Published online Mar 21, 2016. doi: 10.3748/wjg.v22.i11.3261
Peer-review started: August 11, 2015
First decision: October 14, 2015
Revised: November 11, 2015
Accepted: December 8, 2015
Article in press: December 8, 2015
Published online: March 21, 2016
Processing time: 218 Days and 16.5 Hours
AIM: To investigate multiple polyps in a Chinese Peutz-Jeghers syndrome (PJS) infant.
METHODS: A nine-month-old PJS infant was admitted to our hospital for recurrent prolapsed rectal polyps for one month. The clinical characteristics, a colonoscopic image, the pathological characteristics of the polyps and X-ray images of the intestinal perforation were obtained. Serine threonine-protein kinase 11 (STK11) gene analysis was also performed using a DNA sample from this infant.
RESULTS: Here we describe the youngest known Chinese infant with PJS. Five polyps, including a giant polyp of approximately 4 cm × 2 cm in size, were removed from the infant’s intestine. Laparotomy was performed to repair a perforation caused by pneumoperitoneum. The pathological results showed that this child had PJS. Molecular analysis of the STK11 gene further revealed a novel frameshift mutation (c.64_65het_delAT) in exon 1 in this PJS infant.
CONCLUSION: The appropriate treatment method for multiple polyps in an infant must be carefully considered. Our results also show that the STK11 gene mutation is the primary cause of PJS.
Core tip: This is the first report detailing a nine-month-old Chinese Peutz-Jeghers syndrome (PJS) infant with multiple polyps. A perforation and pneumoperitoneum developed after polypectomy and were followed by sepsis. STK11 gene sequencing and pathology results confirmed that this infant had PJS with a novel, de novo mutation. This article also gives some thoughts to PJS management in children, especially in infants.
- Citation: Huang ZH, Song Z, Zhang P, Wu J, Huang Y. Clinical features, endoscopic polypectomy and STK11 gene mutation in a nine-month-old Peutz-Jeghers syndrome Chinese infant. World J Gastroenterol 2016; 22(11): 3261-3267
- URL: https://www.wjgnet.com/1007-9327/full/v22/i11/3261.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i11.3261
Peutz-Jeghers syndrome (PJS) is a rare autosomal dominant disorder characterized by hamartomatous polyps in the gastrointestinal tract, mucocutaneous pigmentation and an increased risk of cancer[1]. A mutation in the tumor suppressor gene serine threonine-protein kinase 11 (STK11) located on 19p13.3 has been found to be primarily responsible for this disease[2]. Presentation of PJS in infancy is rare. This is the first detailed report of a nine-month-old Chinese PJS infant with multiple polyps. A perforation and pneumoperitoneum developed after polypectomy in this patient, followed by sepsis. The STK11 gene sequencing and pathology results confirmed that this infant had PJS.
A nine-month-old infant was admitted to our department with recurrent prolapsed rectal polyps (PRPs) for one month. This infant was female, and she had no family history of PJS.
The infant underwent polyp screening, and the polyps were removed during colonoscopy, which was performed using gastrointestinal endoscopy (GIF-XQ260, Olympus, Japan) and an argon plasma coagulation device (VIO200D + APC2, German) with detachable snares (MAJ339, Olympus, Japan). The polyps that were removed were subject to pathological analysis. In our study, polyps > 1 cm were classified as “large”, and those > 2 cm were classified as “giant”.
A blood sample was collected, and genomic DNA was extracted according to the STK11 gene testing protocol. All of the STK11 coding exons and its boundary regions were amplified by PCR and analyzed by direct sequencing[3].
Three online software packages, including mutation taster (http://mutationtaster.org/) and PolyPhen 2 (http://genetics.bwh.harvard.edu/pph2/), were used to predict the functional significance of the variants.
The body weight of this infant was 9.5 kg. She was delivered by cesarean section due to social factors after 40 wk of gestation and had a birth weight of 3500 g. Her chief complaint was recurrent PRPs for one month. The size of the first PRP was similar to that of a pigeon egg. However, the PRP size increased to as large as a chicken egg after one month. Mucocutaneous pigmentation was not detectable in any part of her body. Her complete blood count (CBC) and fecal occult blood test were normal. Her mother was 28 years of age (1 gravida, 1 para), and her father was 31 years of age. She had no brothers or sisters. Both parents were healthy, and neither had a family history of PJS.
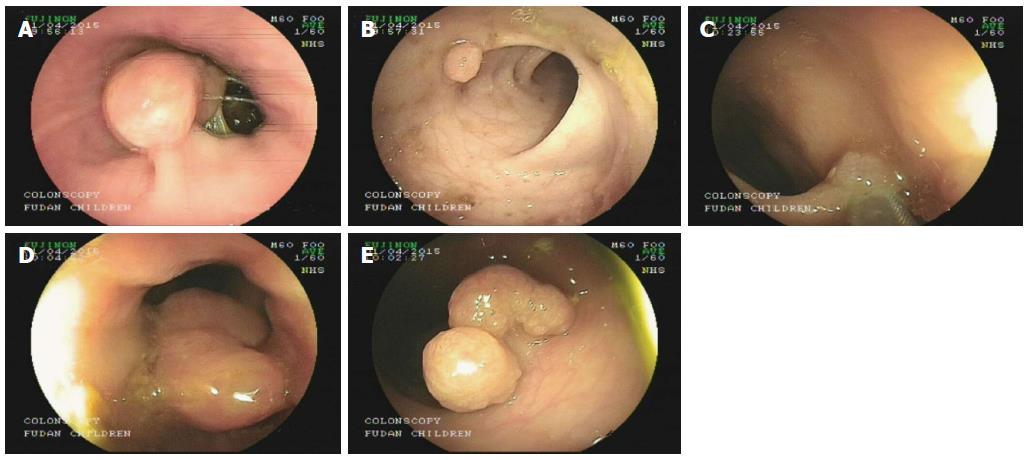
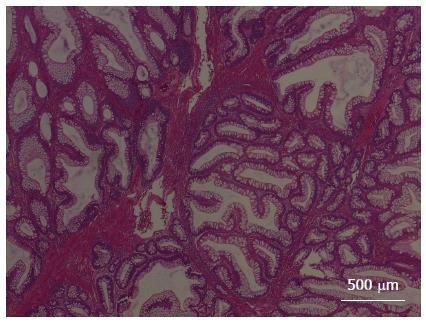
Under general anesthesia, colonoscopic examination revealed five polyps (Figure 1). The first large polyp was located at the outlet of the anus and was approximately 1.5 cm × 1.5 cm in size. The second polyp was small and was located in the sigmoid, with a size of approximately 0.6 cm × 0.8 cm. The third polyp was also small and was located in the sigmoid, with a size of approximately 0.4 cm × 0.5 cm. The fourth polyp was a cucurbit-shaped giant polyp that was located in the descending colon, with a size of 4 cm × 2 cm. The fifth polyp was also a giant polyp with three lobulations and was located in the transverse colon, with a size of approximately 2.5 cm × 2 cm. All of the polyps were removed smoothly during colonoscopy. The polyps were confirmed as hamartomatous polyps and were pathologically diagnosed as PJS (Figure 2).
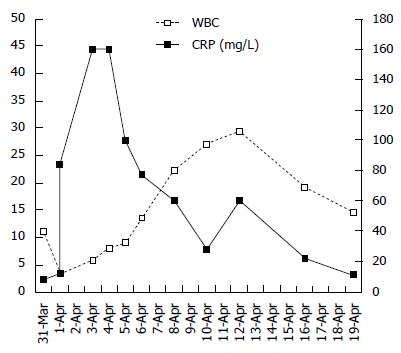
The infant was transferred to the general ward for routine hemostasis with dicynone, p-aminomethyl benzoic acid and supplemental fluid due to fasting. Her blood pressure was normal. The infant had a low fever for approximately 2 h after polypectomy, and her abdomen was soft. We believed that she had an absorption fever due to the extent of polypectomy. Therefore, no further action was taken. The infant still had a low fever and was slightly agitated after 3 h. Physical examination showed that her abdomen was slightly inflated. Still, no further action was taken. Her temperature increased after 4 h, and physical cooling was performed. The infant became more agitated, and after 6 h, her abdomen became more inflated. A CBC test was performed, which revealed that the white blood cell (WBC) count was 3.1 × 109/L and the C-reactive protein (CRP) level was 12 mg/L.
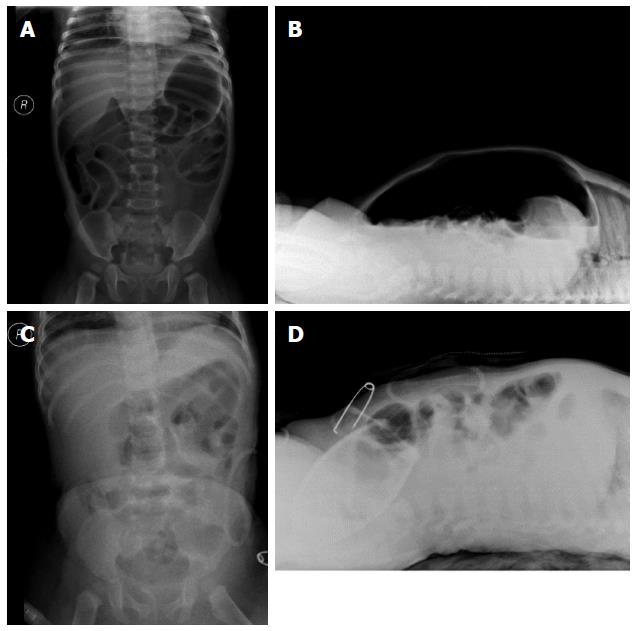
Symptoms of shock were observed in the infant, as evidenced by low spirit and poor peripheral limb circulation after 8 h. A CBC test was performed, and the results showed that the WBC was 3.5 × 109/L and the CRP was 68 mg/L. A crystalloid solution was administered quickly to improve the shock symptoms. After approximately 10 h, the infant underwent abdominal X-ray examination. The X-ray image showed pneumoperitoneum (Figure 3A and B).
The infant immediately underwent emergency surgery. During surgery, a perforation of 0.5 cm2 in size was found in the descending colon where the fourth giant polyp was removed. This part of the intestine was resected by anastomosis.
The infant was transferred to the pediatric intensive care unit for further postoperative support. A CBC test showed that the WBC was 3.0 × 109/L and the CRP was 84 mg/L. The infant was intubated and placed on ventilator support for 4 d. The blood culture was positive for Escherichia coli. Antibiotics, including ceftriaxone, metronidazole, meropenem, and vancomycin, were used for the treatment of sepsis and acute peritonitis. Albumin was used to treat hypoproteinemia caused by inflammatory exudation. Abdominal X-ray showed that the pneumoperitoneum had disappeared five days after surgery (Figure 3C and D).
The infant was discharged from our hospital when her CBC was nearly normal and her blood culture was negative. The changes in the WBC and CRP that occurred during this period are described in Figure 4.
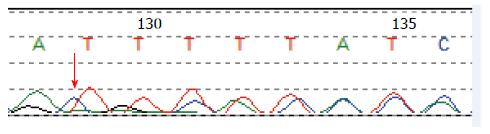
Molecular analysis of the STK11 gene revealed a novel and de novo frameshift mutation (c.64_65het_delAT, p.M22Gfs*140) in exon 1 in this PJS infant (Figure 5). This mutation leads to the partial loss of the kinase domain and the complete loss of the C-terminus, and it has not been previously reported. We also found an SNP (c.920+7G>G/C) in intron 7.
A total of 679 papers were identified (PubMed = 183, Embase = 112, Scopus = 297, Cochrane = 0, Web of Science = 87), and 27 papers were considered potentially relevant. All articles discussing PJS in children were retrieved. The criterion for inclusion in the study was patient age of 0-18 years. Only English-language articles published from 1995, when the first case of neonatal PJS was reported, up to July 31, 2015 were retrieved, and all articles were reviewed independently for suitability (Table 1). The clinical manifestations in PJS vary among patients. In the above-mentioned literature, some children exhibited mucocutaneous pigmentation, abdominal pain, anemia and bloody stool, intussusception or prolapsed rectal polyps. Fernandez Seara et al[4] first reported PJS in a neonate with abdominal distention and bloody stool initially due to multiple polyposis. This patient died at 12 mo of age with multiple complications. Al Faour and Burgmeier respectively reported another neonate with PJS due to an oblong abdominal mass[5,6]. Those neonates with PJS were diagnosed by pathology but STK11 gene information was not collected for these cases. Some of the retrieved studies focused on the clinical information whereas some focused on genetic information related to STK11 and other genes[7,8]. Few studies focused on both clinical features and gene information, and all children in the previous PJS reports were 1 year of age or older[9-11]. Morrison reported a nine-month-old PJS infant with umbilical pigmentation[12]. This infant had a family history of PJS and had a novel mutation (c.88dupG) in exon 1 of the STK11 gene. And in our study, we report the youngest PJS infant in China with recurrent prolapsed rectal polyps, and her clinical information, laboratory data, image data, pathological images and STK11 gene data are also described in detail.
| Ref. | Year | Study type | Country | Age range | Manifestation | Treatment | Gene information |
| Fernandez Seara et al[4] | 1995 | Case report | Spain | 15 d | PRPs | ED and Died | NA |
| Corley et al[21] | 1997 | Case report | United States | 15 yr | Gastric outlet obstruction, abdominal pain | ED and OP | NA |
| Al Faour et al[5] | 2002 | Case report | France | 4 d | Abdominal mass | OP | NA |
| Boseto et al[22] | 2002 | Case report | Australia | 14 mo | Gastric outlet obstruction | OP | NA |
| Homan et al[23] | 2005 | Case report | Slovenia | 10 yr | Abdominal pain, vomiting, intussusception | OP | NA |
| Hearle et al[7] | 2006 | Letter | United Kingdom | 1-38 yr | NA | NA | Yes |
| Zuo et al[8] | 2007 | Article | China | 5-10 yr | MP | NA | Yes |
| Adolph et al[24] | 2008 | Review | United States | Children | MP, intussusception, abdominal pain, etc. | ED and OP | NA |
| Vidal et al[25] | 2009 | Article | France | 4.8-15.1 yr | MP, PRPs, intussusception, etc. | ED and OP | NA |
| Ausavarat et al[26] | 2009 | Case report | Thailand | 14 yr | MP, abdominal pain, intestinal obstruction, etc. | ED or OP | Yes |
| Resta et al[9] | 2010 | Article | Italy | 1-17 yr | NA | NA | Yes |
| Yang et al[27] | 2010 | Article | Korea | 6 mo-13.8 yr | MP, hematochezia, intussusception, anemia,etc. | ED or OP | Yes |
| Liu et al[11] | 2011 | Article | China | 6 yt | MP, abdominal pain, hematochezia | ED and OP | Yes |
| Burgmeier et al[6] | 2012 | Case report | Germany | 2 d | Vomiting, abdominal mass | OP | NA |
| Chen et al[28] | 2012 | Article | China | 14 yr | MP | ED | Yes |
| Thakkar et al[29] | 2012 | Review | United States | children | MP, PRPs, intussusception, etc. | ED and OP | Yes |
| Liu et al[30] | 2012 | Article | China | 12 and 16 yr | MP, abdominal pain, hematochezia, etc. | ED | Yes |
| Goldstein et al[31] | 2013 | Article | United States | 1-14 yr | MP, PRPs, intussusception, etc. | ED and OP | NA |
| Zheng et al[32] | 2013 | Article | China | 1-5 yr | MP, abdominal pain | NA | Yes |
| Vageli et al[33] | 2013 | Case report | Greece | 12 yy | MP | NA | NA |
| Chae et al[34] | 2014 | Case report | Korea | 12 yr | MP, abdominal pain, anemia | OP | Yes |
| Morrison et al[12] | 2014 | Case report | United Kingdom | 9 mo | Umbilical pigmentation | NA | Yes |
| Wang et al[35] | 2014 | Article | China | 1-43 yr | MP, histopathologic examination, etc. | ED and OP | Yes |
| Cohen et al[36] | 2014 | Article | Israel | 4-16 yr | MP, abdominal pain, hematochezia | ED or OP | Yes |
| Kimura et al[37] | 2015 | Case report | Japan | 13 yr | MP, abdominal pain | OP | NA |
| Kuroda et al[10] | 2015 | Article | Japan | 12 yr | MP, intellectual disability | ED | Yes |
| Armijo et al[38] | 2015 | Case report | United States | 4 yr | gynecomastia, MP, testicular lesions | Orchiectomy | Yes |
| Our group | 2015 | Article | China | 9 mo | PRPs | ED and OP | Yes |
In 1995, Fernandez Seara et al[4] first reported on the occurrence of PJS in a neonate. The STK11 gene responsible for PJS was discovered in 1998. However, there are still few reports of PJS presenting in infants, and there are no relevant reports of this syndrome affecting infants in China. This study presents a nine-month-old Chinese PJS infant with multiple intestinal polyps. To our knowledge, this is the first report to describe the clinical characteristics, colonoscopic findings, pathological results, and surgical treatment of the youngest Chinese infant with PJS and the STK11 gene mutation.
PJS may have varying clinical manifestations, and most clinical complications occur in adults who are 20 to 30 years of age; however, 1/3 of patients are younger than 10[13]. Symptoms of PJS include abdominal pain, rectal bleeding, intussusception, and mucocutaneous pigmentation. The nine-month-old infant in this study suffered recurrent PRPs for one month. No mucocutaneous pigmentation was detectable in any part of the patient’s body, and she only had growing PRPs. Colonoscopy revealed five intestinal polyps, two of which were giant polyps.
For large polyp (≥ 2 cm) removal, endoscopic polypectomy is commonly considered safe, efficacious and cost-effective in the management of complex colon polyps compared with surgical removal. However, this procedure is not free from complications[14]. For polyps in children, surgical removal may influence physical and psychological development and endoscopic follow-up. Endoscopic resection of large polyps, which is minimally invasive, is associated with lower morbidity and mortality rates compared with surgery, and it is not only indicated, it is also considered the most appropriate approach. In children, and especially in infants, the lumen size is small and the intestinal wall is thin. Thus, endoscopic polypectomy has a greater risk of perforation in young patients than in adults. For adults, there is still debate as to whether large polyps should be removed under laparoscopy or under colonoscopy[15]. For children, no guidelines are available for removal of a large polyp via laparoscopy, colonoscopy or surgery. We considered that the polyp size, its pedunculated or sessile nature, its location, the age of the child, and the experience of the endoscopist are important factors when selecting between endoscopic or surgical removal of a large polyp[16]. Wiseman et al[17] presented two patients with very large polyps in the proximal colon that were not amenable to colonoscopic removal. These large polyps were removed using minimal-access surgical techniques under laparoscopy. For our patient, surgery may have been the best choice to avoid perforation and sepsis.
Bleeding and perforation are common adverse events in polyposis patients after endoscopy. Bleeding from the large feeding vessels could induce unexpected hypovolemic shock, especially in young toddlers. Perforation can occur during or after resection. Immediate perforation occurs due to deep resection, whereas delayed perforation occurs from a rupture of the wall caused by coagulation necrosis[18]. In our study, the PJS infant had intestinal perforation, serious pneumoperitoneum and sepsis. The large intestine is full of detrimental bacteria, especially Escherichia coli. Migration of Escherichia coli into the blood stream from a perforation site can lead to sepsis. For large polyp removal, careful examination is very important. Performing timely postoperative abdominal examination, abdominal X-ray and ultrasound aids in the early detection of complications. There are fewer risks of sepsis and other complications if bleeding and perforation are found earlier.
For children with PJS, especially infants, mucocutaneous pigmentation may develop after birth or later. It is difficult to distinguish PJS from other polyp syndromes if mucocutaneous pigmentation presents late. Pathological examination and STK11 gene testing are the gold standards for PJS diagnosis[19]. The patient in this study was initially diagnosed with juvenile polyposis syndrome; however, pathological examination of the polyps led to a diagnosis of PJS. STK11 gene testing further confirmed this diagnosis. This PJS patient had a de novo, novel frameshift STK11 gene mutation. This type of truncating mutation in PJS is associated with the presence of more polyps, as well as increased risks of surgical intervention and cancer[20]. Therefore, additional follow-up of this child and similar patients is needed in the future
In conclusion, we have detailed the first case of PJS occurring in a Chinese infant without mucocutaneous pigmentation. For infants with PJS, the treatment strategy should be individualized, and careful postoperative examination should be conducted. Pathological and genetic testing is also indispensable.
Peutz-Jeghers syndrome (PJS) is an uncommon autosomal dominant inherited disease characterized by the occurrence of hamartomatous polyps in the gastrointestinal tract, and mucocutaneous pigmentation. Germline mutations of STK11 gene are responsible for most PJS cases. However, the report of Chinese infant with PJS is rare.
PJS is associated with mutations of the STK11 gene located on chromosome 19p13.3. Germline point mutations are responsible for most patients with PJS and large genomic deletions have also been identified in few patients with PJS. Currently, there is no guideline available on polypectomy by endoscopy or surgical intervention for the children with PJS.
This is the first study to report the youngest PJS infant in China without mucocutaneous pigmentation, and her clinical information, laboratory data, image data, pathological images are also described in detail. STK11 gene sequencing results confirmed that this infant had PJS with a novel, de novo mutation. Further, systematic literature concerning pediatric PJS is analyzed and explained.
The present study suggested that for patients with multiple polyps, histopathological examination and gene testing are essential. This article also gives some thoughts on management of the children, especially infants with PJS cost-effectively.
PJS refers to a rare autosomal dominant inherited disorder characterized by gastrointestinal hamartomatous polyposis, mucocutaneous pigmentations, and high risk of cancer. Germline mutations of STK11 gene are the main cause of PJS.
The authors described a PJS infant with an STK11 gene mutation who developed intestinal perforation following endoscopic polypectomy.
