Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.8
Peer-review started: May 5, 2015
First decision: June 23, 2015
Revised: July 7, 2015
Accepted: November 13, 2015
Article in press: November 13, 2015
Published online: January 7, 2016
Processing time: 246 Days and 18.8 Hours
Alcohol consumption is the principal factor in the pathogenesis of chronic liver diseases. Alcoholic liver disease (ALD) is defined by histological lesions on the liver that can range from simple hepatic steatosis to more advanced stages such as alcoholic steatohepatitis, cirrhosis, hepatocellular carcinoma and liver failure. As one of the oldest forms of liver injury known to humans, ALD is still a leading cause of liver-related morbidity and mortality and the burden is exerting on medical systems with hospitalization and management costs rising constantly worldwide. Although the biological mechanisms, including increasing of acetaldehyde, oxidative stress with induction of cytochrome p450 2E1, inflammatory cytokine release, abnormal lipid metabolism and induction of hepatocyte apoptosis, by which chronic alcohol consumption triggers serious complex progression of ALD is well established, there is no universally accepted therapy to prevent or reverse. In this article, we have briefly reviewed the pathogenesis of ALD and the molecular targets for development of novel therapies. This review is focused on current therapeutic strategies for ALD, including lifestyle modification with nutrition supplements, available pharmacological drugs and new agents that are under development, liver transplantation, application of complementary medicines, and their combination. The relevant molecular mechanisms of each conventional medication and natural agent have been reviewed according to current available knowledge in the literature. We also summarized efficacy vs safety on conventional and herbal medicines which are specifically used for the prevention and treatment of ALD. Through a system review, this article highlighted that the combination of pharmaceutical drugs with naturally occurring agents may offer an optimal management for ALD and its complications. It is worthwhile to conduct large-scale, multiple centre clinical trials to further prove the safety and benefits for the integrative therapy on ALD.
Core tip: The aim of this article is to review the impairment of hepatocellular dysfunction in alcoholic liver diseases and their prospective managements. Specifically, we focused on the natural therapies with their efficacies and safeties. Moreover, we summarized molecular mechanisms of herbal therapy to treat alcoholic liver disease (ALD). With evidence-based natural therapy, this article highlighted that the combination of pharmaceutical drugs with naturally occurring agents may offer an optimal management for this complex liver disease. It is worthwhile to conduct large-scale, multiple centre clinical trials further to prove the safety and benefits for the integrative therapy on ALD.
- Citation: Kim MS, Ong M, Qu X. Optimal management for alcoholic liver disease: Conventional medications, natural therapy or combination? World J Gastroenterol 2016; 22(1): 8-23
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/8.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.8
Alcohol is a psychoactive substance and has been widely used in many cultures for centuries. Alcoholic abuse causes a large range of diseases and is also a social and economic burden in world societies. Reduction of alcohol consumption is now becoming the global strategy, with attempts to define “harmful use” of alcohol by the World Health Organization (WHO) to reduce the associated morbidity and mortality. Alcohol intake is a principal etiological factor in chronic liver diseases. Alcoholic liver disease (ALD), which initially manifests as hepatic fat accumulation, followed by an inflammatory response to induce final stage liver failure, is now a deleterious health problem[1]. Alcohol abuse accelerates various types of liver diseases, such as alcoholic fatty liver disease (AFLD), alcoholic steatohepatitis, alcoholic hepatitis (AH), progressive fibrosis, liver cirrhosis and liver failure[2]. Patients with ALD, especially with alcoholic cirrhosis, are associated with increased risk of hepatocellular carcinoma (HCC)[3,4].
The prevalence of ALD has increased in the last years, parallel with the increasing alcohol consumption in the western world as well as in Asian counties[5]. According to the WHO report in 2011, chronic alcohol consumption results in approximately 2.5 million deaths each year with much of the burden related to ALD[6]. Though ALD significantly contributes to the rising morbidity and mortality statistics and related health expenses, there is a lack of effective treatments for ALD, especially cirrhosis and HCC. Abstention from alcohol may reverse the early stage of ALD to a normal condition. The treatment for ALD with conventional medicines, mainly pharmaceutical medications, has limited success with side-effects. Recently, natural medicines, which mainly apply herb-derived agents, are emphasized as alternative therapies to manage the various alcohol-related liver diseases. It is the aim of this article to provide an overview of understanding the mechanisms of ALD, which could generate therapeutic interventions with conventional medicines, natural therapies and their combinations to reverse or retard the progression of ALD.
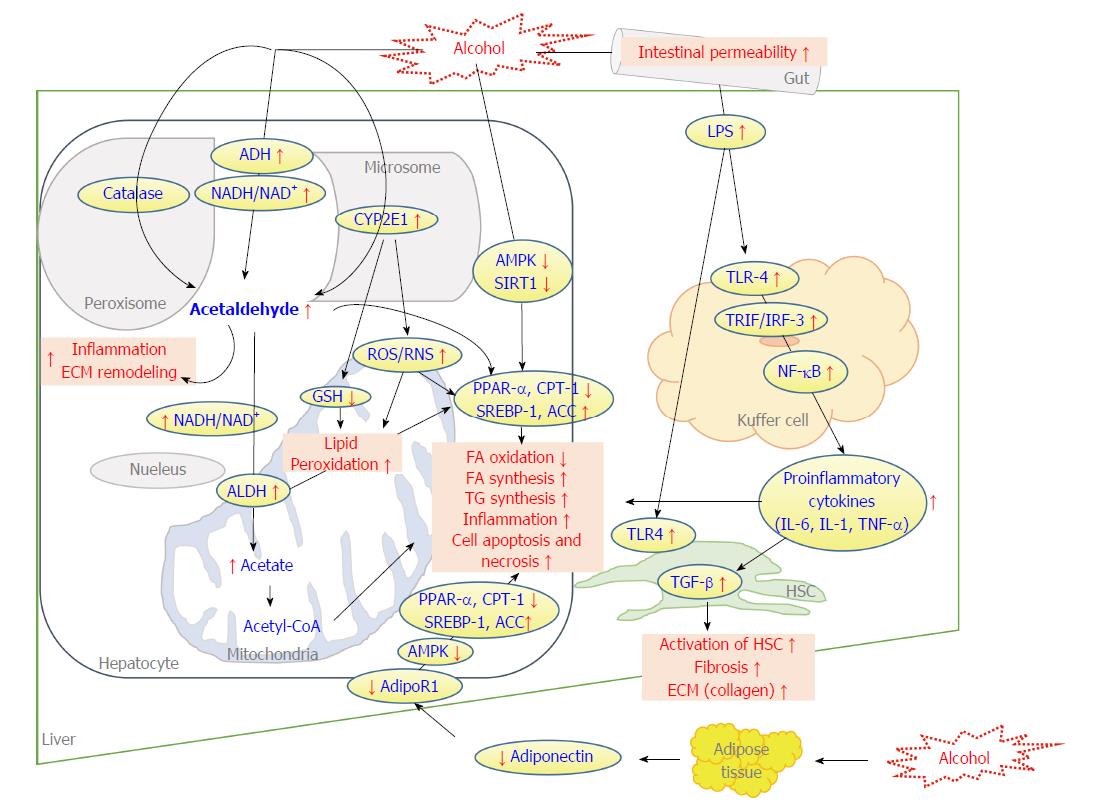
Pathogenesis of ALD is multifactorial. Strategically, the liver is the most important organ to target as about 90% of alcohol intake is metabolized by the liver[7]. Alcohol consumption can cause fatty liver by disrupting hepatic lipids and glucose metabolism. Furthermore, ethanol and its oxidative and non-oxidative metabolites have direct toxic effects on the liver[8]. Histologically, fatty liver, or HS, is a malfunctioned fat accumulation in the parenchymal cells of the liver. HS is a reversible lesion. Fibrosis (or scar formation) is the subsequent result if liver injury persists. Fibrosis determined by biopsy increases the likelihood of progression to cirrhosis and end-stage liver disease. Biochemically, alcohol abuse contributes to a serious complex phenomenon involving different molecular and biological mechanism to induce ALD. Many hypotheses have been investigated and established to explain the pathogenic mechanisms of ALD. These include: (1) activation of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) to cause the over generation of acetate[9]; (2) induction of cytochrome P450 2E1 (CYP2E1) for alcoholic oxidative stress and hepatotoxicity[10]; (3) abnormal lipid metabolism by increasing fatty acid (FA) synthesis and decreasing of FA oxidation[10]; (4) hepatic inflammation indicated by an increase of tumour necrosis factor-α (TNF-α) and cytokine release[11]; (5) induction of hepatocyte apoptosis and subsequent activation of Kupffer cells; (6) increased hepatic levels of cellular fibronectin and tissue growth factor-β (TGF-β) related to the activation of hepatic stellate cells[12]; and (7) ethanol-induction of gut endotoxins[13,14]. Proposed mechanisms impacting hepatocellular dysfunction by alcohol exposure within ALD pathogenesis are summarized in Figure 1. To further improve current strategies for managing ALD, a deeper understanding of the pathomechanisms of the disease is needed. Various curative approaches based on these mechanisms could prevent the progression of ALD and its downstream sequelae.
General management of ALD should initially be abstinence from alcohol[2]. This becomes increasingly vital as the condition progresses. However, this is largely dependent on patient willingness and compliance. Currently, there is no universally accepted therapy to prevent or reverse the progression of ALD. Successful managements include lifestyle modification with the correction of nutritional deficiencies, conventional drugs, liver transplantation, application of complementary medicines, and their combinations[15,16].
Lifestyle intervention initially entails the abstinence from alcohol. Abstinence limits the development of hepatic steatosis (HS) and prevents further ongoing liver injury, fibrosis and the possibility of HCC. It was reported that abstaining from alcohol improves the prognosis of ALD patients and prevents advancement to hepatic cirrhosis by reducing portal pressure and histological lesions[17,18]. However, long-term alcohol abstinence heavily relies on patient compliance and willingness and therefore may need to be accompanied with psychological therapy or social support. Cognitive behavioral therapy, one of the psychotherapies, reduces the risk of alcoholism and motivates patients to take responsibility and avoid relapse[19]. Pharmacotherapy in combination with psychosocial interventions also encourages patients to sustain abstinence from alcohol. Naltrexone and Acamprosate have been used to manage alcohol abuse in chronic heavy drinkers[20]. Disulfiram has also been approved by the FDA for the management of alcoholism and is broadly utilized despite unclear results from clinical trials[21]. Disulfiram is an acetaldehyde dehydrogenase inhibitor and may cause an increase of serum acetaldehyde by changing the alcohol metabolism. Disulfiram, therefore, needs to have supervised administration[21]. Furthermore, reducing alcohol consumption, but not completely stopping has also displayed favourable results for the survival of patients with ALD[22]. Other important lifestyle changes can include smoking cessation, adopting a balanced diet and weight control if relevant. Smoking is an independent risk factor for increasing the severity of ALD and its progression to fibrosis and HCC[23,24]. Obesity is another risk factor that can accelerate the progression of ALD as it alone is linked to fatty liver disease and cirrhosis[25].
Malnutrition is another major complication of ALD as poor dietary intake from anorexia, vomiting, mal-digestion, iatrogenic causes, metabolic disturbance, hypermetabolic state, impaired protein synthesis, or mal-absorption can lead to malnutrition and contribute to the disease[26,27]. Nutritional supplementation may address calorie, protein, and nutrient intake to support hepatocyte regeneration[28]. Protein energy malnutrition can be detected with symptoms of muscle wasting, edema and loss of subcutaneous fat. It is recommended that patients with ALD have a daily intake of 1.5 g/kg and 35-40 kcal/kg per day of protein[29]. Supplementation with vitamins such as folate and thiamine should be considered if their deficiencies are detected[16,30]. A discussion concerning micronutrient supplementation is necessary beyond the scope of this article. Nevertheless, an example of micronutrient deficiency related to ALD is seen in zinc deficiency as zinc supplementation has shown to improved ALD by enhancing the activity of ADH and suppressing CYP2E1 in animal models[31,32]. In summary, nutritional status should be balanced in individual cases of malnutrition.
Although patients living with ALD are mostly treated with strategies to encourage abstinence from alcohol, some patients may need to be accompanied with pharmacological treatment approaches. There are several drugs that have been widely used and reviewed with efficacy, molecular mechanisms (Figure 1), and safety. Table 1 summarizes the effects and mechanism of conventional medicines.
| Medicine/compound | Type of model | Dosage/duration | Effects | Mechanisms | Ref. |
| Corticosteroid | RCT in severe AH | Prednisone (30 mg/d, 30 d) | Lower mortality AH group compared to antioxidant treated group | [37] | |
| RMT and double blind trials in severe AH | Methylprednisolone (32 mg within 7 d) and 28 d treatment and then tapered over 2 wk and discontinued | Decrease short-term mortality of patients with AH | [160] | ||
| Pentoxifylline | A double-blind, placebo-controlled trial | 400 mg orally 3 times daily for 4 wk | Decrease in the risk of Progressing hepatorenal syndrome; Improve short-term survival in patients with AH | [42] | |
| A RCT followed by open study in severe AH patients | 400 mg/d, orally for 4 wk and then tapered by 5 mg/wk for 7 wk | Better reduced mortality compared to prednisolone in AH | [161] | ||
| SAM | Ethanol-LPS-induced fibrotic rats | 10 mg/kg bw, ip | Attenuate oxidative stress and HSC activation | Inhibition of TGF-β signaling pathway | [50] |
| Double-blind, placebo-controlled trial in 220 patients with AH and cirrhosis | 1600 mg/d orally | Decreased serum markers of cholestasis; Improved subjective symptoms such as pruritus, fatigue, and feeling of being unwell | [162] | ||
| PPAR-α agonists | C57BL/6J mice with ethanol (27.5% of total calories) | 0.1% Wy-14643 for 4 wk | Decreased serum and hepatic fat accumulation | Enhancement of hepatic FA oxidation pathway; Increased PPAR-α mRNA expression by 5-fold | [163] |
| C57BL/6J mice with 4 % ethanol (Ethanol feeding for 12 wk) | Wy-14643 (50 mg/kg per day) for last 2 wk | Decreased serum AST and ALT Dropped on score of hepatic steatosis and hepatocyte ballooning | Repress PI3K and COX-2 expression; Enhance adiponectin and HO-1; Increase SIRT1 protein expression | [164] | |
| C57BL/6J mice with ethanol containing diet (29% of total calories) | Rosiglitazone (3 mg/kg bw/d) for 2 wk | Reduced hepatic triglyceride content; Reduced AST and ALT | Upregulated level of adiponectin and its receptors; Enhance SIRT1-AMPK signaling pathway | [55] | |
| Metformin | C57BL/6 mice TNF knockout mice PAI-1 knockout with ethanol (6 g/kg ig) | 200 mg/kg ip for 4 d prior to ethanol administration | Reduced ALT; Prevented hepatic fat accumulation | Prevention of the upregulation of PAI-1 | [57] |
| Metadoxine | HepG2, CFSC-2G and HSC treated with ethanol (50 mM) and acetaldehyde (175 µM) | 10µg/mL for 24 h | Reduced peroxidation and oxidized GSH content | Decrease collagen secretion and IL-6, IL-8 and TNF-α secretion | [69] |
| Carvedilol | Male Wistar rats with ethanol (5%) fed | 10 mg/kg per day for last 7 d of total 49 d | Reduced hepatic TG level and the accumulation of fatty droplets within hepatocytes; | Down-regulated FAS and SREBP-1, and up-regulated PPAR-α; | [56] |
| Inhibited ethanol-induced the thickening of zone 3 vessel walls | Reduced the activation of HSCs with decrease induction of TGF-β1 and collagen | ||||
| Propylthiouracil | Male Sprague-Dawley rat with ethanol consumption (36%) | 50 mg/kg per day of Wy-14643 for last 2 wk for the last 5 d of 4-6 wk diets with alcohol | Increased hepatic blood flow; Reduces oxidative stress | [71] | |
| Colchicine | RCT in 74 patients with cirrhosis | 1 mg/d for 4.4 yr | Reduced serum N-terminal peptide of type III procollagen levels | [70] |
Corticosteroids were one of the first pharmacologic therapies investigated for the treatment of AH and many researchers have extensively studied their use for patients with ALD[33]. The underlying mechanism of action is thought to be the ability of corticosteroids to ameliorate the characteristic inflammatory response by decreasing TNF-α, IL-6, and IL-8[34,35] and also to suppress the formation of acetaldehyde adduct metabolites with inhibition of collagen[36]. Prednisolone, belonging to the corticosteroid family, with a dose of 40 mg/d for 28 d followed by tapering over 2-4 wk is recommended to treat severe alcoholic hepatitis (AH). Another recent published clinical trial randomized 101 acute AH patients to corticosteroids (e.g., prednisone 30 mg/d or methylprednisone 24 mg/d intravenously) and at 30 d, survival was 70% in corticosteroid group when compared to the control (37/53)[37]. Although, corticosteroids have been established as a common treatment for ALD, the data on treatment duration and efficacy of corticosteroids in ALD are conflicting. Long-term usage of corticosteroid seems to enhance the efficacy while others have showed no benefit of corticosteroid therapy after more than 6 mo[38]. Glucocorticoid has been demonstrated to decrease serum bilirubin levels, which is shown to be a clinically useful indicator[39,40]. Using corticosteroids in severe AH, however, carries contraindication including sepsis, hepatitis B, hepatorenal syndrome and gastrointestinal (GI) bleeding[37,41].
Pentoxifylline (PTX), has been thought of as an appropriate substitute to replace corticosteroid treatment in patients with severe AH when there is a contraindication to steroids and is suitable for short-term use[42,43]. The exact mechanism of PTX is not entirely clear. Anti-TNF actions may explain part of its protective effect for ALD. Interesting results have proved that 100 to 1000 μg/mL of PTX inhibited TNF expression by murine peritoneal exudate cells treated with endotoxin 1 μg/mL and PTX treatment significantly reduced serum TNF-α levels in murine in vivo[44]. Despite these positive efficacies, treatment with PTX still remains controversial. It has been reported that no significant differences between PTX and corticosteroid treatment groups, or their combination were observed[45]. Meta-analysis also argued that PTX decreased the hepatic-related mortality, but trial sequential analysis did not support this result[46]. Nevertheless, the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases have both recommended that PTX should be considered when AH patients have contraindications to corticosteroid treatment[47].
S-adenosyl-L-methionine (SAM) operates as a methyl donor and contributes in the synthesis of the major cellular antioxidant, glutathione (GSH). In patients with AH and cirrhosis, decreased hepatic SAM, cysteine, and GSH levels caused by abnormality of hepatic gene expression in methionine and GSH metabolism is observed[48]. SAM deficiency arises in early stages of ALD. SAM supplementation can however increase reduced SAM concentrations and reverse liver injury and mitochondrial damage[49]. SAM works to reduce the severity of oxidative stress and hepatic stellate cell (HSC) activation by inhibiting hepatic TGF-α signalling pathway in an ethanol-lipopolysaccharide-induced fibrotic rat model[50]. SAM supplementation has also shown its benefit in 220 patients with chronic hepatitis and cirrhosis in a prospective, double-blind, placebo-controlled trial. Other studies have contradicted these findings, as one demonstrated that there was no significant difference between SAM and placebo in liver biopsies[51] and no improvement to liver histopathology scores or steatosis, inflammation, fibrosis in ALD patients[52]. Large and high-quality clinical trials are needed to further prove clinical benefits of SAM in ALD.
Peroxisome proliferator-activated receptor-alpha (PPAR-α), a member of the nuclear receptor superfamily and mainly expressed in liver, participates in the regulation of the transcription of genes involved in fatty acid (FA) oxidation, FA transportation, and export of free fatty acid. Alcohol intake inhibits FA oxidation via the suppression of PPAR-α in hepatocytes[53]. PPAR-α agonists, are therefore a potential therapy to reverse fat accumulation in AFLD. Many PPAR-α agonists have been assessed as a therapeutic approach to patients with ALD owing their positive efficacies to their anti-inflammatory and hypolipidaemic effects, as well as the induction of FA oxidation. Adiponectin and its receptors, recently, are a new prospective target to manage ALD through PPAR-α, TNF-α and sirtuin 1 (SIRT-1)-related pathways[54]. Thereby, drug discovery of adiponectin agonists may be a potential strategy to manage ALD. PPAR-α plays a pivotal role in the regulation of adiponectin level and rosiglitazone, one of the PPAR-α, agonists has shown to increase the circulating level of adiponectin and enhance hepatic adiponectin receptors (AdipoRs) in ethanol-fed mice[55]. These increments are correlated with the activation of the SIRT-1-AMPK signalling system. Carvedilol, a beta-blocker, has been recently reported to attenuate the development of AH by decreasing hepatic trigylceride (TG) levels and lipid droplets within hepatocytes through down-regulation of fatty acid synthase (FAS) and sterol regulator element binding protein 1 (SREBP-1), and up-regulated PPAR-α[56]. Carvedilol also prevented ethanol-induced thickening of zone 3 vessel walls and reduced HSC activation, decreased induction of TGF-β1 and collagen[56]. Metformin, an antidiabetic drug, has recently been discovered to improve HS by lowering fat accumulation and liver damage in mouse models of acute and chronic alcohol stimulation. This was through the prevention of the up-regulation of plasminogen activator inhibitor-1 and improvement of insulin resistance and liver injury by increasing PPAR-γ and adiponectin levels[57,58].
Although CYP2E1 inhibitors prevent alcohol-induced liver damage, compounds, which have been available in commercial markets, have been too toxic for clinical use. Polyenylphosphatidylcholine (PPC), an innocuous mixture of polyunsaturated lecithins, extracted from soybeans, was recently showed to reduce CYP2E1 activity and also attenuate hepatic oxidative stress and fibrosis[59]. However, one randomized controlled trial (RCT) did not confirm the clear association with the progression of liver fibrosis[60]. Further studies into the effect of PPC on ALD should be encouraged. Clomethiazole, another potential CYP2E1 inhibitor, has also shown to protect the liver from injury in ethanol-fed rats and humans[61,62].
The liver and GI tract shares a reciprocal relationship and therefore establishing healthy gut microbiota has been put forward as a strategy in the treatment of ALD. Lipopolysaccharide (LPS) binds to CD14 in Kupffer cells which reacts with toll like receptor-4 (TLR-4) to activate and release pro-inflammatory cytokines genes[63]. LPS signaling and gut microbiota can be adjusted by probiotics, prebiotics and TLR-4 antagonists to treat ALD[64]. In animal experiments, treating with antibiotics or lactobacillius improved ALD by reducing the gut microflora which suggests that gut-derived endotoxin/LPS may be important in the study of ALD[65]. The modified probiotic, Escherichia coli Nissle 1917 was shown to secrete pyrroloquinoline quinone (PQQ), which decreased lipid peroxidation and increased GSH to have an anti-inflammatory, anti-oxidant and anti-hyperlipidaemia effect in acute alcohol exposure in rats[66]. LPS antibody may also benefit injury from LPS induced by exposure to microorganisms in the gut. The outcome of experimental studies has provoked the need to compare the effects of LPS antibodies when combined with corticosteroids for translation into clinical trials for AH[67].
As mentioned, oxidative stress is regarded as a key player in the pathogenesis of ALD with increasing evidence of its importance, such as products of lipid peroxidation being detected in both heavy drinkers and ALD patients[68]. It, therefore, has been anticipated that anti-oxidant therapy would benefit the outcomes for ALD patients. In an animal study, metadoxine had preventative effects of lipid peroxidation and GSH depletion in human hepatocyte (HepG2), exposed with ethanol and acetaldehyde[68]. In HSC, metadoxin also prevents the increase of collagen production and TNF-α secretion[69].
In addition, some drugs have been used for alcoholic liver cirrhosis or fibrosis. Colchicine when administered (1 mg/d) to patients with chronic liver cirrhosis over a three year period displayed anti-fibrotic effects[70]. Propylthiouracil (PTU), typically used as an anti-thyroid medication may also ameliorate liver fibrosis. PTU has been suggested as a therapeutic drug to treat alcohol-induced liver cirrhosis as it increases hepatic blood flow and also reduces oxidative stress in rats[71].
Liver transplantation is an effective therapy and is the next viable option for ALD patients with alcoholic cirrhosis who have not responded to abstaining from alcohol. Liver transplantation proposes to be a rescue option for patients with decompensated liver injury and no response from pharmaceutical therapy[72]. Liver transplantation is regarded as a final treatment option for end-stage ALD. However, many reviews have reported that relapse incidences following liver transplantation are common and generally occurs in approximately 10%-52%[73,74] of patients. Moreover, patients who have been treated with liver transplantation related to ALD display an increased likelihood of de novo cancer in other organs, especially, a high rate of cardiovascular complications[75-77]. In addition, the transplantation should be followed after six months of abstinence to avoid histological damage[78,79].
Chronic alcohol uses and subsequent injury to the liver involves various changes to cells and molecules that can compromise the metabolic capabilities of the liver. Therefore, medications that rely mainly on the liver for their metabolism and clearance should be used with caution as there is subsequently less elimination and higher plasma concentrations[80]. Impaired drug metabolism may also relate to changes of the enzyme, CYP2E1[81] which is effected by chronic alcohol use. Additionally, in a cirrhotic liver, drug administration may also be compromised with an increased risk for adverse drug reactions. Furthermore, adverse reactions in the treatment of ALD may often occur due to individual differences such as food intake, genetic differences, age, gender and environmental factors including obesity and diabetes. Although it was found that there was no significantly increased risk of hepatotoxicity in patients with liver disease[80], there still should be considerable attention to dosage when medicating ALD patients to minimise the risk of adverse drug reactions and hepatotoxicity. Caution, for example, should also be taken when using corticosteroids and anti-TNF medications[82] in the hospital setting due to an increased risk of immune-related events including pneumonia, staphylococcus septicaemia, candida septicaemia and GI bleeding[83]. This outcome was seen in a previous study investigating the effects of infliximab and prednisolone which was terminated prematurely due to a significantly higher occurrence of severe infections in the treatment group when compared to placebo and prednisolone[83]. Corticosteroids also increase the risk of coincidental bacterial infections, gastrointestinal bleeding, or renal failure indicating the need for more clinical evidence to confirm this safety issue. Metformin and alcohol combination may produce severe consequences as a side effect of lactic acidosis. This condition can generate when the blood has insufficient oxygen, which is required to transport the glucose throughout the body. Metformin also should be prudent in patients with cirrhosis due to arterial hypoxemia[84]. Other drugs lack evidence in human models beyond preliminary trials due to toxicity or no significant treatment effect. This could perhaps be influenced by the high morbidity rates and short-term mortality associated with end stage ALD, especially acute AH. This highlights the need for complementary and alternative medicine (CAM) where side effects and potential for toxicity are significantly reduced.
CAM encompasses a wide range of approaches with many advantages as it is readily available, has fewer side effects and is a natural way of healing with lasting effects when compared to conventional medicines[85]. With these benefits, natural therapy and herbal medicines have attracted a lot of attention as a potential therapy for the treatment of ALD. It was found that 41% of outpatients with liver disease had utilized some form of CAM[86]. In the US survey, Silymarin and garlic were reported as the most used herbs for liver disease with 12% and 8% respectively. Ginseng (6%), green tea (5%), gingko (5%), echinacea (5%), and St. John’s wort (4%) have also been reported. Other than silymarin, the only herb used specifically for liver diseases was licorice root (1%)[87]. Herbal medicines and formulations are commonly grouped according to country. This includes Traditional Chinese Medicine (TCM), Japanese Herbal Medicine, Ayurvedic Medicine (Indian Subcontinent), Traditional African Medicine, Traditional Medicines of the Amazonian Basin in South America, and Arab Traditional Medicine. In general, the markers to display the benefits of herbal products rely on serum/plasma enzymes such as alanine transaminase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP). Other characteristics, including lipid lowering effects, anti-oxidant, anti- inflammation and anti-fibrotic effects are also used to show the hepatoprotective effects[87]. This review may describe the potential role and effects of natural products in chronic ALD including biological properties and their beneficial effects. A summary list of natural compounds is shown in Table 2.
| Medicine/compound | Type of model | Dosage/duration | Effects | Mechanisms | Ref. |
| Vitamin E and C | Malnourished rats with ethanol | Vitamin E (15 mg/kg) Vitamin C (10 mg/kg); single and combined treatments | Decreased ethanol induced hepatic glutathione peroxidase activity and hepatic fibrosis; Attenuated the development of hepatomegaly and hepatic necroinflammation | [165] | |
| Epigallocatechin-3-gallate (EGCG) | Male albino Wistar rats with Ethanol (6 g/kg per day) for 60 d | EGCG (100 mg/kg per day) for the last of 30 d of ethanol administration | Normalization of activities of enzymatic antioxidants; Reduction of lipid peroxidation | [93] | |
| Female Sprague-Dawley rats with ethanol (56%) | EGCG (100 mg/bw) | Partly blocked the gut leakiness; Reduced endotoxemia and lipid peroxidation | Blunted the elevated expressions of CD14, TNF-α, COX-2 and iNOS | [94] | |
| Male Wistar rats with ethanol for 5 wk | EGCG contained diet (3 g/L) for 2 wk and then ethanol-EGCG diet for 5 wk | Reduced serum AST and ALT | Enhancement of FA oxidation through increasing of CTP-1 and p-ACC expression | [95] | |
| Silymarin (Silybum marianum) | RCT, double-blind, 170 patients with cirrhosis | 140 mg/d for 3 times orally | Reduced the rate of mortality No side effect | [33] | |
| Alcohol induced baboons (50% of calories) | 0.84 mg/calorie for 36 mo | Improve histologic stage of fibrosis | Decrease collagen I and (I) procollagen | [100] | |
| Alcohol and high fat induced rats | 100 mg/kg per day, 150 mg/kg per day, 200 mg/kg per day for 6 wk | 200 mg/kg per day decrease serum ALT and AST and hepatic fat contents | Attenuated NF-κB p65, ICAM-1 and IL-6 were found in silymarin groups (150 mg/kg, 200 mg) | [101] | |
| Betaine | Ethanol diet Wistar rats | 1% (w/v) in diet | Decrease hepatic ballooning and fat contents | Attenuate NOS production; Decrease CYP2E1 protein and activity | [106] |
| High fat containing ethanol diet rats 6 g/kg for 8 more wk | 200 and 400 mg/kg per day for 4 wk | Decrease ALT and AST | Inhibition of TLR-4 expression; Decrease serum endotoxin, TNF-α, IFN-γ and IL-18 | [107] | |
| Glycyrrhizin (Glycyrrhiza glabra) | Ethanol-CCl4 induced male SD rats | Intraperitoneal injections of potenlin (acquired from Hai Ning Pharmaceutical Co., Zhe Jiang, China) | Decreasing serum ALT levels | Normalized NF-κB binding activity | [114] |
| Ginsenosides | Ethanol-feeding mice Ethanol feeding hepatocytes (AML12 cell lines) | Red ginseng extract containing abundant ginsenosides (Rb1, Rb2, and Rd) (250 mg/kg or 500 mg/kg) for 4 wk in mice | Improves chronic alcohol-induced histopathological changes; Decreases in hepatic triglyceride content | Inhibition of lipogenesis pathway; Attenuated EtOH-induced cytochrome; P450 2E1, 4-hydroxynonenal, and nitrotyrosine levels; Activation of AMPK-SIRT1 | [166] |
| Alcohol consumption with high fat diet mice | Red ginseng (200 mg/kg per day) for last 2 wk | Lower ALT levels and no different AST | Reduced level of TNF-α and IL-1 and increase IL-10 | [124] | |
| Fenugreek seed polyphenol | EtOH (30 mM) induced Chang liver cells | 20, 40, 60 mg/mL | Increased GSH/GSSG ratio Reduced EtOH-induced LDH leakage | Inhibition of NF-κB | [129] |
| Ethanol induced rats (6 g/kg per day) for 30 d | 200 mg/kg per day for 30 d | Improved lipid profile and reduced collagen content, crosslinking, aldehyde content and peroxidation | [132] | ||
| Curcumin | Alcohol (100 mM) induced rat primary hepatocytes | 0-50 μmol/L | Ameliorated MDA and AST | Improved GSH and heme oxygenase-1 (HO-1) induction | [133] |
| Alcohol-induced female Sprague-Dawley rats | 400 mg/kg bw | Improved liver pathology, decreasing elevation of hepatic MDA | Suppressing of NF-κB activation | [134] | |
| LIV-52 | Ethanol-induced HepG2 cells (100 and 200 mM ) | 1% or 2% Liv.52 for 24 h | Normalized suppressed PPAR-α and induced TNF-α | [148] |
Green tea is derived from the leaves and buds of Camellia sinensis. Green tea contains rich polyphenolic compounds and has pleiotropic effects including anti-oxidant, hypolipidaemic and anti-inflammatory actions. Green tea polyphenols (GTP) significantly ameliorated ethanol-induced hepatic necrosis. Furthermore, while ethanol significantly increased the accumulation of 4- hydroxynonenal, a product of lipid peroxidation and an index of oxidative stress, green tea extract down regulated this effect. This indicated that GTP protects alcohol-induced liver injury through preventing oxidative stress[88]. The major tea catechins include epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin gallate (EGCG) with EGCG being the major constituent of the catechins with numerous biological functions[89-91]. Our previous study showed total GTP and EGCG enhance glycogen synthesis and inhibit lipogenesis in hepatocytes[92]. EGCG treatment also significantly decreased elevated serum ALT in alcohol-induced rats[88] and normalised the activities of enzymatic anti- oxidants[93]. Another study has reported that EGCG ameliorated plasma endotoxin levels in alcohol-induced female rats with endotoxemia, displaying anti- inflammatory effects through the inhibition of TNF-α, COX-2, and iNOS mRNA expression[94]. EGCG also protected against liver impairment through the enhancement of FA oxidation with increased carnitine palmitoyl transferase 1 (CPT-1) levels[95]. Despite much evidence supporting the effects of EGCG on ALD, clinical studies are still limited and large-scale human studies may be needed to confirm this effect. GTP, catechins may also increase HO-1 activity to reduce oxidative stress in human liver tissue (hHeps). Administering polyphenols prior to ethanol exposure reduced the diminishing of GSH to display a protective effect on liver cells by regulating the immune system and pro-inflammatory mediators such as TGF-β1[96].
Silymarin (Milk thistle), which is one of the active compounds extracted from Silybum marianum (Asteraceae) is the most common type of CAM therapy for treatment of liver diseases[85]. Silymarin is a complex mixture of polyphenolic molecules including 7 flavonolignans (silybinA, B, isosilybin A, B, silychristin, isosilychristin, silydianin) and 1 flavonoid (taxifolin)[97]. Silibinin, which is a semi-purified fraction of silymarin, has been shown to be a radical scavenger[98] and a RCT with silymarin also improved survival from patients with alcoholic and non-alcoholic cirrhosis without any side effect[33]. Another RCT was also conducted with the prospective effect of silymarin (140 mg/d) in AH[99]. Mechanisms by which silymarin extracts ameliorate liver disease include anti- inflammatory, anti-oxidative, antifibrotic, and immune-modulating activities[100]. Silymarin has retarded the development of alcohol-induced hepatic fibrosis in baboons by preventing collagen type I and decreasing histological progression to fibrosis[100]. Silymarin also reduces the elevated serum AST and ALT levels, and decreases hepatic lipid contents in alcohol-induced fatty liver in rats. These effects may be through down-regulating the expression of NF-κB p65, ICAM-1 and IL-6 in liver tissue[101]. However, Cochrane Database reviews have been unsuccessful in demonstrating the beneficial effect of silymarin on ALD. Further studies are needed to provide the appropriate data concerning efficacy.
Betaine is another natural dietary compound, synthesized in vivo from choline and converts homocysteine to methionine[102]. Betaine (trimethylglycine), a pivotal nutrient for humans, can be acquired from a wide range of foods and nutritional supplements[103]. It attenuates AH by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway[104]. Betaine also increases SAM, normalizing hepatocellular SAM:S-adenosyl-L-homocysteine ratio[105], resulting in the attenuation of fatty liver[103]. The study of mitochondrial dysfunction in the progression of ALD has also been enforced by many research areas. Betaine gives a beneficial effect against loss of oxidative phosphorylation system proteins by alcohol, by preventing NOS2 induction and NO generation in male Wister rats with exposed to ethanol[106]. The mechanism of betaine in liver is also involved in the suppression of endotoxin/TLR-4 signaling pathways. Betaine treatment decreased the levels of serum ALT, AST, endotoxin, TNF-α, IFN-γ, IL-18, and TLR-4, and improved the degree of HS and inflammation in liver tissues in rats with alcohol-induced liver injury[107].
Glycyrrhiza glabra (Leguminosae), commonly named licorice root, is a perennial herb cultivated in temperate and subtropical regions of the world as well as central and South-Western Asia[108]. Glycyrrhizin, as a main constituent in aqueous extract, is a conjugate of two molecules of glucuronic acid and one of 18β-glycyrrhetic acid[109]. Glycyrrhizin is hydrolysed by intestinal bacterial into 18β-glycyrrhetic acid, which is reabsorbed into the bloodstream. Glycyrrhizin suppressed serum AST and ALT levels and histologically inhibited the spread of deteriorating areas in hepatocytes in an animal model of concanavalin A-induced liver injury[110]. Moreover, the effects of Glycyrrhizin has extended to the attenuation of inflammation response by regulation of NF-κB and MAPK pathway[111] and inhibition of TNF-α, ROS, and proinflammatory interleukins such as IL-6 and IL-1β[112,113]. Another beneficial effect of glycyrrhizin, from Potenlini compound, was shown in ethanol-CCl4 induced liver cirrhosis rats by decreasing serum ALT levels and normalising NF-κB binding activity[114,115]. In clinical trials, glycyrrhizin is mostly used to treat hepatitis C and interferon treatment[16]. One clinical trial showed to lower level of ALT with 200 mg of glycyrrhizin for 5 d per week for approximately 10 years in hepatitis C patients[116]. Glycyrrhizin has potential effects to cure various chronic liver diseases but large-scale, rigorously designed clinical trials for ALD is warranted.
Ginseng, an ancient medicinal herb, has been popular as a tonic for the treatment of various diseases including diabetes and hepatic diseases[117]. Ginsenosides, the main active constituent groups of all ginseng species, has been used as a supplementary medicine and is mostly responsible for the beneficial pharmacological effects in metabolic disorders and various cancer via its ability to boost immunity and its anti- inflammation function[118,119]. Ginsenoside Rb1, as the most abundant ginsenoside in P. ginseng has improved hepatic fibrosis induced by CCl4 by down regulation of hepatic prostaglandin E2 and TIMP-1[120]. Ginsenoisde Rg1 showed to inhibit TNF-α-mediated NF-κB transcriptional activity in HepG2 cells with IC50 of 28.14 μmol/L and gene expression of iNOS and COX-2 inducible inflammatory enzymes[121]. It was also reported that ginsenoside Rc (40 mg/kg) attenuates AFLD in alcoholic-fed ICR mice through the regulation of AMPK and MAPK pathways[122]. Red ginsengs, which contain an abundance of ginsenosides, showed to improve chronic alcohol-induced histopathological changes and hepatic TG content through inhibition of the lipogenesis pathway and AMPK-SIRT1 activation in alcohol-fed mice[123]. Red ginseng also reduced levels of TNF-α and IL-1β which were increased by alcohol consumption with a high fat diet in mice[124]. Recently, compound K which is a metabolite form of ginsenoside Rb1 and Rc has been identified as an inducer to increase AMPK expression, resulting in reduction of lipid droplets and hepatic TG accumulation in high FA exposed human hepatocytes (Huh7). This compound had better efficacy to treat HS compared to metformin[125]. With the hepatoprotective effects of many types of ginsenosides, these herbal components should be potential targets to improve liver damage caused by alcohol abuse.
Fenugreek (Trigonella foenum graecum) seeds have been reported to have beneficial effects on enhancing the antioxidant apparatus[126]. Fenugreek seeds also have been used in remedies for diabetes and high cholesterol in various traditional medicines and proven experimentally in diabetic humans[127]. The polyphenolic extract of the seeds is the main constituent beneficial for the treatment of ALD according to many articles. In general, fenugreek seed polyphenols contain five different polyphenolic flavonoids, vitexin, tricin, naringenin, quercetin, and tricin-7-O-b-D-glucopyranoside[128]. These polyphenols prevent the alcohol-induced toxic effect in human Chang liver cells through improvement of GSH/GSSG ratio[129]. Interestingly, quercetin ameliorated ethanol-stimulated mitochondrial dysfunction by induced permeability transition though suppressing GSH depletion. This indicates a promising preventive strategy for ALD[130,131]. Fenugreek seed polyphenols also have demonstrated improvement to lipid profiles and collagen content in alcohol-fed rats[132]. Compared to other herbal medicines, however, the clinical studies of fenugreek seed polyphenols are lacking evidence in metabolic disorders, especially in relation to ALD, suggesting the need for accurate standardization of polyphenols and their individual efficacy for the treatment of ALD.
Curcumin, commonly called turmeric yellow and compound of curcuma longa, is a low-molecular weight polyphenol derived agent. It is commonly used in Ayurvedic medicine and has various pharmacological effects including anti-oxidative, anti- inflammatory, and hepatoprotective activities[105]. This historical use of curcumin has prompted investigation into the hepatoprotective effects against liver damage induced by alcohol abuse and the molecular mechanisms, however the research in this area is still lacking. Curcumin has ameliorated malondialdehyde (MDA) and AST and improved GSH and heme oxygenase-1 (HO-1) induction in alcohol (100 mM) induced rat primary hepatocytes[133]. Curcumin (400 mg/kg bw) has also been examined in alcohol-induced female Sprague-Dawley rats to show improvement by reducing the elevation of hepatic MDA, and the suppressing of NF-κB activation[134]. Another similar result confirmed the inhibition of the expression of NF-κB-dependent genes by the supplementation of curcumin (75 mg/kg per day) for 4 wk in rats[135]. Recently, low dosage of curcumin prevented HS compared with the alcohol control group with inhibition of dehydrogenase, ALDH2 as well as CYP2E1 activation[136]. Curcumin also proved its anti-oxidant effect against ethanol-exposed mice by decreasing ROS generation[137]. Despite the beneficial effects of curcumin in ALD, it is unfavourable to apply as a medical strategy due to its decreased bioavailability and rapid metabolism with systemic elimination in animals and humans, suggesting an investigation of more sophisticated pharmacokinetic improvements of curcumin such as curcumin phospholipid complex or curcumin nanoparticles[105,138].
Historically, some herbal blends have been commonly used for hepatic disorders around the world. Clinical trials to evaluate their efficacy are, however, challenging to demonstrate due to heterogeneous formulations and dosages. Moreover, these blends have not been regulated by the Food and Drug Administration. In this review, a few blends are evaluated in ALD. Herbal Medicine861 (HM 861) consists of 10 herbs, based on TCM including Salvia miltiorrhiza, Astragalus membranaceus and Spatholobus suberectus[139]. HM861 has shown the inhibition of HSC proliferation and induction of HSC apoptosis in patients with chronic hepatitis[140].
Moreover, the compound also suppressed tissue inhibitor metalloprotease 1 (TIMP-1) mRNA expression in HSC-T6 cells[141]. In clinical trials, this herbal blend was tested for anti-fibrotic activity encompassing 107 patients with hepatitis B resulting in the drop of ALT levels to the normal range in 73% of patients[142]. In a clinical trial, HM861 showed to improved fibrosis and early cirrhosis in 52 patients with HBV-related fibrotic patients with the hepatic inflammatory scores also decreased[143]. More Cochrane reviews, however, are needed to confirm the efficacy of the blend in the treatment of ALD. TJ-9, also referred to as Sho-saiko-to in Japan or xiao-chai-hu- tang in China, containing 7 herbal constituents including: bupleurum root, pinellia tuber, scutellaria toot, jujube fruit, ginger rhizome, ginseng root, and glycyrrhiza root. The formula has been traditionally used for the treatment of liver diseases. Usually, the preparation contains 7.5 g of TJ-9 with the active components, baicalin and baicalein, responsible for the anti-oxidant activity[144]. Although the exact of mechanism of TJ-9 is unknown, one anticipated mechanism may be exposed in the observation of stellate cells which has shown an inhibition of α-smooth muscle actin, type I collagen production, and cell spreading, indicating the suppression of HSC activation[144]. TJ-9 has been associated with several cases of interstitial pneumonitis, especially when used with interferon for the treatment of chronic hepatitis[145]. Liv-52, which is an Indian Ayurvedic medicine that has been used for the treatment of liver diseases, is a herbal preparation including Capparis spinosa (Himsara), Cichorium intybus (Kasani), Mandur bhasma, Solanum nigrum (Kakamachi), Terminalia arjuna (Arjuna), Cassia occidentalis (Kasamarda), Achillea millefolium (Biranjasipha), and Tamarix gallica (Jhavaka)[146]. It was originally used to treat ALD, but a recent RCT from Europe demonstrated a detrimental effect on advanced alcohol-induced cirrhosis[147]. Contrastingly, an in vitro study, reported that Liv.52 improved PPAR-γ suppression and TNF-α expression in HepG2 cells[148]. Another clinical study also showed the efficacy of LIV.52 in 26 cirrhotic patients for 6 mo with normalized serum ALT and AST levels compared to the placebo group[149]. Although there are many positive effects of LIV.52 for the improvement of ALD, underlying molecular mechanisms are still necessary to support its potential function. In summary, many compounds are available on the open market to treat ALD, but their efficacy data is still not supported by clinical trials. Comprehension of herbal interaction with pharmacological medicines is also necessary to improve the treatment of ALD.
More readily available on the market and popular than ever, CAM and herbal therapy are increasingly attractive treatments for chronic liver diseases with many advantages, such as low side effect and toxicity. Augmentation of herb-drug interaction has consequently followed stipulating dialogue between pharmaceutical experts and practician. Although there is strong evidence, supporting the use of herbal medicine for the treatment of ALD, combination therapy should be speculated for any safety issues. Magnesium isoglycyrrhizinate injection is one example of a drug containing glycyrrhizin and possesses effective and safe treatment for chronic liver diseases[150] and down regulates the progress of pulmonary fibrosis[151]. Glycyrrhizin also has been combined with matrine to improve CCL4-induced liver fibrosis through lowering levels of collagen and less HSC proliferation[152], suggesting the positive effect of their combination to protect against liver fibrosis. Recently, a combination of silymarin and SAM was evaluated in ALD markets with much promise[153]. There have been some cases of adverse events and hepatotoxicity caused by herbal medicines[154]. Xiao Chaihu Tang, alone or in combination with interferon, may induce acute interstitial pneumonia in chronic hepatitis patients[155]. Despite the emerging of CAM on the markets, documented herb-drug interactions are still sparse, especially in relation to ALD with a reliance only on individual case reports. Although some combinations of CAMs and conventional drugs contribute many beneficial efficacies, some herb-drug interaction also may be associated with toxicity in certain situations such as the combination of silymarin with indinavir therapy in AIDS patients[156].
It is undeniable that chronic alcohol abuse leads to deleterious implications for the liver regardless of age, gender or other factors. The more advanced the stage of alcoholic liver disease, the more morbid and life-threatening the complications become. Various therapies should, therefore, aim to reverse these complications but are often only palliative. As an initial therapy of ALD, abstinence from alcohol accompanied with basic lifestyle modifications when appropriate. When the injury becomes decompensated, pharmacological therapy should be accompanied to reverse the more severe stages of ALD. The present studies are still inconclusive with humble methodological quality and design, high heterogeneous patient populations, and poorly defined point of progression of ALD. These flaws may partly explain the conflicting reports in the literature to support the effectiveness of treatment for ALD for many conventional medications, such as Corticosteroids, PTX, and SAM. Mechanistic studies are still insufficient to prove their pharmaceutical potency and future studies will be thirsty to investigate the molecular mechanisms related in the complex relationships between ethanol metabolism, oxidative stress, immune response, HSC activation and extracellular matrix remodelling. Larger RCTs with a longer follow-up period are required for further evaluation.
Increasing attention has been paid to herbal medicines as a newly emerging treatment strategy for ALD. In this review, we summarized functionalities of CAM for the prevention and treatment of ALD. Most single herbs and formulae have resulted in improvement of ALD-related conditions with multiple and diverse mechanisms of actions in spite of their mild side effects. To date, only a few active compounds from herbal extracts have been identified as candidates to treat ALD and alcohol induced liver injury. Although many herbal constituents have shown promising potential for the treatment of ALD with multi-targets, the underlying molecular mechanisms, especially that of single herbal compounds, have not been completely elucidated. Moreover, clinical trials to adjust concurrent systematic review are still required in the area of ALD research. More profound studies underlying the prospective effect of herbal medicines should be further investigated. In addition, some unique therapies are now increasing to develop the treatment of ALD. Epigenetic regulation underlying alcohol metabolism, such as histone modification may be a new trend to advance effective treatment strategies for ALD[157]. Acetate may affect histone modification by up-regulating acetyl-CoA and enhance the inflammation in ethanol-exposed macrophages by interfering histone deacetylase activity[158]. With many natural products potentially possessing this ability of epigenetic modification, it would be a particularly beneficial breakthrough for ALD patients.
P- Reviewer: Wan JB S- Editor: Kong JX L- Editor: Filipodia E- Editor: Wang CH
| 1. | Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466-478. [PubMed] |
| 2. | European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 455] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 3. | Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87-S96. [PubMed] |
| 4. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] |
| 5. | Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 537] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 6. | World Health Organization. Global status report on alcohol and health 2011. Available from: http://www.who.int/substance_abuse/publications/global_alcohol_report/msbgsruprofiles.pdf. |
| 7. | Everitt H, Hu M, Ajmo JM, Rogers CQ, Liang X, Zhang R, Yin H, Choi A, Bennett ES, You M. Ethanol administration exacerbates the abnormalities in hepatic lipid oxidation in genetically obese mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G38-G47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1495] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 9. | Zakhari S, Li TK. Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 235] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1-G6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 11. | Gao B. Cytokines, STATs and liver disease. Cell Mol Immunol. 2005;2:92-100. [PubMed] |
| 12. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2244] [Cited by in RCA: 2198] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 13. | Nagata K, Suzuki H, Sakaguchi S. Common pathogenic mechanism in development progression of liver injury caused by non-alcoholic or alcoholic steatohepatitis. J Toxicol Sci. 2007;32:453-468. [PubMed] |
| 14. | Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15 Suppl:D20-D25. [PubMed] |
| 15. | Veldt BJ, Lainé F, Guillygomarc’h A, Lauvin L, Boudjema K, Messner M, Brissot P, Deugnier Y, Moirand R. Indication of liver transplantation in severe alcoholic liver cirrhosis: quantitative evaluation and optimal timing. J Hepatol. 2002;36:93-98. [PubMed] |
| 16. | Hanje AJ, Fortune B, Song M, Hill D, McClain C. The use of selected nutrition supplements and complementary and alternative medicine in liver disease. Nutr Clin Pract. 2006;21:255-272. [PubMed] |
| 17. | Luca A, García-Pagán JC, Bosch J, Feu F, Caballería J, Groszmann RJ, Rodés J. Effects of ethanol consumption on hepatic hemodynamics in patients with alcoholic cirrhosis. Gastroenterology. 1997;112:1284-1289. [PubMed] |
| 18. | Kadden R. Cognitive-behavioral Coping Skills Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence. US: Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism 1995; . |
| 19. | Bouza C, Angeles M, Muñoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 301] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 20. | Williams SH. Medications for treating alcohol dependence. Am Fam Physician. 2005;72:1775-1780. [PubMed] |
| 21. | Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT. Pharmacological treatment of alcohol dependence: a review of the evidence. JAMA. 1999;281:1318-1325. [PubMed] |
| 22. | Sofair AN, Barry V, Manos MM, Thomas A, Zaman A, Terrault NA, Murphy RC, Stabach N, Huie S, Van Ness G. The epidemiology and clinical characteristics of patients with newly diagnosed alcohol-related liver disease: results from population-based surveillance. J Clin Gastroenterol. 2010;44:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Corrao G, Lepore AR, Torchio P, Valenti M, Galatola G, D’Amicis A, Aricó S, di Orio F. The effect of drinking coffee and smoking cigarettes on the risk of cirrhosis associated with alcohol consumption. A case-control study. Provincial Group for the Study of Chronic Liver Disease. Eur J Epidemiol. 1994;10:657-664. [PubMed] |
| 24. | Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol. 1992;136:1248-1257. [PubMed] |
| 25. | Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 436] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 26. | Singal AK, Charlton MR. Nutrition in alcoholic liver disease. Clin Liver Dis. 2012;16:805-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | McClain CJ, Barve SS, Barve A, Marsano L. Alcoholic liver disease and malnutrition. Alcohol Clin Exp Res. 2011;35:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Hirsch S, de la Maza MP, Gattás V, Barrera G, Petermann M, Gotteland M, Muñoz C, Lopez M, Bunout D. Nutritional support in alcoholic cirrhotic patients improves host defenses. J Am Coll Nutr. 1999;18:434-441. [PubMed] |
| 29. | Frazier TH, Stocker AM, Kershner NA, Marsano LS, McClain CJ. Treatment of alcoholic liver disease. Therap Adv Gastroenterol. 2011;4:63-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol Aspects Med. 2005;26:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Das I, Burch RE, Hahn HK. Effects of zinc deficiency on ethanol metabolism and alcohol and aldehyde dehydrogenase activities. J Lab Clin Med. 1984;104:610-617. [PubMed] |
| 32. | Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol. 2005;166:1681-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, Meryn S, Base W, Schneider B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105-113. [PubMed] |
| 34. | Spahr L, Rubbia-Brandt L, Pugin J, Giostra E, Frossard JL, Borisch B, Hadengue A. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule-1 in hepatic venous blood. J Hepatol. 2001;35:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Taïeb J, Mathurin P, Elbim C, Cluzel P, Arce-Vicioso M, Bernard B, Opolon P, Gougerot-Pocidalo MA, Poynard T, Chollet-Martin S. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. J Hepatol. 2000;32:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Tome S, Lucey MR. Review article: current management of alcoholic liver disease. Aliment Pharmacol Ther. 2004;19:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O’Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis--a randomised clinical trial. J Hepatol. 2006;44:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Ramond MJ, Poynard T, Rueff B, Mathurin P, Théodore C, Chaput JC, Benhamou JP. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 258] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Mathurin P, Abdelnour M, Ramond MJ, Carbonell N, Fartoux L, Serfaty L, Valla D, Poupon R, Chaput JC, Naveau S. Early change in bilirubin levels is an important prognostic factor in severe alcoholic hepatitis treated with prednisolone. Hepatology. 2003;38:1363-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Mathurin P, Beuzin F, Louvet A, Carrié-Ganne N, Balian A, Trinchet JC, Dalsoglio D, Prevot S, Naveau S. Fibrosis progression occurs in a subgroup of heavy drinkers with typical histological features. Aliment Pharmacol Ther. 2007;25:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 693] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 42. | Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648. [PubMed] |
| 43. | De B, Mandal S, Sau D, Mani S, Chatterjee S, Mondal S, Bhattacharya K, Sil K, Bhattacharya R. Pentoxifylline Plus Prednisolone versus Pentoxifylline Only for Severe Alcoholic Hepatitis: A Randomized Controlled Clinical Trial. Ann Med Health Sci Res. 2014;4:810-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Doherty GM, Jensen JC, Alexander HR, Buresh CM, Norton JA. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery. 1991;110:192-198. [PubMed] |
| 45. | Parker R, Armstrong MJ, Corbett C, Rowe IA, Houlihan DD. Systematic review: pentoxifylline for the treatment of severe alcoholic hepatitis. Aliment Pharmacol Ther. 2013;37:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev. 2009;CD007339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105:14-32; quiz 33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 48. | Lee TD, Sadda MR, Mendler MH, Bottiglieri T, Kanel G, Mato JM, Lu SC. Abnormal hepatic methionine and glutathione metabolism in patients with alcoholic hepatitis. Alcohol Clin Exp Res. 2004;28:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Lieber CS. S-Adenosyl-L-methionine and alcoholic liver disease in animal models: implications for early intervention in human beings. Alcohol. 2002;27:173-177. [PubMed] |
| 50. | Karaa A, Thompson KJ, McKillop IH, Clemens MG, Schrum LW. S-adenosyl-L-methionine attenuates oxidative stress and hepatic stellate cell activation in an ethanol-LPS-induced fibrotic rat model. Shock. 2008;30:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Le MD, Enbom E, Traum PK, Medici V, Halsted CH, French SW. Alcoholic liver disease patients treated with S-adenosyl-L-methionine: an in-depth look at liver morphologic data comparing pre and post treatment liver biopsies. Exp Mol Pathol. 2013;95:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Medici V, Virata MC, Peerson JM, Stabler SP, French SW, Gregory JF, Albanese A, Bowlus CL, Devaraj S, Panacek EA. S-adenosyl-L-methionine treatment for alcoholic liver disease: a double-blinded, randomized, placebo-controlled trial. Alcohol Clin Exp Res. 2011;35:1960-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Wagner M, Zollner G, Trauner M. Nuclear receptors in liver disease. Hepatology. 2011;53:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 54. | Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life. 2008;60:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G364-G374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 56. | Liu J, Takase I, Hakucho A, Okamura N, Fujimiya T. Carvedilol attenuates the progression of alcohol fatty liver disease in rats. Alcohol Clin Exp Res. 2012;36:1587-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Bergheim I, Guo L, Davis MA, Lambert JC, Beier JI, Duveau I, Luyendyk JP, Roth RA, Arteel GE. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006;130:2099-2112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 58. | Zhu Z, Jiang Z, Zhou J, Zhou D, Wang W, Zhao C, Zhen Z, Nanji AA. Involvement of insulin resistance in the protective effect of metformin against alcoholic liver injury. Alcohol Clin Exp Res. 2014;38:1510-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Lieber CS. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968-1998)--a review. Alcohol Clin Exp Res. 1999;23:991-1007. [PubMed] |
| 60. | Lieber CS, Weiss DG, Groszmann R, Paronetto F, Schenker S. II. Veterans Affairs Cooperative Study of polyenylphosphatidylcholine in alcoholic liver disease. Alcohol Clin Exp Res. 2003;27:1765-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Gebhardt AC, Lucas D, Ménez JF, Seitz HK. Chlormethiazole inhibition of cytochrome P450 2E1 as assessed by chlorzoxazone hydroxylation in humans. Hepatology. 1997;26:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, Fang C, Ingelman-Sundberg M, Donohue TM, French SW. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med. 2000;224:302-308. [PubMed] |
| 63. | Kinde H, Bettey RL, Ardans A, Galey FD, Daft BM, Walker RL, Eklund MW, Byrd JW. Clostridium botulinum type-C intoxication associated with consumption of processed alfalfa hay cubes in horses. J Am Vet Med Assoc. 1991;199:742-746. [PubMed] |
| 64. | Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 268] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 65. | Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med. 1994;205:243-247. [PubMed] |
| 66. | Singh AK, Pandey SK, Naresh Kumar G. Pyrroloquinoline quinone-secreting probiotic Escherichia coli Nissle 1917 ameliorates ethanol-induced oxidative damage and hyperlipidemia in rats. Alcohol Clin Exp Res. 2014;38:2127-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 68. | Clot P, Tabone M, Aricò S, Albano E. Monitoring oxidative damage in patients with liver cirrhosis and different daily alcohol intake. Gut. 1994;35:1637-1643. [PubMed] |
| 69. | Gutiérrez-Ruiz MC, Bucio L, Correa A, Souza V, Hernández E, Gómez-Quiroz LE, Kershenobich D. Metadoxine prevents damage produced by ethanol and acetaldehyde in hepatocyte and hepatic stellate cells in culture. Pharmacol Res. 2001;44:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Muntoni S, Rojkind M, Muntoni S. Colchicine reduces procollagen III and increases pseudocholinesterase in chronic liver disease. World J Gastroenterol. 2010;16:2889-2894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 71. | Carmichael FJ, Orrego H, Saldivia V, Israel Y. Effect of propylthiouracil on the ethanol-induced increase in liver oxygen consumption in awake rats. Hepatology. 1993;18:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Dureja P, Lucey MR. The place of liver transplantation in the treatment of severe alcoholic hepatitis. J Hepatol. 2010;52:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Pageaux GP, Bismuth M, Perney P, Costes V, Jaber S, Possoz P, Fabre JM, Navarro F, Blanc P, Domergue J. Alcohol relapse after liver transplantation for alcoholic liver disease: does it matter? J Hepatol. 2003;38:629-634. [PubMed] |
| 74. | Tang H, Boulton R, Gunson B, Hubscher S, Neuberger J. Patterns of alcohol consumption after liver transplantation. Gut. 1998;43:140-145. [PubMed] |
| 75. | Dumortier J, Guillaud O, Adham M, Boucaud C, Delafosse B, Bouffard Y, Paliard P, Scoazec JY, Boillot O. Negative impact of de novo malignancies rather than alcohol relapse on survival after liver transplantation for alcoholic cirrhosis: a retrospective analysis of 305 patients in a single center. Am J Gastroenterol. 2007;102:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 76. | Burra P, Senzolo M, Adam R, Delvart V, Karam V, Germani G, Neuberger J. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry). Am J Transplant. 2010;10:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 77. | Zanus G, Carraro A, Vitale A, Gringeri E, D’Amico F, Valmasoni M, D’Amico FE, Brolese A, Boccagni P, Neri D. Alcohol abuse and de novo tumors in liver transplantation. Transplant Proc. 2009;41:1310-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Tomé S, Martinez-Rey C, González-Quintela A, Gude F, Brage A, Otero E, Abdulkader I, Forteza J, Bustamante M, Varo E. Influence of superimposed alcoholic hepatitis on the outcome of liver transplantation for end-stage alcoholic liver disease. J Hepatol. 2002;36:793-798. [PubMed] |
| 79. | Lucey MR, Brown KA, Everson GT, Fung JJ, Gish R, Keeffe EB, Kneteman NM, Lake JR, Martin P, McDiarmid SV. Minimal criteria for placement of adults on the liver transplant waiting list: a report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver Transpl Surg. 1997;3:628-637. [PubMed] |
| 80. | Westphal JF, Brogard JM. Drug administration in chronic liver disease. Drug Saf. 1997;17:47-73. [PubMed] |
| 81. | French SW. The importance of CYP2E1 in the pathogenesis of alcoholic liver disease and drug toxicity and the role of the proteasome. Subcell Biochem. 2013;67:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Spengler EK, Dunkelberg J, Schey R. Alcoholic hepatitis: current management. Dig Dis Sci. 2014;59:2357-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 83. | Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, Davion T, Oberti F, Broët P, Emilie D. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 322] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 84. | Brackett CC. Clarifying metformin’s role and risks in liver dysfunction. J Am Pharm Assoc (2003). 2010;50:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Cortez-Pinto H, Alexandrino P, Camilo ME, Gouveia-Oliveira A, Santos PM, Alves MM, Moura MC. Lack of effect of colchicine in alcoholic cirrhosis: final results of a double blind randomized trial. Eur J Gastroenterol Hepatol. 2002;14:377-381. [PubMed] |
| 86. | Seeff LB, Lindsay KL, Bacon BR, Kresina TF, Hoofnagle JH. Complementary and alternative medicine in chronic liver disease. Hepatology. 2001;34:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 87. | Strader DB, Bacon BR, Lindsay KL, La Brecque DR, Morgan T, Wright EC, Allen J, Khokar MF, Hoofnagle JH, Seeff LB. Use of complementary and alternative medicine in patients with liver disease. Am J Gastroenterol. 2002;97:2391-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 88. | Arteel GE, Uesugi T, Bevan LN, Gäbele E, Wheeler MD, McKim SE, Thurman RG. Green tea extract protects against early alcohol-induced liver injury in rats. Biol Chem. 2002;383:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1190] [Article Influence: 54.1] [Reference Citation Analysis (1)] |
| 90. | Tedeschi E, Suzuki H, Menegazzi M. Antiinflammatory action of EGCG, the main component of green tea, through STAT-1 inhibition. Ann N Y Acad Sci. 2002;973:435-437. [PubMed] |
| 91. | Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003;133:3262S-3267S. [PubMed] |
| 92. | Kim JJ, Tan Y, Xiao L, Sun YL, Qu X. Green tea polyphenol epigallocatechin-3-gallate enhance glycogen synthesis and inhibit lipogenesis in hepatocytes. Biomed Res Int. 2013;2013:920128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 93. | Kaviarasan S, Sundarapandiyan R, Anuradha CV. Epigallocatechin gallate, a green tea phytochemical, attenuates alcohol-induced hepatic protein and lipid damage. Toxicol Mech Methods. 2008;18:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Yuan G, Gong Z, Zhou X, Zhangq P, Sun X, Li X. Epigallocatechin-3-Gallate Ameliorates Alcohol-Induced Liver Injury in Rats. Int J Mol Sci. 2006;7:204. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Yun JW, Kim YK, Lee BS, Kim CW, Hyun JS, Baik JH, Kim JJ, Kim BH. Effect of dietary epigallocatechin-3-gallate on cytochrome P450 2E1-dependent alcoholic liver damage: enhancement of fatty acid oxidation. Biosci Biotechnol Biochem. 2007;71:2999-3006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 96. | Nussler AK, Hao L, Knobeloch D, Yao P, Nussler NC, Wang Z, Liu L, Ehnert S. Protective role of HO-1 for alcohol-dependent liver damage. Dig Dis. 2010;28:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 98. | Comoglio A, Tomasi A, Malandrino S, Poli G, Albano E. Scavenging effect of silipide, a new silybin-phospholipid complex, on ethanol-derived free radicals. Biochem Pharmacol. 1995;50:1313-1316. [PubMed] |
| 99. | El-Kamary SS, Shardell MD, Abdel-Hamid M, Ismail S, El-Ateek M, Metwally M, Mikhail N, Hashem M, Mousa A, Aboul-Fotouh A. A randomized controlled trial to assess the safety and efficacy of silymarin on symptoms, signs and biomarkers of acute hepatitis. Phytomedicine. 2009;16:391-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 100. | Lieber CS, Leo MA, Cao Q, Ren C, DeCarli LM. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol. 2003;37:336-339. [PubMed] |
| 101. | Zhang W, Hong R, Tian T. Silymarin’s Protective Effects and Possible Mechanisms on Alcoholic Fatty Liver for Rats. Biomol Ther (Seoul). 2013;21:264-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 102. | IDel Prete A, Scalera A, Iadevaia MD, Miranda A, Zulli C, Gaeta L, Tuccillo C, Federico A, Loguercio C. Herbal products: benefits, limits, and applications in chronic liver disease. Evid Based Complement Alternat Med. 2012;2012:837939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 103. | Purohit V, Abdelmalek MF, Barve S, Benevenga NJ, Halsted CH, Kaplowitz N, Kharbanda KK, Liu QY, Lu SC, McClain CJ. Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: summary of a symposium. Am J Clin Nutr. 2007;86:14-24. [PubMed] |
| 104. | Kharbanda KK, Mailliard ME, Baldwin CR, Beckenhauer HC, Sorrell MF, Tuma DJ. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J Hepatol. 2007;46:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 105. | Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 533] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 106. | Kharbanda KK, Todero SL, King AL, Osna NA, McVicker BL, Tuma DJ, Wisecarver JL, Bailey SM. Betaine treatment attenuates chronic ethanol-induced hepatic steatosis and alterations to the mitochondrial respiratory chain proteome. Int J Hepatol. 2012;2012:962183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 107. | Shi QZ, Wang LW, Zhang W, Gong ZJ. Betaine inhibits toll-like receptor 4 expression in rats with ethanol-induced liver injury. World J Gastroenterol. 2010;16:897-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 108. | Li YJ, Chen J, Li Y, Li Q, Zheng YF, Fu Y, Li P. Screening and characterization of natural antioxidants in four Glycyrrhiza species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Chromatogr A. 2011;1218:8181-8191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 109. | Gwak GY, Moon TG, Lee DH, Yoo BC. Glycyrrhizin attenuates HMGB1-induced hepatocyte apoptosis by inhibiting the p38-dependent mitochondrial pathway. World J Gastroenterol. 2012;18:679-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 110. | Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, Armanini D, Bielenberg J. Antiviral effects of Glycyrrhiza species. Phytother Res. 2008;22:141-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 301] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 111. | Wang CY, Kao TC, Lo WH, Yen GC. Glycyrrhizic acid and 18β-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-κB through PI3K p110δ and p110γ inhibitions. J Agric Food Chem. 2011;59:7726-7733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 112. | Thiyagarajan P, Chandrasekaran CV, Deepak HB, Agarwal A. Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents. Inflammopharmacology. 2011;19:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 113. | Li XL, Zhou AG. Evaluation of the immunity activity of glycyrrhizin in AR mice. Molecules. 2012;17:716-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 114. | Wang J, Guo J, Liu S. [Inhibitory effect of glycyrrhizin on NF-kappa B binding activity in CCl4 plus ethanol induced liver cirrhosis in rats]. Zhonghua Ganzangbing Zazhi. 1999;7:42-43. [PubMed] |
| 115. | Wang JY, Guo JS, Li H, Liu SL, Zern MA. Inhibitory effect of glycyrrhizin on NF-kappaB binding activity in CCl4- plus ethanol-induced liver cirrhosis in rats. Liver. 1998;18:180-185. [PubMed] |
| 116. | Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494-1500. [PubMed] |
| 117. | Huu Tung N, Uto T, Morinaga O, Kim YH, Shoyama Y. Pharmacological effects of ginseng on liver functions and diseases: a minireview. Evid Based Complement Alternat Med. 2012;2012:173297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 118. | Cho WC, Chung WS, Lee SK, Leung AW, Cheng CH, Yue KK. Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006;550:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 119. | Yokozawa T, Kobayashi T, Oura H, Kawashima Y. Hyperlipemia-improving effects of ginsenoside-Rb2 in streptozotocin-diabetic rats. Chem Pharm Bull (Tokyo). 1985;33:3893-3898. [PubMed] |
| 120. | Hou YL, Tsai YH, Lin YH, Chao JC. Ginseng extract and ginsenoside Rb1 attenuate carbon tetrachloride-induced liver fibrosis in rats. BMC Complement Altern Med. 2014;14:415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 121. | Song SB, Tung NH, Quang TH, Ngan NT, Kim KE, Kim YH. Inhibition of TNF-α-mediated NF-κB Transcriptional Activity in HepG2 Cells by Dammarane-type Saponins from Panax ginseng Leaves. J Ginseng Res. 2012;36:146-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 122. | Yuan HD, Kim SJ, Quan HY, Kim DY, Kim GW, Chung SH. Ginsenoside Re attenuates alcoholic fatty liver disease via regulation of AMPK and MAPK pathways in alcohol-fed ICR mice. Proceedings of the Proceedings of the Spring International Ginseng Conference. Jeju, Korea: Spring 2012; 114. |
| 123. | Han JY, Lee S, Yang JH, Kim S, Sim J, Kim MG, Jeong TC, Ku SK, Cho IJ, Ki SH. Korean Red Ginseng attenuates ethanol-induced steatosis and oxidative stress via AMPK/Sirt1 activation. J Ginseng Res. 2015;39:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 124. | Hong M, Kim SW, Han SH, Kim DJ, Suk KT, Kim YS, Kim MJ, Kim MY, Baik SK, Ham YL. Probiotics (Lactobacillus rhamnosus R0011 and acidophilus R0052) reduce the expression of toll-like receptor 4 in mice with alcoholic liver disease. PLoS One. 2015;10:e0117451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 125. | Kim MS, Lee KT, Iseli TJ, Hoy AJ, George J, Grewal T, Roufogalis BD. Compound K modulates fatty acid-induced lipid droplet formation and expression of proteins involved in lipid metabolism in hepatocytes. Liver Int. 2013;33:1583-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 126. | Anuradha CV, Ravikumar P. Restoration on tissue antioxidants by fenugreek seeds (Trigonella Foenum Graecum) in alloxan-diabetic rats. Indian J Physiol Pharmacol. 2001;45:408-420. [PubMed] |
| 127. | Kochhar A, Nagi M. Effect of supplementation of traditional medicinal plants on blood glucose in non-insulin-dependent diabetics: a pilot study. J Med Food. 2005;8:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 128. | Shang M, Cai S, Han J, Li J, Zhao Y, Zheng J, Namba T, Kadota S, Tezuka Y, Fan W. [Studies on flavonoids from Fenugreek (Trigonella foenumgraecum L.)]. Zhongguo Zhongyao Zazhi. 1998;23:614-66, 639. [PubMed] |
| 129. | Kaviarasan S, Ramamurty N, Gunasekaran P, Varalakshmi E, Anuradha CV. Fenugreek (Trigonella foenum graecum) seed extract prevents ethanol-induced toxicity and apoptosis in Chang liver cells. Alcohol Alcohol. 2006;41:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 130. | Nickel T, Hanssen H, Sisic Z, Pfeiler S, Summo C, Schmauss D, Hoster E, Weis M. Immunoregulatory effects of the flavonol quercetin in vitro and in vivo. Eur J Nutr. 2011;50:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 131. | Tang Y, Gao C, Xing M, Li Y, Zhu L, Wang D, Yang X, Liu L, Yao P. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem Toxicol. 2012;50:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 132. | Kaviarasan S, Viswanathan P, Anuradha CV. Fenugreek seed (Trigonella foenum graecum) polyphenols inhibit ethanol-induced collagen and lipid accumulation in rat liver. Cell Biol Toxicol. 2007;23:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 133. | Bao W, Li K, Rong S, Yao P, Hao L, Ying C, Zhang X, Nussler A, Liu L. Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. J Ethnopharmacol. 2010;128:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 134. | Samuhasaneeto S, Thong-Ngam D, Kulaputana O, Suyasunanont D, Klaikeaw N. Curcumin decreased oxidative stress, inhibited NF-kappaB activation, and improved liver pathology in ethanol-induced liver injury in rats. J Biomed Biotechnol. 2009;2009:981963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 135. | Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Thomas P, Dannenberg AJ. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G321-G327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 136. | Lee HI, McGregor RA, Choi MS, Seo KI, Jung UJ, Yeo J, Kim MJ, Lee MK. Low doses of curcumin protect alcohol-induced liver damage by modulation of the alcohol metabolic pathway, CYP2E1 and AMPK. Life Sci. 2013;93:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 137. | Rong S, Zhao Y, Bao W, Xiao X, Wang D, Nussler AK, Yan H, Yao P, Liu L. Curcumin prevents chronic alcohol-induced liver disease involving decreasing ROS generation and enhancing antioxidative capacity. Phytomedicine. 2012;19:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 138. | Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3258] [Cited by in RCA: 3631] [Article Influence: 201.7] [Reference Citation Analysis (0)] |
| 139. | Wang L, Wang BE, Wang J, Xiao PG, Tan XH. Herbal compound 861 regulates mRNA expression of collagen synthesis- and degradation-related genes in human hepatic stellate cells. World J Gastroenterol. 2008;14:1790-1794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 140. | You H, Wang B, Wang T. [Proliferation and apoptosis of hepatic stellate cells and effects of compound 861 on liver fibrosis]. Zhonghua Ganzangbing Zazhi. 2000;8:78-80. [PubMed] |
| 141. | Yin C, Ma H, Wang A, Ma X, Jia J, Wang B. [Effect of compound 861 on tissue inhibitor of metalloprotenase 1 gene expression of HSC-T6 cells]. Zhonghua Ganzangbing Zazhi. 2002;10:197-199. [PubMed] |
| 142. | Baoen W, Tailing W, Jidong J, Hong M, Zhongping D, Xinmin L, Jia L, Aimin W, Linxue Q. Experimental and clinical study on inhibition and reversion of Liver fibrosis with integrated Chinese and western medicine. CJIM. 1999;5:6-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 143. | Yin SS, Wang BE, Wang TL, Jia JD, Qian LX. [The effect of Cpd 861 on chronic hepatitis B related fibrosis and early cirrhosis: a randomized, double blind, placebo controlled clinical trial]. Zhonghua Ganzangbing Zazhi. 2004;12:467-470. [PubMed] |
| 144. | Shimizu I, Ma YR, Mizobuchi Y, Liu F, Miura T, Nakai Y, Yasuda M, Shiba M, Horie T, Amagaya S. Effects of Sho-saiko-to, a Japanese herbal medicine, on hepatic fibrosis in rats. Hepatology. 1999;29:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 170] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 145. | Cohen MR. Herbal and complementary and alternative medicine therapies for liver disease. A focus on Chinese traditional medicine in hepatitis C virus. Clin Liver Dis. 2001;5:461-478, vii. [PubMed] |
| 146. | Dhiman RK, Chawla YK. Herbal medicines for liver diseases. Dig Dis Sci. 2005;50:1807-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 147. | Schuppan D, Jia JD, Brinkhaus B, Hahn EG. Herbal products for liver diseases: a therapeutic challenge for the new millennium. Hepatology. 1999;30:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 146] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 148. | Mitra SK, Varma SR, Godavarthi A, Nandakumar KS. Liv.52 regulates ethanol induced PPARgamma and TNF alpha expression in HepG2 cells. Mol Cell Biochem. 2008;315:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 149. | Huseini HF, Alavian SM, Heshmat R, Heydari MR, Abolmaali K. The efficacy of Liv-52 on liver cirrhotic patients: a randomized, double-blind, placebo-controlled first approach. Phytomedicine. 2005;12:619-624. [PubMed] |
| 150. | Mao YM, Zeng MD, Chen Y, Chen CW, Fu QC, Cai X, Wu SM, Chen YG, Sun Y, Li J. [Magnesium isoglycyrrhizinate in the treatment of chronic liver diseases: a randomized, double-blind, multi-doses, active drug controlled, multi-center study]. Zhonghua Ganzangbing Zazhi. 2009;17:847-851. [PubMed] |
| 151. | Xiao ZW, Zhang W, Ma L, Qiu ZW. Therapeutic effect of magnesium isoglycyrrhizinate in rats on lung injury induced by paraquat poisoning. Eur Rev Med Pharmacol Sci. 2014;18:311-320. [PubMed] |
| 152. | Zhao J, Wan XY, Luo M, Chen TS, He P. Antifibrotic effects of glycyrrhizin and matrine in vitro and in vivo. Biomedicine Preventive Nutrition. 2012;2:132-137. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 153. | Testino G, Leone S, Ansaldi F, Borro P. Silymarin and S-adenosyl-L-methionine (SAMe): two promising pharmacological agents in case of chronic alcoholic hepathopathy. A review and a point of view. Minerva Gastroenterol Dietol. 2013;59:341-356. [PubMed] |
| 154. | Stickel F, Patsenker E, Schuppan D. Herbal hepatotoxicity. J Hepatol. 2005;43:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 155. | Nakagawa A, Yamaguchi T, Takao T, Amano H. [Five cases of drug-induced pneumonitis due to Sho-saiko-to or interferon-alpha or both]. Nihon Kyobu Shikkan Gakkai Zasshi. 1995;33:1361-1366. [PubMed] |
| 156. | DiCenzo R, Shelton M, Jordan K, Koval C, Forrest A, Reichman R, Morse G. Coadministration of milk thistle and indinavir in healthy subjects. Pharmacotherapy. 2003;23:866-870. [PubMed] |
| 157. | Singal AK, Anand BS. Recent trends in the epidemiology of alcoholic liver disease. Clinical Liver Disease. 2013;2:53-56. [RCA] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (3)] |
| 158. | Moghe A, Joshi-Barve S, Ghare S, Gobejishvili L, Kirpich I, McClain CJ, Barve S. Histone modifications and alcohol-induced liver disease: are altered nutrients the missing link? World J Gastroenterol. 2011;17:2465-2472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 159. | You M, Crabb DW. Molecular mechanisms of alcoholic fatty liver: role of sterol regulatory element-binding proteins. Alcohol. 2004;34:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 160. | Carithers RL, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, Maddrey WC. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med. 1989;685-690. [PubMed] |
| 161. | De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 162. | Frezza M, Surrenti C, Manzillo G, Fiaccadori F, Bortolini M, Di Padova C. Oral S-adenosylmethionine in the symptomatic treatment of intrahepatic cholestasis. A double-blind, placebo-controlled study. Gastroenterology. 1990;99:211-215. [PubMed] |
| 163. | Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. 2003;278:27997-28004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 164. | Kong L, Ren W, Li W, Zhao S, Mi H, Wang R, Zhang Y, Wu W, Nan Y, Yu J. Activation of peroxisome proliferator activated receptor alpha ameliorates ethanol induced steatohepatitis in mice. Lipids Health Dis. 2011;10:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 165. | Soylu AR, Altaner S, Aydodu N, Basaran UN, Tarcin O, Gedik N, Umit H, Tezel A, Ture M, Kutlu K. Effects of vitamins E and C supplementation on hepatic glutathione peroxidase activity and tissue injury associated with ethanol ingestion in malnourished rats. Curr Ther Res Clin Exp. 2006;67:118-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 166. | Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 942] [Article Influence: 85.6] [Reference Citation Analysis (4)] |