Published online Feb 21, 2015. doi: 10.3748/wjg.v21.i7.2242
Peer-review started: July 2, 2014
First decision: July 21, 2014
Revised: August 10, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: February 21, 2015
Processing time: 224 Days and 16.5 Hours
Myeloid sarcomas (MS) involve extramedullary blast proliferation from one or more myeloid lineages that replace the original tissue architecture, and these neoplasias are called granulocytic sarcomas, chloromas or extramedullary myeloid tumors. Such tumors develop in lymphoid organs, bones (e.g., skulls and orbits), skin, soft tissue, various mucosae, organs, and the central nervous system. Gastrointestinal (GI) involvement is rare, while the occurrence of myeloid sarcomas in patients without leukemia is even rare. Here, we report a case of a 38-year-old man who presented with epigastric pain and progressive jaundice. An upper GI endoscopy had shown extensive multifocal hyperemic fold thickening and the spread of nodular lesions in the body of the stomach. Biopsies from the gastric lesions indicated myeloid sarcoma of the stomach. However, concurrent peripheral blood and bone marrow examinations showed no evidence of acute myeloid leukemia. For diagnosis, the immunohistochemical markers must be checked when evaluating a suspected myeloid sarcoma case. Accurate MS diagnosis determines the appropriate therapy and prognosis.
Core tip: Myeloid sarcomas (MS) are extramedullary tumors of myeloblasts, myeloid precursors, and neutrophils in various combinations. MS may be an initial indication for acute myeloid leukemia (AML), or they present during leukemic relapses. The small intestine is a relatively common location for these tumors, and their usual presentation includes abdominal pain from a small bowel obstruction. Gastric MS are rarely reported. Here, we report a rare case of gastric MS without AML. The diagnosis was verified by biopsies of the gastric tissues and bone marrow and immunohistochemical staining.
- Citation: Huang XL, Tao J, Li JZ, Chen XL, Chen JN, Shao CK, Wu B. Gastric myeloid sarcoma without acute myeloblastic leukemia. World J Gastroenterol 2015; 21(7): 2242-2248
- URL: https://www.wjgnet.com/1007-9327/full/v21/i7/2242.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i7.2242
Myeloid sarcomas (MS) are extramedullary tumors of myeloblasts, myeloid precursors, and neutrophils in various combinations, and they involve extramedullary blast proliferation from one or more myeloid lineages that replace the original tissue architecture. These tumors are also known as granulocytic sarcomas, chloromas or extramedullary myeloid tumors[1]. These tumors may be an initial indication for acute myeloid leukemia, indicate a leukemic relapse, or signal impending blast crisis in the setting of a myeloproliferative disorder or leukemic transformation in myelodysplastic syndromes, and they have also been found after allogeneic bone marrow transplantation. MS have been reported at nearly every anatomic site, but they are most commonly found in bones, periosteum, lymph nodes, and skin, and they have also been reported in the gastrointestinal (GI) tract, breast, testis, uterus, cervix, vagina, salivary glands, mediastinum, pleura, and peritoneum[2]. The GI tract is also a relatively common location for these tumors, and their typical presentation includes abdominal pain from a small bowel obstruction. Other regions of the GI system that have been reported to be involved include the stomach, colon, appendix, mesentery, and biliary tract. Many MS patients have either coexisting acute myeloid leukemia (AML) or a myeloproliferative or myelodysplastic disorder at the time of diagnosis, or these tumors appear at the first sign of relapse from one of these disorders. Gastric MS without AML involvement is rare[3]. Here, we report a rare case of gastric MS presenting as a spread of nodular lesions without myeloblastic leukemia.
A 38-year-old man who presented with intermittent dull epigastric pain, anorexia and weight loss for 20 d was admitted into our hospital. Pain was obvious at night and not associated with food ingestion, but it was not relieved after pharmacological treatment with a proton pump inhibitor and a mucosal protective agent. The patient also complained of persistent jaundice, abdominal distension and whole body itching. General physical examination revealed no abnormalities with the exception of moderate tenderness over the epigastrium and liver region pain. No rebound tenderness was present.
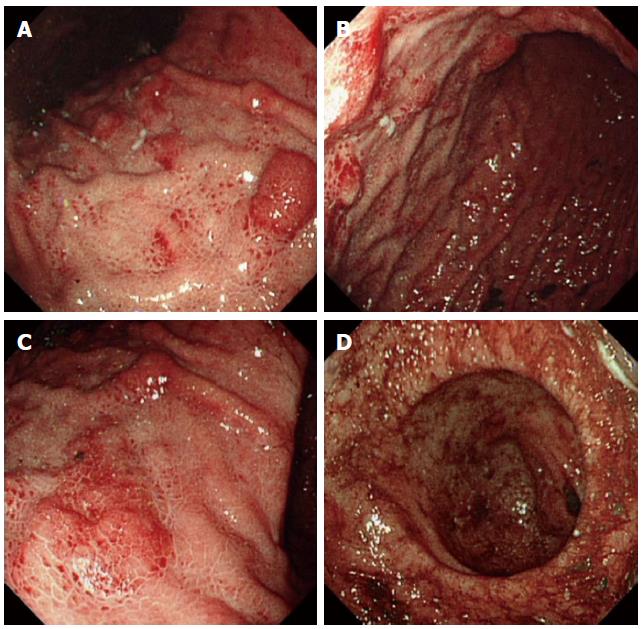
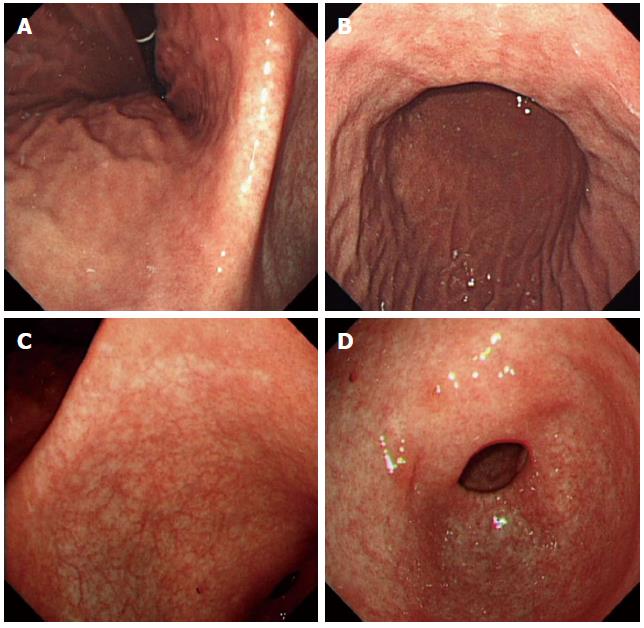
Clinical laboratory tests indicated that the patient’s total bilirubin significantly increased (mainly direct bilirubin) and that his lipase was slightly elevated. The tumor marker carbohydrate antigen 199 had increased approximately ten-fold. The complete blood count, liver enzymes, renal function, and electrolytes were all within normal limits. Ultrasonic examination and a computed tomography (CT) scan of the abdomen showed pancreatitis and intrahepatic and extrahepatic bile duct dilation. Contrast-enhanced ultrasonic examination showed abnormalities in the proximal common bile duct and a suspected cholangiocarcinoma. An upper GI endoscopy showed extensive multifocal hyperemic fold thickening and the spread of nodular lesions in the body of the stomach (Figure 1). A colonoscopy and capsule endoscopy showed smooth intestinal mucosa without erosion, an ulcer, or a neoplasm (data not shown).
