Published online Feb 28, 2015. doi: 10.3748/wjg.v21.i8.2387
Peer-review started: August 8, 2014
First decision: October 14, 2014
Revised: October 28, 2014
Accepted: December 1, 2014
Article in press: December 1, 2014
Published online: February 28, 2015
Processing time: 205 Days and 17.2 Hours
AIM: To investigate the prognostic usefulness of several existing scoring systems in predicting the severity of acute pancreatitis (AP).
METHODS: We retrospectively analyzed the prospectively collected clinical database from consecutive patients with AP in our institution between January 2011 and December 2012. Ranson, Acute Physiology and Chronic Health Evaluation (APACHE)-II, and bedside index for severity in acute pancreatitis (BISAP) scores, and computed tomography severity index (CTSI) of all patients were calculated. Serum C-reactive protein (CRP) levels were measured at admission (CRPi) and after 24 h (CRP24). Severe AP was defined as persistent organ failure for more than 48 h. The predictive accuracy of each scoring system was measured by the area under the receiver-operating curve (AUC).
RESULTS: Of 161 patients, 21 (13%) were classified as severe AP, and 3 (1.9%) died. Statistically significant cutoff values for prediction of severe AP were Ranson ≥ 3, BISAP ≥ 2, APACHE-II ≥ 8, CTSI ≥ 3, and CRP24≥ 21.4. AUCs for Ranson, BISAP, APACHE-II, CTSI, and CRP24 in predicting severe AP were 0.69 (95%CI: 0.62-0.76), 0.74 (95%CI: 0.66-0.80), 0.78 (95%CI: 0.70-0.84), 0.69 (95%CI: 0.61-0.76), and 0.68 (95%CI: 0.57-0.78), respectively. APACHE-II demonstrated the highest accuracy for prediction of severe AP, however, no statistically significant pairwise differences were observed between APACHE-II and the other scoring systems, including CRP24.
CONCLUSION: Various scoring systems showed similar predictive accuracy for severity of AP. Unique models are needed in order to achieve further improvement of prognostic accuracy.
Core tip: Only a few studies have evaluated the comparison of various scoring systems including bedside index for severity in acute pancreatitis in predicting the severity of acute pancreatitis (AP) according to the revised Atlanta Classification. Based on our study, Acute Physiology and Chronic Health Evaluation (APACHE)-II score appeared to have highest accuracy for prediction of severe AP, although the predictive accuracy of APACHE-II was not significantly different compared to that of the other scoring systems, including C-reactive protein. Various scoring systems most widely used for early prediction of severity of AP showed similar predictive accuracy for severity of AP, and unique models are needed in order to achieve further improvement of predictive accuracy.
- Citation: Cho JH, Kim TN, Chung HH, Kim KH. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol 2015; 21(8): 2387-2394
- URL: https://www.wjgnet.com/1007-9327/full/v21/i8/2387.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i8.2387
Acute pancreatitis (AP) is an inflammatory process with a highly variable clinical course. Most patients with AP have a mild disease that resolves spontaneously without sequelae, however, 10%-20% of patients experience a severe attack with high mortality up to 30%[1,2]. This high risk group of patients may benefit from aggressive fluid resuscitation, close monitoring for development of organ failure, proper administration of antibiotics and specific therapeutic procedures, such as endoscopic sphincterotomy and radiologic intervention[3]. Therefore, early assessment of the severity and identification of patients at risk is important for early intensive therapy and timely intervention, and has been shown to improve prognosis and survival.
The Atlanta Classification has been considered the global standard tool for the assessment of AP severity since its establishment in 1992[4]. However, as time goes on, some of the definitions in the original Atlanta Classification has been proved to be confusing, especially its definition of “severity”. In 2012, the Atlanta classification was revised with an emphasis on persistent organ failure[5].
Multi-factorial scoring systems, including Ranson et al[6] and Acute Physiology and Chronic Health Evaluation (APACHE)-II scores[7] have been used since the 1970s for assessment of the severity of AP. Balthazar computed tomography severity index (CTSI)[8] was developed in 1990. These predictive methods have been established as an important tool for assessment of the severity of AP. However, these multi-factorial scoring systems, which are complex and difficult to use in clinical bases, have been shown to perform with high negative predictive value but only moderate overall sensitivity[3,9,10]. A new prognostic scoring system, the Bedside Index for Severity in Acute Pancreatitis (BISAP), has recently been proposed as an accurate and simple method for early identification of patients at risk of in-hospital mortality[11,12]. There have been a few studies concerning the comparison of various scoring systems including BISAP in predicting the severity of AP based on the revised Atlanta Classification[9,13].
This study was conducted for assessment and comparison of the early predictability of various parameters most widely used in AP, such as multi-factorial scoring systems (Ranson, APACHE-II, and BISAP), CTSI and one single laboratory parameter [C-reactive protein (CRP)] in a tertiary care center.
Demographic, radiographic, and laboratory data from 161 consecutive patients with AP who were admitted or transferred to our institution were prospectively collected during a two-year-period between January 2011 and December 2012. Analysis of this clinical database was performed retrospectively. The mean age of a total of 161 patients was 62.3 ± 16.1 years and 102 patients (63%) were male. Sixteen patients (10%) had a history of previous pancreatitis attack. Causes of AP included biliary (54%), alcohol (22%), idiopathic (21%), and others (3%). Twenty one patients (13%) developed persistent organ failure for more than 48 h and were classified as severe AP according to the Atlanta Classification. Thirteen patients (8%) were classified as moderately severe AP and 127 patients (79%) as mild AP.
Laboratory tests were performed upon arrival at the hospital and at 48 h after admission. Computed tomography (CT) scan was performed in all patients within 48 h after arrival at the hospital for detection of the development of fluid collections, the extent of inflammation, and necrotic changes. Oral feeding was permitted when abdominal pain subsided and patients felt hunger sensation. When patients remained asymptomatic with oral intake, patients were discharged or underwent cholecystectomy if indicated.
The following parameters for each episode of AP were collected: length of hospital stay, in-hospital mortality, duration of nil per os (NPO), presence of organ failure and local complications such as peripancreatic fluid collections, pseudocyst and necrosis. APACHE-II and BISAP scores were calculated using data from the first 24 h after admission and the Ranson score using data from the first 48 h. Serum CRP levels were measured at admission (CRPi) and after 24 h (CRP24). CTSI was calculated in patients who underwent contrast-enhanced computed tomography (CECT) within 48 h after admission. All CT scans were reviewed by radiologists, who were blinded to laboratory data and clinical course.
This study was approved by the Institutional Review Board of Yeungnam University Hospital.
The diagnosis of AP was based on the presence of two or more of the following three features: (1) abdominal pain consistent with AP (acute onset of a persistent and severe epigastric pain often radiating to the back); (2) elevation of serum amylase and/or lipase levels three or more times of the upper limit of normal; and (3) characteristic findings of AP on CECT[5]. Pancreatic fluid collections and pseudocysts were defined according to the Atlanta Classification. Alcoholic AP was defined when patients had a history of alcohol consumption within 48 h before symptom onset with no signs of other possible causes. Biliary pancreatitis was defined when there was a gallstone or biliary sludge on ultrasonogram or CT. The etiology was considered to be idiopathic when causative factors could not be identified from a detailed clinical and drug history or after initial investigations.
Severity of AP was determined according to the most recently revised Atlanta Classification. Mild AP was defined by the absence of organ failure and the absence of local or systemic complications. Moderately severe AP was defined by the presence of transient organ failure, local complications, or exacerbation of co-morbid diseases. Severe AP was defined by persistent organ failure for more than 48 h. Organ failure was defined as a score of 2 or more for one of the three systems (respiratory, cardiovascular, and renal) using the modified Marshall scoring system[14]. The major difference between the new and former definition of clinical severity is that the presence of local complications or transient organ failure is no longer regarded as clinically severe disease, unless organ failure exceeds 48 h in duration.
Data were collected prospectively in a Microsoft Excel database. After completion of data collection, the database was imported into SPSS for Windows (20.0, SPSS, Chicago, IL, United States). Continuous baseline descriptive variables were expressed as mean with standard deviation (SD) and were compared using the Mann-Whitney test. Categorical variables were expressed as absolute numbers and proportions. Bivariate relationship for categorical variables was assessed using odds ratio (OR) calculated based on Pearson’s χ2 test or Fisher’s exact test. Spearman rank correlation analysis was used for evaluation of the correlation between each pair of scoring systems, and between each scoring system and length of hospital stay. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for individual scoring systems and biochemical markers (CRPi, CRP24). Receiver-operating characteristic (ROC) curves for severe AP were calculated for Ranson, BISAP, APACHE-II scores, CTSI, CRPi, and CRP24 using cutoff values, and the predictive accuracy of each scoring system was measured by the area under the receiver-operating curve (AUC) with standard error and 95% confidence intervals (CIs). A P value of < 0.05 was considered statistically significant.
Age and BMI did not differ significantly between the mild to moderately severe AP group and the severe AP group (P = 0.968, and P = 0.607, respectively). The number of males was significantly higher in patients with severe AP compared to patients with mild to moderately severe AP (P = 0.023). Among the etiologies of AP, alcohol showed significant association with patients with severe AP (P = 0.030). Initial laboratory findings of blood urea nitrogen (BUN) and creatinine were significantly higher in patients with severe AP compared to those with mild to moderately severe AP (P = 0.031, and P = 0.003, respectively). Significantly longer duration of NPO and length of hospital stay were observed for patients with severe AP compared to patients with mild to moderately severe AP (6.9 ± 5.1 vs 4.3 ± 2.2, P = 0.038 and 11.7 ± 6.2 vs 8.4 ± 4.8, P = 0.008, respectively). There were three mortalities (1.9%) during hospitalization. Two mortality patients were diagnosed with persistent organ failure for more than 48 h in the early days of hospitalization. The other one mortality patient had pancreatic necrosis (CTSI 6) without organ failure at the time of admission, however, developed organ failure during treatment.
Ranson, BISAP, and APACHE-II scores, and CTSI were significantly higher in the severe AP group, compared with the mild to moderately severe AP group (3.7 ± 1.4 vs 2.7 ± 1.4, P = 0.005; 1.9 ± 0.9 vs 1.0 ± 0.8, P < 0.001; 10.8 ± 4.8 vs 6.5 ± 3.5, P < 0.001; and 3.5 ± 2.2 vs 2.2 ± 1.4, P < 0.001; respectively). Significantly higher serum CRP24 level was observed in patients with severe AP (P = 0.026). Serum CRPi level did not differ significantly between the two groups (P = 0.259) (Table 1). The highest score of each scoring system of the mortality patients was Ranson of 6, BISAP of 3, APACHE-II of 17 and CTSI of 6.
| Mild to moderately severe AP (n = 140) | Severe AP(n = 21) | P value | |
| Age, yr | 62.2 ± 16.5 | 63.4 ± 13.1 | 0.968 |
| Male | 84 (60.0) | 18 (85.7) | 0.023 |
| BMI (kg/m2) | 23.4 ± 4.4 | 23.9 ± 4.1 | 0.607 |
| Hospital visit from symptom onset (d) | 5.7 ± 12.8 | 3.3 ± 6.9 | 0.388 |
| Etiology | |||
| Biliary | 78 (55.7) | 9 (42.8) | 0.270 |
| Alcoholic | 26 (18.6) | 10 (47.6) | 0.030 |
| Idiopathic | 33 (23.6) | 1 (4.8) | 0.130 |
| Others | 3 (2.1) | 1 (4.8) | 0.472 |
| Laboratory finding (initial) | |||
| Hematocrit (%) | 39.1 ± 6.2 | 40.3 ± 7.4 | 0.422 |
| Amylase (IU/L) | 1687.9 ± 1415.4 | 1668.2 ± 1668.8 | 0.954 |
| Lipase (IU/L) | 1949.7 ± 1951.3 | 2108.2 ± 2750.3 | 0.744 |
| AST (IU/L) | 203.2 ± 240.4 | 362.6 ± 618.4 | 0.256 |
| ALT (IU/L) | 155.6 ± 182.1 | 184.7 ± 275.9 | 0.645 |
| BUN (mg/dL) | 14.5 ± 8.6 | 31.2 ± 32.9 | 0.031 |
| Creatinine (mg/dL) | 1.0 ± 0.5 | 2.2 ± 1.6 | 0.003 |
| CRPi (mg/dL) | 4.4 ± 6.8 | 6.9 ± 10.4 | 0.259 |
| CRP24 (mg/dL) | 9.0 ± 7.9 | 17.4 ± 12.7 | 0.026 |
| Peripancreatic fluid collection | 46 (32.9) | 10 (47.6) | 0.158 |
| Duration of NPO (d) | 4.3 ± 2.2 | 6.9 ± 5.1 | 0.038 |
| Hospital stay (d) | 8.4 ± 4.8 | 11.7 ± 6.2 | 0.008 |
| Scoring systems | |||
| Ranson | 2.7 ± 1.4 | 3.7 ± 1.4 | 0.005 |
| BISAP | 1.0 ± 0.8 | 1.9 ± 0.9 | < 0.001 |
| APACHE-II | 6.5 ± 3.5 | 10.8 ± 4.8 | < 0.001 |
| CTSI | 2.2 ± 1.4 | 3.5 ± 2.2 | < 0.001 |
| Mortality | 1 (0.7) | 2 (9.5) | 0.005 |
According to Spearman ranked correlations, Ranson, BISAP, and APACHE-II scores, and CRPi, and CRP24 levels showed positive correlation with each pair of them, whereas CTSI showed positive correlation with CRP24 level (correlation coefficient; 0.44, P < 0.001) but not with other scores (Table 2). Ranson, BISAP, and APACHE-II scores, and CTSI showed significant correlation with length of hospital stay (correlation coefficients; 0.21 (P = 0.008), 0.17 (P = 0.037), 0.23 (P = 0.003) and 0.26 (P = 0.001), respectively). CRPi and CRP24 levels did not show correlation with length of hospital stay (correlation coefficients: 0.01 (P = 0.925) and 0.07 (P = 0.056), respectively).
| Ranson | BISAP | APACHE-II | CTSI | CRPi | CRP24 | ||
| Ranson | r | 1 | 0.58 | 0.60 | -0.03 | 0.28 | 0.22 |
| P-value | < 0.001 | < 0.001 | 0.693 | 0.001 | 0.045 | ||
| BISAP | r | 1 | 0.54 | -0.05 | 0.33 | 0.28 | |
| P-value | < 0.001 | 0.498 | < 0.001 | 0.009 | |||
| APACHE-II | r | 1 | -0.01 | 0.32 | 0.37 | ||
| P-value | 0.905 | < 0.001 | < 0.001 | ||||
| CTSI | r | 1 | 0.07 | 0.44 | |||
| P-value | 0.427 | < 0.001 | |||||
| CRPi | r | 1 | 0.59 | ||||
| P-value | < 0.001 | ||||||
| CRP24 | r | 1 | |||||
| P-value | |||||||
On the basis of the highest sensitivity and specificity values generated from the ROC curves, the following cutoffs were selected for prediction of severe AP: Ranson ≥ 3, BISAP ≥ 2, APACHE-II ≥ 8, CTSI ≥ 3, and CRP24≥ 21.4. The observed incidences of severe AP when above cutoff value of each scoring system was applied along with the corresponding ORs are shown in Table 3. On χ2 test, patients with APACHE-II of 8 or greater and CRP24 of 21.4 or greater had a 8 times and 22 times higher likelihood of developing severe AP (OR = 8.2 and 22.0), respectively.
| Patients | Severe AP | |
| Ranson | ||
| < 2 | 65 (40.4) | 3 (4.6) |
| ≥ 3 | 96 (59.6) | 18 (18.8) |
| OR (95%CI) | 4.8 (1.3-16.9) | |
| BISAP | ||
| < 1 | 109 (67.7) | 8 (7.3) |
| ≥ 2 | 52 (22.3) | 13 (25.0) |
| OR (95%CI) | 4.2 (1.6-10.9) | |
| APACHE-II | ||
| < 7 | 65 (40.4) | 4 (6.2) |
| ≥ 8 | 96 (59.6) | 17 (17.7) |
| OR (95%CI) | 8.2 (2.6-25.6) | |
| CTSI | ||
| < 2 | 101 (62.7) | 7 (6.9) |
| ≥ 3 | 60 (37.3) | 14 (23.3) |
| OR(95%CI) | 4.1 (1.5-10.8) | |
| CRP24 | ||
| < 21.4 | 72 (85.7) | 6 (8.3) |
| ≥ 21.4 | 12 (14.3) | 8 (66.7) |
| OR (95%CI) | 22.0 (5.1-95.0) |
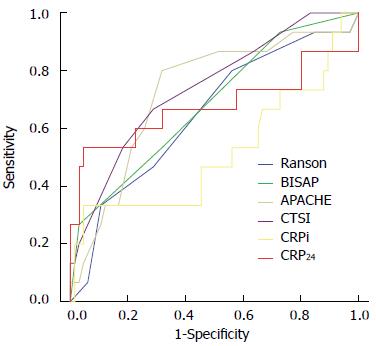
Figure 1 shows the comparisons of ROC curves for severe AP among all scoring systems and CRP. AUCs for Ranson, BISAP, APACHE-II, CTSI, CRPi, and CRP24 in predicting severe AP were 0.69 (95%CI: 0.62-0.76), 0.74 (95%CI: 0.66-0.80), 0.78 (95%CI: 0.70-0.84), 0.69 (95%CI: 0.61-0.76), 0.52 (95%CI: 0.33-0.70), and 0.68 (95%CI: 0.57-0.78), respectively. All scoring systems and CRP24 were found to be reliable in prediction of severe AP, except CRPi. The sensitivity, specificity, PPV, and NPV of different scoring systems and CRP in prediction of severe AP using cutoff values of Ranson ≥ 3, BISAP ≥ 2, APACHE-II ≥ 8, CTSI ≥ 3, and CRP24≥ 21.4 are shown in Table 4. APACHE-II score demonstrated the highest accuracy for prediction of severe AP (AUC = 0.78), however, no statistically significant pairwise differences were observed between APACHE-II and the other score systems, including CRP24.
| Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | |
| Ranson | 85.7 (63.7-97.0) | 44.3 (35.9-52.9) | 18.8 (11.5-28.0) | 95.3 (87.1-99.0) |
| BISAP | 61.9 (38.4-81.9) | 72.1 (63.9-79.4) | 25.0 (14.0-38.9) | 92.7 (86.0-96.8) |
| APACHE-II | 81.0 (58.1-94.6) | 65.7 (57.2-73.5) | 26.2 (16.0-38.5) | 95.8 (89.7-98.9) |
| CTSI | 66.7 (43.0-85.4) | 67.1 (58.7-74.8) | 23.3 (13.4-36.0) | 93.1 (86.2-97.2) |
| CRP24 | 53.3 (26.6-78.7) | 94.3 (86.0-98.4) | 66.7 (34.9-90.1) | 90.4 (81.2-96.1) |
AP is a disease with variable severity and an evolving process that may involve multiple organ systems. Although approximately 80% of patients have mild disease that resolves spontaneously with little morbidity, the remaining 20% suffer from severe attack with mortality rates as high as 30%[1,2]. In this study, 21 patients (13%) were classified as severe AP, and two (10%) of these patients died during hospitalization. Some studies have reported that the cause of AP was not related to disease severity[15-17]. However, in this study, among the etiologies of AP, alcohol showed a significant association with patients with severe AP (P = 0.030). Severe AP is usually observed at the initial stage of AP and slow progression from mild to severe disease is uncommon[4,18]. Therefore, early evaluation of its severity is considered to be a critical concern in the prognosis and management of AP. Since the 1970s, many studies for development of a widely available prognostic scoring system in AP for prediction of which patients are at the highest risk of developing clinically severe AP and require aggressive therapy have been reported[19]. An ideal prognostic scoring system should be simple, noninvasive, accurate, and quantitative, and the assessment methods should be easily applicable at the time of diagnosis.
Early in the course of AP, systemic inflammatory response syndrome or organ failure suggests potentially severe disease and poor prognosis[5]. Morbidity and mortality in the earlier stage of AP are associated with the systemic inflammatory response and persistent organ failure rather than local complications[20], and are the most common cause of death within the first two weeks of disease onset[21]. In 2012, the Atlanta Classification was revised with an emphasis on persistent organ failure[5]. In this study, the severity of AP was determined according to this revised Atlanta Classification.
The Ranson score represented a major advancement in evaluation of disease severity in AP and has been used clinically for more than three decades[6,22]. Since its development, several other scoring systems, such as the Glasgow criteria[23-25], APACHE-II score[7,26], BISAP[11], and CTSI[8], have been developed. These scoring systems incorporate physiologic, laboratory, and radiographic parameters using cut-off values and converting continuous variables into binary values. Because of their complexity, attention has also focused on the role of individual laboratory parameters, such as CRP, hematocrit, BUN, and creatinine, in assessment of disease severity. In this study, accuracy of three clinical scoring systems and CTSI, including CRP, in prediction of severe AP, were compared.
In the previous study, the degree of correlation between the length of hospital stay and APACHE-II and modified Glasgow scores was larger than that between the length of hospital stay and Ranson score[27]. Results of this study demonstrated significant correlation of Ranson, BISAP, and APACHE-II scores, and CTSI with the length of hospital stay, however, CRPi and CRP24 did not show correlation with the length of hospital stay. In this study, correlations of different scoring systems were evaluated. The Ranson, BISAP, and APACHE-II scores showed positive correlation with each pair of them, whereas CTSI did not show correlation with any scoring system. These results may be related to the fact that pancreatic parenchymal necrosis on CECT may not appear within 48 h[28].
Serum CRP (after 48 h) was reported to be a predictor of severe AP[10]. Despite the simplicity and easy availability of CRP in clinical practice, many studies have described limitation of clinical utility of CRP in the early phase of AP, and revealed that usage of CRP alone was potentially failing to detect severe cases of AP at an earlier stage[27,29-31]. In this study, the CRP24 was significantly higher in severe AP compared to mild to moderately severe AP; however CRPi did not differ significantly between the two groups. This study again demonstrated that CRP showed significantly higher value in severe AP at a later stage, however, it has limitations in prediction of the severity of AP at an earlier stage.
In one meta-analysis, including 1300 patients with AP, Ranson score had an overall sensitivity of 75%, specificity of 77%, PPV of 49%, and NPV of 91%[3]. In this study, sensitivity and NPV of Ranson score was 85.7% and 95.3%, respectively, however, specificity and PPV were low (44.4% and 18.8%, respectively). Based on this result, there was a high false positive rate of severe AP with Ranson score, and approximately 80% of patients with a Ranson score of more than 3 were not severe AP actually.
BISAP, a recently developed prognostic scoring system, has been proposed as a simple method for prediction of severe AP compared to traditional scoring systems. Results of this study demonstrated that predictive accuracy of severe AP was similar to that of the other scoring systems, however, against expectations, the process of calculation of the BISAP score was not simple compared to Ranson and CTSI.
The CTSI was reported to be useful in identification of patients with severe AP and poor prognosis in selected patients in 1990[8]. However, only a few studies have investigated whether CTSI is superior to the APACHE-II or Ranson score in prediction of severe AP[32]. Although pancreatic parenchymal necrosis has been shown to correlate with development of organ failure and local complications that require intervention[20,33,34], major limitation of CTSI is that pancreatic parenchymal necrosis may be unrecognized on an early CT performed within 24 h after admission and development of local complications, such as abscess or hemorrhage, usually occur late in the course of AP[35]. The results of CTSI for prediction of mortality and prognosis in patients with AP were different from those of other studies and the cut off values were variable greatly[4,36,37]. In this study, when CTSI ≥ 3 was selected for prediction of severe AP, sensitivity and specificity were 66.7% and 67.1%, respectively.
In this study, APACHE-II score appeared to be a more influential tool than other scoring systems, including CRP, although no statistically significant pairwise differences were observed between APACHE-II and other scoring systems. The AUCs of Ranson, BISAP, and APACHE-II scores, and CTSI and CRP24 were 0.69 (95%CI: 0.62-0.76), 0.74 (95%CI: 0.66-0.80), 0.78 (95%CI: 0.70-0.84), 0.69 (95%CI: 0.61-0.76), and 0.68 (95%CI: 0.57-0.78), respectively. Current practice guidelines have suggested that APACHE-II score was the most helpful test at admission in distinguishing severe from mild AP, and, according to recommendation, it should be generated during the first three days of hospitalization[1]. Although the process of calculating APACHE-II score was complex, it might be easier in the era of computerized calculation systems.
There were some limitations in this study. Although the data used in this study were collected prospectively, some clinical data, including CRP, were missing due to lack of availability. In this study, the number of cases of severe AP and mortalities was lower compared to other large scale clinical studies; therefore, comparison of prognostic value of various scoring systems was somewhat difficult. Nevertheless, this study was one of the few studies to compare different prognostic scoring systems including BISAP on the basis that severe AP was defined by persistent organ failure for more than 48 h, especially in a non-Western area. In addition, in this study, the prevalence of mild and severe cases (87% and 13%, respectively) was similar to the prevalence of clinical severity of AP commonly reported in the literature.
If the pathophysiologic mechanism of organ failure in severe AP and factors play a key role in development, and the course of AP is clearly identified in the near future, appropriate and specific therapy for severe AP will be developed. At this time, development of a simple predictive method for accurate identification of individual patients who develop clinically severe disease will become the main issue in AP.
In conclusion, results of this study demonstrate that the APACHE-II scoring system seems to have the highest accuracy in assessment of the severity and outcome of AP, although the predictive accuracy of APACHE-II was not significantly different compared to that of the other scoring systems, including CRP. No simple scoring system capable of reaching maximal utility is available, and unique models are needed in order to achieve further improvement of predictive accuracy.
Severe acute pancreatitis (AP) usually discloses itself just after the onset of the disease and slow progression from mild to severe disease is uncommon. Therefore, early assessment of its severity is crucial for the prognosis and management of AP. In 2012, the Atlanta classification was revised with an emphasis on persistent organ failure. The major difference between the new and former definition of clinical severity is that the presence of local complications or transient organ failure is no longer regarded as clinically severe disease, and severe AP was defined by persistent organ failure for more than 48 h.
Recently, bedside index for severity in acute pancreatitis (BISAP) has been proposed as an accurate and simple method for prediction of severe AP. Several studies demonstrated that the accuracy of BISAP in predicting severe AP and death was similar to that of the other scoring systems, including Ranson and Acute Physiology and Chronic Health Evaluation (APACHE)-II.
Based on this study, APACHE-II score appeared to have highest accuracy for prediction of severe AP, although the predictive accuracy of APACHE-II score was not significantly different compared to that of the other scoring systems. Various existing scoring systems most widely used including BISAP, showed similar predictive accuracy for severity of AP and against expectations, the process of calculation of the BISAP score was not simple compared to Ranson and CTSI.
No simple scoring system capable of reaching maximal utility for prediction of severe AP is available. If the pathophysiologic mechanism of organ failure in severe AP and factors play a key role in development, and the course of AP is clearly identified in the near future, appropriate and specific therapy for severe AP will be developed. At this time, development of a simple predictive method for accurate identification of individual patients who develop clinically severe disease will become the main issue in AP.
The present manuscript demonstrated that Ranson, APACHE-II, BISAP, CTSI, and CRP showed similar predictive accuracy in predicting the severity of AP. Overall, this manuscript appears to be a carefully designed and conducted study. And the manuscript is logical and the conclusion is in general well supported. The data and English language are well controlled.
P- Reviewer: Ker CG, Lee KT, Wronski M, Wu WJ, Zhao JB S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1150] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 2. | Fagenholz PJ, Castillo CF, Harris NS, Pelletier AJ, Camargo CA. Increasing United States hospital admissions for acute pancreatitis, 1988-2003. Ann Epidemiol. 2007;17:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Forsmark CE, Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 505] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 4. | Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1929] [Cited by in RCA: 1736] [Article Influence: 54.3] [Reference Citation Analysis (1)] |
| 5. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4342] [Article Influence: 361.8] [Reference Citation Analysis (45)] |
| 6. | Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Localio SA. Objective early identification of severe acute pancreatitis. Am J Gastroenterol. 1974;61:443-451. [PubMed] |
| 7. | Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;2:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 426] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 960] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 9. | Papachristou GI, Muddana V, Yadav D, O’Connell M, Sanders MK, Slivka A, Whitcomb DC. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435-41; quiz 442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 10. | Neoptolemos JP, Kemppainen EA, Mayer JM, Fitzpatrick JM, Raraty MG, Slavin J, Beger HG, Hietaranta AJ, Puolakkainen PA. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study. Lancet. 2000;355:1955-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 338] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57:1698-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 520] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 12. | Singh VK, Wu BU, Bollen TL, Repas K, Maurer R, Johannes RS, Mortele KJ, Conwell DL, Banks PA. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol. 2009;104:966-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Mounzer R, Langmead CJ, Wu BU, Evans AC, Bishehsari F, Muddana V, Singh VK, Slivka A, Whitcomb DC, Yadav D. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142:1476-182; quiz 1476-182;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 283] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 14. | Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1949] [Cited by in RCA: 1746] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 15. | Woo SM, Noh MH, Kim BG, Hsing CT, Han JS, Ryu SH, Seo JM, Yoon HA, Jang JS, Choi SR. Comparison of serum procalcitonin with Ranson, APACHE-II, Glasgow and Balthazar CT severity index scores in predicting severity of acute pancreatitis. Korean J Gastroenterol. 2011;58:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Uhl W, Isenmann R, Curti G, Vogel R, Beger HG, Büchler MW. Influence of etiology on the course and outcome of acute pancreatitis. Pancreas. 1996;13:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Gullo L, Migliori M, Oláh A, Farkas G, Levy P, Arvanitakis C, Lankisch P, Beger H. Acute pancreatitis in five European countries: etiology and mortality. Pancreas. 2002;24:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 194] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Yeung YP, Lam BY, Yip AW. APACHE system is better than Ranson system in the prediction of severity of acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2006;5:294-299. [PubMed] |
| 19. | McKay CJ, Imrie CW. Staging of acute pancreatitis. Is it important? Surg Clin North Am. 1999;79:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Perez A, Whang EE, Brooks DC, Moore FD, Hughes MD, Sica GT, Zinner MJ, Ashley SW, Banks PA. Is severity of necrotizing pancreatitis increased in extended necrosis and infected necrosis? Pancreas. 2002;25:229-233. [PubMed] |
| 21. | Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6591] [Cited by in RCA: 7778] [Article Influence: 268.2] [Reference Citation Analysis (1)] |
| 22. | Ranson JH, Pasternack BS. Statistical methods for quantifying the severity of clinical acute pancreatitis. J Surg Res. 1977;22:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 179] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Imrie CW, Benjamin IS, Ferguson JC, McKay AJ, Mackenzie I, O’Neill J, Blumgart LH. A single-centre double-blind trial of Trasylol therapy in primary acute pancreatitis. Br J Surg. 1978;65:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 338] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | McKay CJ, Evans S, Sinclair M, Carter CR, Imrie CW. High early mortality rate from acute pancreatitis in Scotland, 1984-1995. Br J Surg. 1999;86:1302-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Blamey SL, Imrie CW, O’Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut. 1984;25:1340-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 469] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 26. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 185] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 27. | Williams M, Simms HH. Prognostic usefulness of scoring systems in critically ill patients with severe acute pancreatitis. Crit Care Med. 1999;27:901-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Ryu JK. [Evaluation of severity in acute pancreatitis]. Korean J Gastroenterol. 2009;54:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 29. | Müller CA, Uhl W, Printzen G, Gloor B, Bischofberger H, Tcholakov O, Büchler MW. Role of procalcitonin and granulocyte colony stimulating factor in the early prediction of infected necrosis in severe acute pancreatitis. Gut. 2000;46:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Puolakkainen P, Valtonen V, Paananen A, Schröder T. C-reactive protein (CRP) and serum phospholipase A2 in the assessment of the severity of acute pancreatitis. Gut. 1987;28:764-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 123] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Chen CC, Wang SS, Lee FY, Chang FY, Lee SD. Proinflammatory cytokines in early assessment of the prognosis of acute pancreatitis. Am J Gastroenterol. 1999;94:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Leung TK, Lee CM, Lin SY, Chen HC, Wang HJ, Shen LK, Chen YY. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II scoring system in predicting acute pancreatitis outcome. World J Gastroenterol. 2005;11:6049-6052. [PubMed] |
| 33. | Isenmann R, Rau B, Beger HG. Bacterial infection and extent of necrosis are determinants of organ failure in patients with acute necrotizing pancreatitis. Br J Surg. 1999;86:1020-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 207] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Ocampo C, Zandalazini H, Kohan G, Silva W, Szelagowsky C, Oría A. Computed tomographic prognostic factors for predicting local complications in patients with pancreatic necrosis. Pancreas. 2009;38:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, Mortele KJ. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 36. | Casas JD, Díaz R, Valderas G, Mariscal A, Cuadras P. Prognostic value of CT in the early assessment of patients with acute pancreatitis. AJR Am J Roentgenol. 2004;182:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Simchuk EJ, Traverso LW, Nukui Y, Kozarek RA. Computed tomography severity index is a predictor of outcomes for severe pancreatitis. Am J Surg. 2000;179:352-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |