Copyright
©The Author(s) 2016.
World J Gastroenterol. Mar 28, 2016; 22(12): 3418-3431
Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3418
Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3418
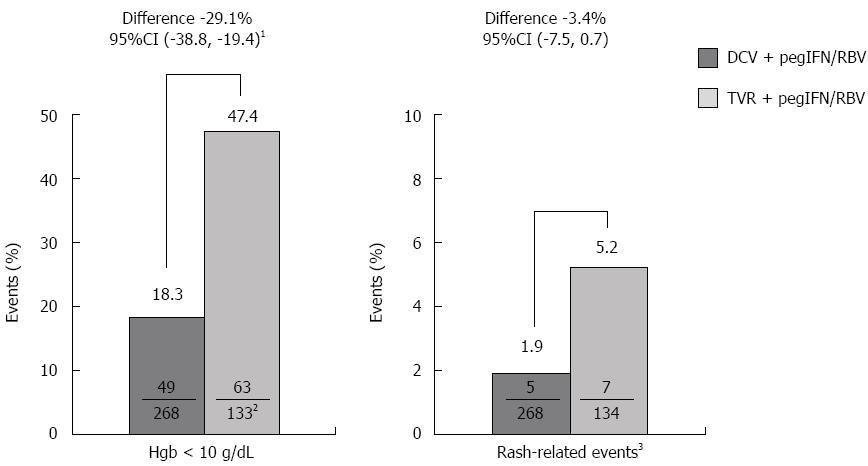
Figure 2 Safety secondary endpoints in GT1b-infected patients (first 12 wk).
mITT analysis (patients with missing data at posttreatment week 12 were considered failures). 1Superior at the 5% significance level; upper bound of the 95%CI was 0%; 2One patient in the TVR arm had no measurement due to discontinuation prior to on-treatment laboratory assessment; 3Includes rash-related SAEs, AEs leading to discontinuation, and grade 3 or 4 AEs. AE: Adverse event; CI: Confidence interval; DCV: Daclatasvir; GT: Genotype; Hgb: Hemoglobin; pegIFN: Peginterferon alfa-2a; RBV: Ribavirin; SAE: Serious adverse event; TVR: Telaprevir.
- Citation: Jacobson I, Zeuzem S, Flisiak R, Knysz B, Lueth S, Zarebska-Michaluk D, Janczewska E, Ferenci P, Diago M, Zignego AL, Safadi R, Baruch Y, Abdurakhmanov D, Shafran S, Thabut D, Bruck R, Gadano A, Thompson AJ, Kopit J, McPhee F, Michener T, Hughes EA, Yin PD, Noviello S. Daclatasvir vs telaprevir plus peginterferon alfa/ribavirin for hepatitis C virus genotype 1. World J Gastroenterol 2016; 22(12): 3418-3431
- URL: https://www.wjgnet.com/1007-9327/full/v22/i12/3418.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i12.3418