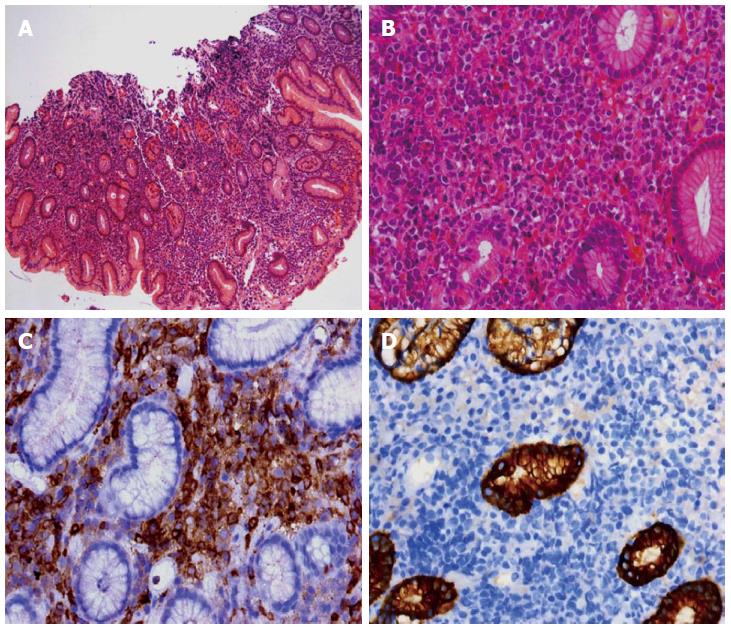
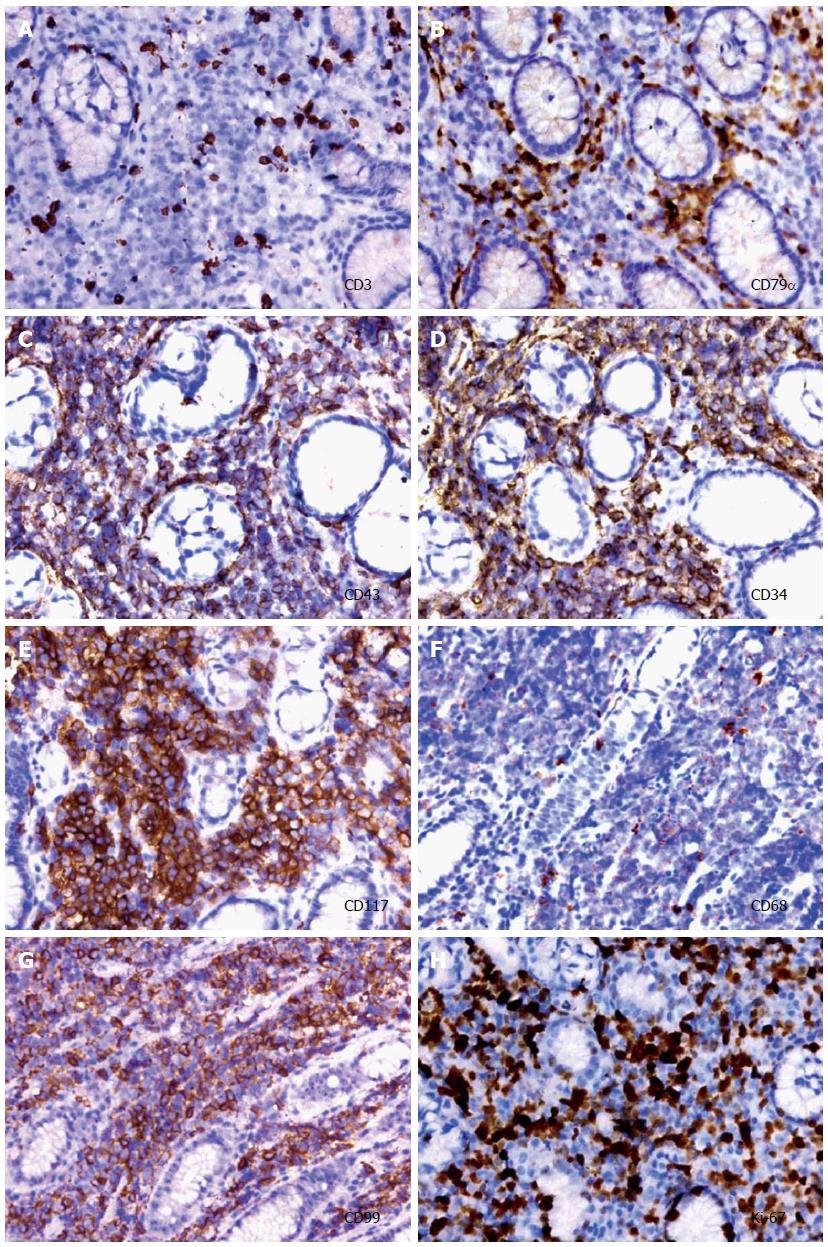
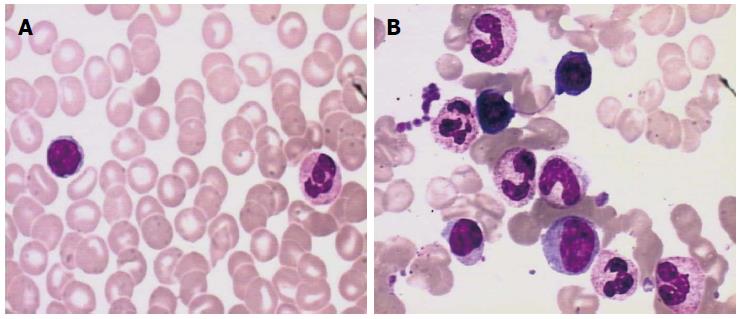
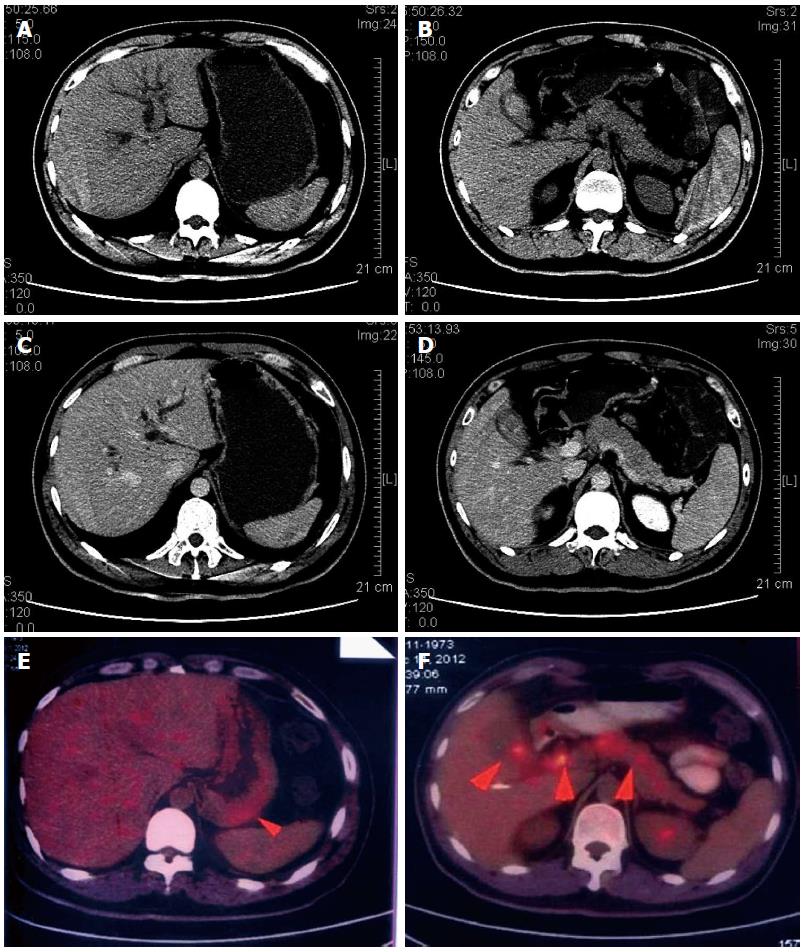
Gastric biopsies showed a decrease in mucosal glands in the lamina propria, the infiltration of many lymphocytes, including atypical cells, and strong myeloperoxidase (MPO) expression with negative cytokeratin staining (Figure 2). The pathological characteristics did not support a diagnosis of Crohn’s disease. Immunohistochemistry indicated that a gastric specimen was negative for CD3, CD15, CD20, CD79α, CD45RO, EMA and Pax-5, but was positive for CD99 (strongly +), CD43(+), CD34 (strongly +), CD117 (strongly +), CD68 (weakly +), Vim(+), κ (weakly +), and λ (weakly +), and the percentage of Ki-67-positive cells was greater than 70% (Figure 3), which is consistent with a diagnosis of gastric MS. A peripheral blood smear and bone marrow biopsy revealed that the patient’s hemocytes and bone marrow were normal without myeloblastic leukemia (Figure 4). Positron emission tomography (PET)-CT imaging with 18F-fluorodeoxyglucose (18F-FDG) showed a significant uptake in the gastric greater curvature (2.0 cm × 2.3 cm), and the tumor infiltrated the gallbladder (1.8 cm × 1.4 cm), pancreas (1.0 cm × 0.9 cm), common bile duct (1.4 cm × 1.4 cm), gastric lesser omental bursa (0.8 cm × 0.9 cm) and retroperitoneal lymph nodes (0.4 cm × 0.9 cm), and partial bones were involved, including the left humerus (0.5 cm × 0.8 cm), left ribs (0.3 cm × 0.5 cm), vertebra (0.4 cm × 0.6 cm), sacrum (0.5 cm × 0.8 cm), and right ilium (0.6 cm × 0.8 cm) (Figure 5).
After a definitive diagnosis of gastric MS, the patient received percutaneous transhepatic cholangiography drainage with ultrasonic guidance and was treated with standard systemic idarubicin and cytarabine (Ara-C) chemotherapy. Follow-up FDG-PET-CT reexamination 3 mo after chemotherapy demonstrated that most of the hypermetabolic lesions were gone. An upper GI endoscopy showed that the gastric mucosa was smooth without nodular lesions at 3 mo (data not shown) and 1 year after the completion of chemotherapy (Figure 6). In addition, gastric biopsies showed the infiltration of a few lymphocytes in the mucosa without atypical cells and MPO expression (data not shown).
MS involve extramedullary blast proliferation from one or more myeloid lineages, which replace the original tissue architecture. MS was first described by Burns in 1811, it was originally called a chloroma by King in 1853 because of its production of MPO, and its association with leukemia was established by Dock in 1893. MS have been reported at nearly every anatomic site. In an analysis of 92 patient cases, Pileri et al[4] found that the most common sites for MS are the skin (28.2%), lymph nodes (16.3%), testes (6.5%), intestines (6.5%), bones (3.25%) and the central nervous system (3.25%). MS occur over a wide range of ages, and it is more common in children than in adults (in some studies pediatric patients have an incidence of up to 30% vs 2%-5% for adult patients)[5]. MS usually present with AML, chronic myeloid leukemia, myeloproliferative neoplasms, or myelodysplastic syndrome either at their initial diagnosis or relapse[6].
In the present case, the patient presented with epigastric pain and progressive jaundice with no fever, anemia, enlarged lymph nodes or any other symptom of leukemia and no bone marrow involvement; thus, the diagnosis was difficult. The identification of immunohistochemical markers will be helpful for diagnosis. Several markers have been employed for MS identification. Menasce et al[7] suggested that histochemical staining for chloroacetate esterase and an immunohistochemical assay of MPO, lysozyme and CD43 are capable of indicating myeloid differentiation, and immunohistochemical staining for CD79a and CD3 is able to exclude the B- and T-cell lineages, respectively. With the exception of anti-CD43 and anti-lysozyme, more specific markers for myeloid disease, such as CD33, MPO, CD34 and CD117, are necessary for establishing a correct diagnosis[7]. Other antibodies may be added depending on differential diagnoses[8]. Differential diagnoses for MS are difficult, particularly for an isolated MS in the absence of a history of leukemia, myelodysplastic syndrome, a myeloproliferative neoplasm, or negative bone morrow biopsy. The most important differential diagnoses include non-Hodgkin lymphoma of the lymphoblastic type, Burkitt’s lymphoma, large-cell lymphoma and small round cell tumors[9]. MS are easily misdiagnosed in patients without a history of leukemic disease. For MS diagnoses, clinical features, radiological characteristics, immunohistochemical markers and cytogenetics must be considered. PET-CT is a useful tool for diagnoses and determining the location of MS[10].
Clinical behavior and therapeutic response are not influenced by age, sex, anatomic site, presentation, histotype, phenotype or cytogenetic findings[4]. For MS treatment, chemotherapy had been found more effective than radiation therapy or surgical resection for improving disease-free intervals or disease-free survival[11,12]. Treatment strategies are largely dependent on the development of the disease at the initial diagnosis or relapse, e.g., intensive chemotherapy is necessary for initial MS treatment, and inductive chemotherapy may be more effective for relapsed MS[13]. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) may provide brighter prospects for MS[14]. However, the effects of allo-HSCT for MS remain unknown.
The prognosis of MS is difficult to establish and is related to the AML clinical course, and the long-term prognosis of MS is poor. The median survival for MS patients without AML has been reported to be 36 mo, while for those progressing toward AML, it is 6 to 14 mo[15]. Thus, early diagnosis and appropriate treatment strategies should be used for patients before they progress to AML, thus ensuring a better prognosis.
The myeloid sarcoma patient had no acute myeloblastic leukemia.
Myeloid sarcoma (MS) lesions were found by upper gastrointestinal endoscopy, and they were identified by gastric biopsies.
Because myeloid sarcoma usually presents with acute myeloblastic leukemia, a bone marrow biopsy was inevitably used to exclude acute myeloblastic leukemia.
Positron emission tomography and computerized tomography imaging with 18F-fluorodeoxyglucose showed a significant uptake in the gastric greater curvature; the tumor infiltrated the gallbladder, pancreas, common bile duct, gastric lesser omental bursa and retroperitoneal lymph nodes; partial bones were involved including the left humerus, left ribs, vertebra, sacrum, and right ilium; and the primary gastric myeloid sarcoma identified was invasive and metastasized.
Hematoxylin and eosin staining revealed the infiltration of many lymphocytes including some atypical cells in a gastric specimen, and immunohistochemical staining suggested the diagnosis of a myeloid sarcoma.
The patient was treated with standard systemic idarubicin and cytarabine chemotherapy.
Myeloid sarcoma could occur at any site in the body. However, cases of gastric myeloid sarcoma without acute myeloblastic leukemia are quite rare.
The prognosis for myeloid sarcomas is difficult and related to the clinical course of acute myeloid leukemia (AML), and the long-term prognosis for MS is poor. Early diagnosis and appropriate treatment strategies should be performed in patients before MS progress to AML, thus ensuring a better prognosis.
This is a very interesting manuscript about a rare tumor occurring in the stomach. Its particular feature as its appearance without acute myeloblastic leukemia makes it more interesting.
P- Reviewer: Cimpean AM, Kitagawa M, Unal E S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Vardiman JW. The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: an overview with emphasis on the myeloid neoplasms. Chem Biol Interact. 2010;184:16-20. [PubMed] [DOI] [Full Text] |
| 2. | Chennareddy SB, Chennareddy SP, Polidori G, Eisenberg L, Saleh HA, Bergsman KL. Gastric granulocytic sarcoma as a cause of acute upper gastrointestinal bleeding. Am J Gastroenterol. 1996;91:609-611. [PubMed] |
| 3. | Narayan P, Murthy V, Su M, Woel R, Grossman IR, Chamberlain RS. Primary myeloid sarcoma masquerading as an obstructing duodenal carcinoma. Case Rep Hematol. 2012;2012:490438. [PubMed] [DOI] [Full Text] |
| 4. | Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero D. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21:340-350. [PubMed] |
| 5. | Byrd JC, Edenfield WJ, Shields DJ, Dawson NA. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol. 1995;13:1800-1816. [PubMed] |
| 6. | Sivan-Hoffmann R, Waksman I, Cohen HI, Eitan A. Small bowel obstruction as a presenting sign of granulocytic sarcoma. Isr Med Assoc J. 2011;13:507-509. [PubMed] |
| 7. | Menasce LP, Banerjee SS, Beckett E, Harris M. Extra-medullary myeloid tumour (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathology. 1999;34:391-398. [PubMed] |
| 8. | Klco JM, Welch JS, Nguyen TT, Hurley MY, Kreisel FH, Hassan A, Lind AC, Frater JL. State of the art in myeloid sarcoma. Int J Lab Hematol. 2011;33:555-565. [PubMed] [DOI] [Full Text] |
| 9. | Audouin J, Comperat E, Le Tourneau A, Camilleri-Broët S, Adida C, Molina T, Diebold J. Myeloid sarcoma: clinical and morphologic criteria useful for diagnosis. Int J Surg Pathol. 2003;11:271-282. [PubMed] |
| 10. | Ueda K, Ichikawa M, Takahashi M, Momose T, Ohtomo K, Kurokawa M. FDG-PET is effective in the detection of granulocytic sarcoma in patients with myeloid malignancy. Leuk Res. 2010;34:1239-1241. [PubMed] [DOI] [Full Text] |
| 11. | Paydas S, Zorludemir S, Ergin M. Granulocytic sarcoma: 32 cases and review of the literature. Leuk Lymphoma. 2006;47:2527-2541. [PubMed] |
| 12. | Schäfer HS, Becker H, Schmitt-Gräff A, Lübbert M. Granulocytic sarcoma of Core-binding Factor (CBF) acute myeloid leukemia mimicking pancreatic cancer. Leuk Res. 2008;32:1472-1475. [PubMed] [DOI] [Full Text] |
| 13. | Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785-3793. [PubMed] [DOI] [Full Text] |
| 14. | Chevallier P, Mohty M, Lioure B, Michel G, Contentin N, Deconinck E, Bordigoni P, Vernant JP, Hunault M, Vigouroux S. Allogeneic hematopoietic stem-cell transplantation for myeloid sarcoma: a retrospective study from the SFGM-TC. J Clin Oncol. 2008;26:4940-4943. [PubMed] [DOI] [Full Text] |