P- Reviewer: Urganci N, Watanabe T S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Jeghers H, Mckusick VA, Katz KH. Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N Engl J Med. 1949;241:993, illust; passim. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 332] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, Prescott AR, Clevers HC, Alessi DR. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102-5114. [PubMed] |
| 3. | Yoo JH, Yoo JH, Choi YJ, Kang JG, Sun YK, Ki CS, Lee KA, Choi JR. A novel de novo mutation in the serine-threonine kinase STK11 gene in a Korean patient with Peutz-Jeghers syndrome. BMC Med Genet. 2008;9:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Fernandez Seara MJ, Martinez Soto MI, Fernandez Lorenzo JR, Trabazo S, Gamborino E, Forteza Vila J. Peutz-Jeghers syndrome in a neonate. J Pediatr. 1995;126:965-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Al Faour A, Vrsansky P, Abouassi F, Dabbagh H, Gross P, Retbi JM. Peutz-Jeghers colonic tumour in a newborn. Eur J Pediatr Surg. 2002;12:138-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Burgmeier C, Schier F, Staatz G. Gastric outlet obstruction in a neonate because of Peutz-Jeghers syndrome. J Pediatr Surg. 2012;47:e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Hearle NC, Rudd MF, Lim W, Murday V, Lim AG, Phillips RK, Lee PW, O’donohue J, Morrison PJ, Norman A. Exonic STK11 deletions are not a rare cause of Peutz-Jeghers syndrome. J Med Genet. 2006;43:e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Zuo YG, Xu KJ, Su B, Ho MG, Liu YH. Two novel STK11 mutations in three Chinese families with Peutz-Jeghers syndrome. Chin Med J (Engl). 2007;120:1183-1186. [PubMed] |
| 9. | Resta N, Giorda R, Bagnulo R, Beri S, Della Mina E, Stella A, Piglionica M, Susca FC, Guanti G, Zuffardi O. Breakpoint determination of 15 large deletions in Peutz-Jeghers subjects. Hum Genet. 2010;128:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Kuroda Y, Saito T, Nagai J, Ida K, Naruto T, Masuno M, Kurosawa K. Microdeletion of 19p13.3 in a girl with Peutz-Jeghers syndrome, intellectual disability, hypotonia, and distinctive features. Am J Med Genet A. 2015;167A:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Liu WL, Li F, He ZX, Jiang HY, Ai R, Zhu XP, Chen XX, Ma HW. Identification of a novel de novo STK11 mutation in a Chinese child with Peutz-Jeghers syndrome. J Int Med Res. 2011;39:2033-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Morrison PT, Donnelly DE, Morrison PJ. Umbilical pigmentation in Peutz-Jeghers syndrome. Clin Dysmorphol. 2014;23:114-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Kitagawa S, Townsend BL, Hebert AA. Peutz-Jeghers syndrome. Dermatol Clin. 1995;13:127-133. [PubMed] |
| 14. | Aziz Aadam A, Wani S, Kahi C, Kaltenbach T, Oh Y, Edmundowicz S, Peng J, Rademaker A, Patel S, Kushnir V. Physician assessment and management of complex colon polyps: a multicenter video-based survey study. Am J Gastroenterol. 2014;109:1312-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Church JM. Laparoscopic vs. colonoscopic removal of a large polyp. Am J Gastroenterol. 2009;104:2633-2634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Spychalski M, Buczyński J, Cywiński J, Dziki Ł, Langner E, Sygut A, Trzciński R, Dziki A. Large colorectal polyps--endoscopic polypectomy as an alternative to surgery. Pol Przegl Chir. 2011;83:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Wiseman J, Emil S. Minimal access surgical management of large juvenile polyps in children. J Pediatr Surg. 2009;44:e9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Kaltenbach T, Soetikno R. Endoscopic resection of large colon polyps. Gastrointest Endosc Clin N Am. 2013;23:137-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Beggs AD, Latchford AR, Vasen HF, Moslein G, Alonso A, Aretz S, Bertario L, Blanco I, Bülow S, Burn J. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 456] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 20. | Salloch H, Reinacher-Schick A, Schulmann K, Pox C, Willert J, Tannapfel A, Heringlake S, Goecke TO, Aretz S, Stemmler S. Truncating mutations in Peutz-Jeghers syndrome are associated with more polyps, surgical interventions and cancers. Int J Colorectal Dis. 2010;25:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Corley DA, Uyeki TM, Cello JP. Gastrointestinal bleeding and gastric outlet obstruction from Peutz-Jeghers polyposis. Diagnosis and treatment. West J Med. 1997;166:350-352. [PubMed] |
| 22. | Boseto F, Shi E, Mitchell J, Preddy J, Adams S. Gastroduodenal intussusception due to Peutz-Jeghers syndrome in infancy. Pediatr Surg Int. 2002;18:178-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Homan M, Dolenc Strazar Z, Orel R. Peutz-Jeghers syndrome. A case report. Acta Dermatovenerol Alp Pannonica Adriat. 2005;14:26-29. [PubMed] |
| 24. | Adolph VR, Bernabe K. Polyps in children. Clin Colon Rectal Surg. 2008;21:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Vidal I, Podevin G, Piloquet H, Le Rhun M, Frémond B, Aubert D, Leclair MD, Héloury Y. Follow-up and surgical management of Peutz-Jeghers syndrome in children. J Pediatr Gastroenterol Nutr. 2009;48:419-425. [PubMed] |
| 26. | Ausavarat S, Leoyklang P, Vejchapipat P, Chongsrisawat V, Suphapeetiporn K, Shotelersuk V. Novel mutations in the STK11 gene in Thai patients with Peutz-Jeghers syndrome. World J Gastroenterol. 2009;15:5364-5367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Yang HR, Ko JS, Seo JK. Germline mutation analysis of STK11 gene using direct sequencing and multiplex ligation-dependent probe amplification assay in Korean children with Peutz-Jeghers syndrome. Dig Dis Sci. 2010;55:3458-3465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Chen C, Zhang X, Wang F, Liu C, Lu H, Wan H, Wei J, Liu J. One novel deletion and one splicing mutation of the LKB1 gene in two Chinese patients with Peutz-Jeghers syndrome. DNA Cell Biol. 2012;31:1535-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Thakkar K, Fishman DS, Gilger MA. Colorectal polyps in childhood. Curr Opin Pediatr. 2012;24:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Liu D, Guo H, Xu X, Yu Y, Bai Y. Two variants in STK11 gene in Chinese patients with Peutz-Jeghers syndrome. J Genet. 2012;91:205-208. [PubMed] |
| 31. | Goldstein SA, Hoffenberg EJ. Peutz-Jegher syndrome in childhood: need for updated recommendations? J Pediatr Gastroenterol Nutr. 2013;56:191-195. [PubMed] |
| 32. | Zheng B, Pan J, Wang Y, Li M, Lian M, Zheng Y, Jin Y. Analysis of STK11 gene variant in five Chinese patients with Peutz-Jeghers syndrome. Dig Dis Sci. 2013;58:2868-2872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Vageli DP, Doukas SG, Markou A. Mismatch DNA repair mRNA expression profiles in oral melanin pigmentation lesion and hamartomatous polyp of a child with Peutz-Jeghers syndrome. Pediatr Blood Cancer. 2013;60:E116-E117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Chae HD, Jeon CH. Peutz-Jeghers syndrome with germline mutation of STK11. Ann Surg Treat Res. 2014;86:325-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Wang Z, Wu B, Mosig RA, Chen Y, Ye F, Zhang Y, Gong W, Gong L, Huang F, Wang X. STK11 domain XI mutations: candidate genetic drivers leading to the development of dysplastic polyps in Peutz-Jeghers syndrome. Hum Mutat. 2014;35:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Cohen S, Gorodnichenco A, Weiss B, Lerner A, Ben-Tov A, Yaron A, Reif S. Polyposis syndromes in children and adolescents: a case series data analysis. Eur J Gastroenterol Hepatol. 2014;26:972-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Kimura J, Sasaki K, Okabayashi T, Shima Y, Iwata J, Morita S. Duodeno-jejunal Intussusception with Acute Pancreatitis in Peutz-Jeghers Syndrome: The First Case Presentation in Childhood. J Gastrointest Surg. 2015;19:1922-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Armijo B, Bocklage T, Heideman R. Intratubular Large Cell Hyalinizing Sertoli Cell Tumor of the Testes in a 4-Year-Old Male With Peutz-Jeghers Syndrome. J Pediatr Hematol Oncol. 2015;37:e184-e187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |