAA-only therapies.
In conclusion, this first and only head-to-head comparison of two classes of DAAs in treatment-naive patients demonstrates that daclatasvir plus pegIFN/RBV is noninferior to telaprevir plus pegIFN/RBV for SVR12 for treatment of HCV GT1b infection. Daclatasvir plus pegIFN/RBV was generally well-tolerated, with a significantly lower rate of anemia, and an observed lower rate of rash-related events compared with telaprevir plus pegIFN/RBV. The results of this study and other studies support the role of daclatasvir as an effective and well-tolerated component of interferon-free, all-oral HCV regimens across multiple genotypes.
The authors thank Nancy Beckert, Gail Denisky, Sharon Igoe, and Megan Wind-Rotolo of Bristol-Myers Squibb for their contributions.
Chronic infection with hepatitis C virus (HCV) affects 130-150 million people globally and is a major cause of cirrhosis and hepatocellular carcinoma. Peginterferon alfa plus ribavirin (pegIFN/RBV) has historically been used to treat HCV, although this regimen achieves only limited sustained virologic response (SVR) rates of 40%-50% in genotype 1 (GT1) infection. Whilst pegIFN/RBV-based therapies remain the standard of care in some countries, HCV treatment has evolved toward direct-acting antiviral agents (DAAs) that target specific viral proteins.
The combination of pegIFN/RBV with the NS3/4A protease inhibitor telaprevir was one of the first approved DAA-based regimens, and replaced pegIFN/RBV as the standard-of-care for GT1-infected patients. Skin rash and anemia are frequent, and at times severe, adverse events (AEs) observed with telaprevir. No direct comparison of telaprevir vs a non-protease inhibitor DAA has been performed to date. Daclatasvir is a potent, once-daily, pangenotypic NS5A inhibitor; this phase 3 study compared the safety and efficacy of daclatasvir with that of telaprevir, each in combination with pegIFN/RBV, in treatment-naive patients with GT1 infection, with a focus on GT1b-infected patients.
Among GT1b-infected patients, daclatasvir plus pegIFN/RBV was noninferior to telaprevir plus pegIFN/RBV for SVR12 [85% (228/268) vs 81% (109/134); difference, 4.3% (95%CI: -3.3% to 11.9%)]. Daclatasvir was generally well-tolerated: anemia (hemoglobin < 10 g/dL) was significantly less frequent with daclatasvir than with telaprevir [difference, -29.1% (95%CI: -38.8% to -19.4%)], and rash-related events were less frequent with daclatasvir plus pegIFN/RBV compared with telaprevir plus pegIFN/RBV. Discontinuations of all 3 agents due to an adverse event were more frequent with telaprevir than with daclatasvir, whereas discontinuations due to lack of efficacy were more frequent with daclatasvir, due, in part, to differences in futility criteria.
Although all-oral regimens have become the new standard of care in chronic HCV management - for which daclatasvir is currently approved for the treatment of HCV GT1, GT3, and GT4 infections (approvals vary by country) - pegIFN/RBV-based therapies will remain a potentially important treatment option in such settings as low-income countries or possible for patients who have failed DAA-only therapies. The results of this study and other studies support the role of daclatasvir as an effective and well-tolerated component of interferon-free, all-oral HCV regimens across multiple genotypes.
SVR12/24, sustained virologic response [HCV-RNA < lower limit of quantitation (LLOQ)] at posttreatment week 12/24; Relapse, undetectable HCV-RNA at the end of treatment followed by confirmed HCV-RNA ≥ LLOQ at any follow-up visit. In the daclatasvir group, treatment futility, which mandated discontinuation of all study drugs, was defined as (1) virologic breakthrough (> 1-log10 increase in HCV-RNA over nadir or confirmed HCV-RNA ≥ LLOQ after confirmed undetectable HCV-RNA while on treatment beginning at week 2 of therapy); (2) week 12 HCV-RNA >1000 IU/mL; or (3) week 24 HCV-RNA ≥ LLOQ. In the telaprevir group, treatment futility was defined per prescribing information as HCV-RNA > 1000 IU/mL (at weeks 4 or 12) or HCV-RNA detectable confirmed (at week 24).
The arena within which HCV is treated has moved at a very rapid pace over the past 5 years. The range of DAA options has expanded since telaprevir and boceprevir were the first protease inhibitors to become available. While globally, the world is focused on DAAs as the treatments of choice for HCV, these drugs are expensive and not all countries will be in a position to treat infection vs disease. In fact, some first-world countries are currently only treating patients with advanced disease due to the cost of these drugs. The authors make this point nicely and rightly state that there may be a place for IFN based anti-viral strategies for the management and treatment of HCV.
P- Reviewer: Fanning LJ, Quarleri J S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | World Health Organization. Hepatitis C key facts. WHO factsheet No 164. (Updated April 2014, accessed June 23, 2015). Available from: http://www.who.int/mediacentre/factsheets/fs164/en/. |
| 2. | Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 965] [Cited by in RCA: 982] [Article Influence: 89.3] [Reference Citation Analysis (1)] |
| 3. | Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1077] [Cited by in RCA: 1146] [Article Influence: 114.6] [Reference Citation Analysis (0)] |
| 4. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [PubMed] |
| 5. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 655] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 6. | American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. (Updated July 7, 2014, accessed June 23, 2015). Available from: http://www.hcvguidelines.org/fullreport7. |
| 7. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2242] [Article Influence: 140.1] [Reference Citation Analysis (1)] |
| 8. | Kretzer IF, do Livramento A, da Cunha J, Gonçalves S, Tosin I, Spada C, Treitinger A. Hepatitis C worldwide and in Brazil: silent epidemic--data on disease including incidence, transmission, prevention, and treatment. ScientificWorldJournal. 2014;2014:827849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Bristol-Myers Squibb. Japan approves first all-oral, interferon- and ribavirin-free hepatitis C treatment, Daklinza® (daclatasvir) and Sunvepra® (asunaprevir) dual regimen. (Updated July 7, 2014, accessed June 23, 2015). Available from: http://news.bms.com/press-release/japan-approves-first-all-oral-interferon-and-ribavirin-free-hepatitis-c-treatment-dakl. |
| 10. | Bristol-Myers Squibb. European Commission approves Bristol-Myers Squibb’s Daklinza (daclatasvir) across multiple genotypes for the treatment of chronic hepatitis C infection. (Updated August 27, 2014, accessed June 23, 2015). Available from: http://news.bms.com/press-release/rd-news/european-commission-approves-bristol-myers-squibbs-daklinza-daclatasvir-across. |
| 11. | Gilead Sciences, Inc . Harvoni (ledipasvir and sofosbuvir) prescribing information. (Updated March 2015, accessed June 23 2015). Available from: http://www.gilead.com/~/media/Files/pdfs/medicines/liverdisease/harvoni/harvoni_pi.pdf. |
| 12. | Gilead Sciences, Inc . Harvoni (ledipasvir and sofosbuvir) [summary of product characteristics]. Cambridge, United Kingdom: Gilead Sciences International Ltd 2014; . |
| 13. | Janssen Therapeutics. OLYSIO™ (simeprevir) prescribing information. (Updated April 2015, accessed June 23, 2015). Available from: https://www.olysio.com/shared/product/olysio/prescribing-information.pdf. |
| 14. | Janssen Therapeutics. Olysio (simeprevir) summary of product characteristics. (Updated April 7, 2015, accessed June 23, 2015). Available from: http://ec.europa.eu/health/documents/community-register/2014/20140514128513/anx_128513_en.pdf. |
| 15. | AbbVie , Inc . Viekira Pak (ombitasvir, paritaprevir, and ritonavir tablets; dasabuvir tablets) prescribing information. (Updated March 2015, accessed June 23, 2015). Available from: http://www.rxabbvie.com/pdf/viekirapak_pi.pdf. |
| 16. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1862] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 17. | Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 602] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 18. | Buti M, Agarwal K, Horsmans Y, Sievert W, Janczewska E, Zeuzem S, Nyberg L, Brown RS, Hézode C, Rizzetto M. Telaprevir twice daily is noninferior to telaprevir every 8 hours for patients with chronic hepatitis C. Gastroenterology. 2014;146:744-753.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Vertex Pharmaceuticals. Incivek (telaprevir) prescribing information. (Updated October 2013, accessed June 23, 2015). Available from: http://pi.vrtx.com/files/uspi_telaprevir.pdf. |
| 20. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1325] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 21. | Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, Craxi A, Peeters M, Lenz O. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403-413. [PubMed] |
| 22. | Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Scott J, Peeters M. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414-426. [PubMed] |
| 23. | Reddy KR, Zeuzem S, Zoulim F, Weiland O, Horban A, Stanciu C, Villamil FG, Andreone P, George J, Dammers E. Simeprevir versus telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial. Lancet Infect Dis. 2015;15:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Gao M, Nettles RE, Belema M, Snyder LB, Nguyen VN, Fridell RA, Serrano-Wu MH, Langley DR, Sun JH, O’Boyle DR. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 762] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 25. | Nettles RE, Gao M, Bifano M, Chung E, Persson A, Marbury TC, Goldwater R, DeMicco MP, Rodriguez-Torres M, Vutikullird A. Multiple ascending dose study of BMS-790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology. 2011;54:1956-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Dore GJ, Lawitz E, Hézode C, Shafran SD, Ramji A, Tatum HA, Taliani G, Tran A, Brunetto MR, Zaltron S. Daclatasvir plus peginterferon and ribavirin is noninferior to peginterferon and ribavirin alone, and reduces the duration of treatment for HCV genotype 2 or 3 infection. Gastroenterology. 2015;148:355-366.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Hézode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez-Torres M, Shafran SD, Thuluvath PJ, Tatum HA, Waked I, Esmat G. Daclatasvir plus peginterferon alfa and ribavirin for treatment-naive chronic hepatitis C genotype 1 or 4 infection: a randomised study. Gut. 2015;64:948-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, Davis MN, Galati JS, Gordon SC, Ravendhran N. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 517] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 29. | Bristol-Myers Squibb. Health Canada approves Daklinza TM (daclatasvir) for the treatment of chronic hepatitis C infection across multiple genotypes including genotype 3. (Accessed October 14, 2015). Available from: http://www.bmscanada.ca/en/news/release/health-canada-approves-daklinza-daclatasvir-for-the-treatment-of-chronic-hepatitis-c-infection-acros. |
| 30. | Bristol-Myers Squibb; Daklinza™ (daclatasvir) Prescribing Information 2015. (Accessed October 14, 2015). Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206843Orig1s000lbl.pdf. . |
| 31. | Fleiss J, Levin B, Paik M. Statistical Methods for Rates and Proportions. 3rd ed. Hoboken: John Wiley & Sons 2013; . |
| 32. | Asselah T, Estrabaud E, Bieche I, Lapalus M, De Muynck S, Vidaud M, Saadoun D, Soumelis V, Marcellin P. Hepatitis C: viral and host factors associated with non-response to pegylated interferon plus ribavirin. Liver Int. 2010;30:1259-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Karino Y, Toyota J, Ikeda K, Suzuki F, Chayama K, Kawakami Y, Ishikawa H, Watanabe H, Hernandez D, Yu F. Characterization of virologic escape in hepatitis C virus genotype 1b patients treated with the direct-acting antivirals daclatasvir and asunaprevir. J Hepatol. 2013;58:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | McPhee F, Hernandez D, Yu F, Ueland J, Monikowski A, Carifa A, Falk P, Wang C, Fridell R, Eley T. Resistance analysis of hepatitis C virus genotype 1 prior treatment null responders receiving daclatasvir and asunaprevir. Hepatology. 2013;58:902-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Reddy KR, Thuluvath PJ, Kumada Y, Toyota J, Chayama K, Levin J, Lawitz E, Gadano A, Ghesquiere W, Gerken G. Long-term follow-up of patients treated with daclatasvir-based regimens in phase 2 and 3 studies. Hoboken: Wiley-Blackwell 2014; 1154A-1155A. |
| 36. | Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 911] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 37. | Manns M, Pol S, Jacobson IM, Marcellin P, Gordon SC, Peng CY, Chang TT, Everson GT, Heo J, Gerken G. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014;384:1597-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 38. | Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, Pearlman B, Rabinovitz M, Gitlin N. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 597] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 39. | Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 548] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 40. | Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 656] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 41. | Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 683] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 42. | Poordad F, Sievert W, Mollison L, Bennett M, Tse E, Bräu N, Levin J, Sepe T, Lee SS, Angus P. Fixed-dose combination therapy with daclatasvir, asunaprevir, and beclabuvir for noncirrhotic patients with HCV genotype 1 infection. JAMA. 2015;313:1728-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Muir AJ, Poordad F, Lalezari J, Everson G, Dore GJ, Herring R, Sheikh A, Kwo P, Hézode C, Pockros PJ. Daclatasvir in combination with asunaprevir and beclabuvir for hepatitis C virus genotype 1 infection with compensated cirrhosis. JAMA. 2015;313:1736-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |