Published online Feb 21, 2015. doi: 10.3748/wjg.v21.i7.2260
Peer-review started: July 7, 2014
First decision: August 6, 2014
Revised: September 21, 2014
Accepted: November 7, 2014
Article in press: November 11, 2014
Published online: February 21, 2015
Processing time: 220 Days and 13.4 Hours
There are several reports of anti-tumor necrosis factor (TNF)-induced lung disease, especially in patients with rheumatologic diseases. Adalimumab is an anti-TNF drug used to induce and maintain remission in patients with immune-mediated diseases, such as Crohn’s disease. Although pulmonary disorders could be an extra-intestinal manifestation of inflammatory bowel disease, biologic therapy could also be a cause of lung injury. Only few cases of adalimumab-induced lung toxicity have been reported, and the majority of them were in patients with rheumatologic diseases. Lung injury secondary to anti-TNF therapy should, after ruling out other etiologies, be considered in patients who have a temporal association between the onset of respiratory symptoms and the exposure to these drugs. A compatible pattern in the biopsy and the clinical improvement after discontinuation of the anti-TNF drug would strongly support the diagnosis.
Core tip: Lung injury secondary to anti-tumor necrosis factor (TNF) drugs could cause severe respiratory symptoms in patients exposed to this therapy, and it should be suspected in patients who: have a temporal association between the onset of respiratory symptoms and the exposure to anti-TNF drugs, show a compatible pattern in the biopsy, and offer negative results for infection. There are a few cases reported of adalimumab-lung toxicity in patients with inflammatory bowel disease. Clinical improvement after biologic therapy discontinuation strongly supports the diagnosis. The mechanism by which anti-TNF drugs induce lung injury remains unclear; therefore, the use of another anti-TNF drug should be discouraged.
- Citation: Casanova MJ, Chaparro M, Valenzuela C, Cisneros C, Gisbert JP. Adalimumab-induced interstitial pneumonia in a patient with Crohn’s disease. World J Gastroenterol 2015; 21(7): 2260-2262
- URL: https://www.wjgnet.com/1007-9327/full/v21/i7/2260.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i7.2260
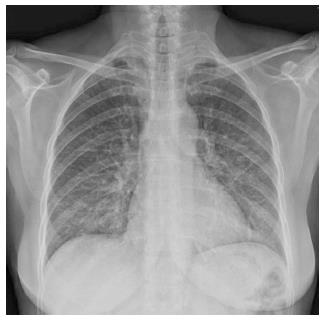
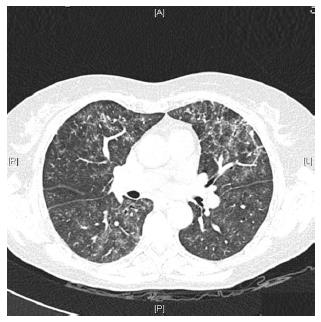
Caccaro et al[1] have recently published a case report of a patient with Crohn’s disease (CD) diagnosed with noninfectious interstitial lung disease secondary to infliximab therapy. The patient had been exposed to adalimumab (ADA) before receiving infliximab, without presenting any respiratory symptoms. The authors pointed out that lung injury secondary to ADA is not well established because, although there are a few reported cases of ADA-induced interstitial pneumonia in patients with rheumatologic diseases, this drug is also effective in the treatment of rheumatologic-associated lung diseases. In this respect, we present a case of a 59-year-old woman diagnosed with CD in May 2013. She had been a 20 pack-year-smoker for the last 40 years. However, from the moment of the diagnosis, she gave up smoking. The patient had no history of asthma, allergy or other pulmonary diseases. Two months later, the patient started the treatment with azathioprine due to steroid-refractoriness. However, she had to stop the treatment because of digestive intolerance, and the treatment with anti-tumor necrosis factor (anti-TNF) drugs was recommended. The patient had a positive Mantoux test (with a normal chest X-ray), so she started isoniazide 300 mg per day before the anti-TNF drug. In September 2013, ADA was started and she achieved clinical remission. One month later, the patient complained of progressive dyspnea, cough and fatigue. A chest X-ray showed a left predominant interstitial pattern (Figure 1) and she was referred to our hospital and admitted in the Pneumology Department. High-resolution computed tomography showed areas of diffuse ground glass opacities and cylindrical bronchiectasis in both lungs (Figure 2). These findings were probably related to drug-induced lung injury. Pulmonary function tests revealed a moderated restrictive pattern with a severe reduction of diffusing capacity of the lung for carbon monoxide. A fibro bronchoscopy was performed without endobronchial findings, and the analysis of bronchoalveolar lavage fluid and bronchial aspirate were negative for bacterial, fungi and alcohol-acid resistant bacilli. In addition, polymerase chain reaction for several respiratory viruses was negative. Bronchoalveolar lavage fluid cell count of 400 cells showed 8% of lymphocytes, 12% eosinophils, 68% of alveolar macrophages. The transbronchial biopsy showed a slight thickening of the alveolar septa and mild to moderate lymphocytic interstitial cellularity, consistent with interstitial lung disease and organizing pneumonia.
Based on these results, and especially in the biopsy’s findings, an organizing pneumonia probably related to ADA was suspected. ADA was discontinued, and prednisone at a dose of 40 mg per day was started. One month later, the respiratory symptoms disappeared and she was referred to our Inflammatory Bowel Disease Unit. As azathioprine had been interrupted due to digestive symptoms, mercaptopurine was started in order to maintain CD remission, presenting good tolerance by the patient. Three months later, the patient showed a significant functional and clinical recovery with normalization of spirometry and plethysmography and only a mild decrease in diffusing capacity of the lung for carbon monoxide.
There are several case reports of anti-TNF-induced lung disease, especially in patients with rheumatologic diseases[1-7]. The spectrum of lung disease has variable patterns, but interstitial pneumonia seems to be the most frequent[5,8,9]. Perez-Alvarez et al[7] reported 122 cases of anti-TNF-induced lung injury in a rheumatologic cohort. Interstitial pneumonia was confirmed by pulmonary biopsy in 26 cases, although a specific histopathologic description was detailed in only 20: 7 patients were classified as usual interstitial pneumonia, 6 as nonspecific interstitial pneumonia, 5 as organizing pneumonia, 1 as diffuse alveolar damage, and 1 as lymphoid interstitial pneumonia.
Reid et al[5] reported a case of a patient with CD who developed ADA-related lung injury. The patient presented a non-specific interstitial pneumonia. As in our case, the patient improved after discontinuing ADA and receiving a course of steroids. The organizing pneumonia pattern has been observed as an adverse event of some drugs, and its resolution after stopping the drug is the main argument to establish causality[10]. However, before establishing that the pulmonary toxicity is secondary to anti-TNF drug, it is essential to rule out other causes of lung-injury, such as infectious diseases. Our case supports the evidence that ADA, as infliximab, may induce lung toxicity in patients with inflammatory bowel disease exposed to this drug.
The exact mechanism by which anti-TNF drugs induce pulmonary disease is unclear[6]. TNF-α is a pro-inflammatory cytokine involved in the apoptosis of inflammatory cells, limiting pulmonary inflammation. The treatment with anti-TNF prevents the apoptosis and the inflammatory cells remain in the lungs, which may result in the characteristic changes of interstitial pneumonia[6,7,11]. Moreover, TNF-α prevents the fibroblast proliferation in the presence of interleukin-1 or interferon, but it also has a profibrotic effect trough the transforming growth factor-β1 regulation. The fact that only some patients develop anti-TNF-induced lung disease may be explained by the contribution of other factors, such as genetic predisposition[6,7].
According to previously published data, and to our case, stopping anti-TNF drugs and starting steroids seems to be a successful therapeutic options in patients with lung injury secondary to these drugs, since a significant number of patients presented clinical and radiological improvement after a few months. The use of immunosuppressive agents according to the clinical evolution may be an option[1,3,5-7].
A poor prognosis of anti-TNF-induced interstitial pneumonia, especially in patients with previous lung disease, has been described; with an overall mortality rate around one third, even when the anti-TNF drug had been suspended[7]. The limited available data suggest that rechallenge of the anti-TNF drug or the use of another anti-TNF agent, particularly those with the same therapeutic target, should be discouraged. We decided not to change from ADA to infliximab because both drugs are monoclonal anti-TNF antibodies that share the same fragment crystallizable region and bind to the same region of TNF.
In conclusion, causation of lung injury due to anti-TNF therapy should be considered, after ruling out other causes of pulmonary disease, specially infectious diseases, in patients who: have a temporal association between the onset of respiratory symptoms and the exposure to anti-TNF drugs; and show a compatible pattern in the biopsy. The clinical improvement after biologic therapy discontinuation strongly supports the diagnosis. The mechanism by which anti-TNF drugs induce lung injury remains unclear. In a patient who develops severe respiratory symptoms secondary to one anti-TNF drug, the use of another anti-TNF drug should be discouraged.
P- Reviewer: Buisson A, Araya J, Tanida S S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Caccaro R, Savarino E, D’Incà R, Sturniolo GC. Noninfectious interstitial lung disease during infliximab therapy: case report and literature review. World J Gastroenterol. 2013;19:5377-5380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007;86:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 516] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 3. | Weatherhead M, Masson S, Bourke SJ, Gunn MC, Burns GP. Interstitial pneumonitis after infliximab therapy for Crohn’s disease. Inflamm Bowel Dis. 2006;12:427-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Yousem SA, Dacic S. Pulmonary lymphohistiocytic reactions temporally related to etanercept therapy. Mod Pathol. 2005;18:651-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Reid JD, Bressler B, English J. A case of adalimumab-induced pneumonitis in a 45-year-old man with Crohn’s disease. Can Respir J. 2011;18:262-264. [PubMed] |
| 6. | Sen S, Peltz C, Jordan K, Boes TJ. Infliximab-induced nonspecific interstitial pneumonia. Am J Med Sci. 2012;344:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C, Pego-Reigosa JM, Retamozo S, Bove A, Brito-Zeron P, Bosch X, Ramos-Casals M. Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthritis Rheum. 2011;41:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Komiya K, Ishii H, Fujita N, Oka H, Iwata A, Sonoda H, Kadota J. Adalimumab-induced interstitial pneumonia with an improvement of pre-existing rheumatoid arthritis-associated lung involvement. Intern Med. 2011;50:749-751. [PubMed] |
| 9. | Yamazaki H, Isogai S, Sakurai T, Nagasaka K. A case of adalimumab-associated interstitial pneumonia with rheumatoid arthritis. Mod Rheumatol. 2010;20:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |