Published online Feb 21, 2015. doi: 10.3748/wjg.v21.i7.2183
Peer-review started: June 1, 2014
First decision: July 21, 2014
Revised: August 12, 2014
Accepted: September 5, 2014
Article in press: September 5, 2014
Published online: February 21, 2015
Processing time: 255 Days and 21.8 Hours
AIM: To perform a profiling analysis of changes in intestinal microRNA (miRNA) expression during hypothermic circulatory arrest (HCA).
METHODS: A total of eight piglets were randomly divided into HCA and sham operation (SO) groups. Under general anesthesia, swine in the HCA group were subjected to hypothermic cardiopulmonary bypass at 24 °C followed by 80 min of circulatory arrest, and the reperfusion lasted for 180 min after cross-clamp removal. The counterparts in the SO group were only subjected to median sternotomy. Histopathological analysis was used to detect mucosal injury, and Pick-and-Mix custom miRNA real-time polymerase chain reaction (PCR) panels containing 306 unique primer sets were utilized to assay unpooled intestinal samples harvested from the two groups.
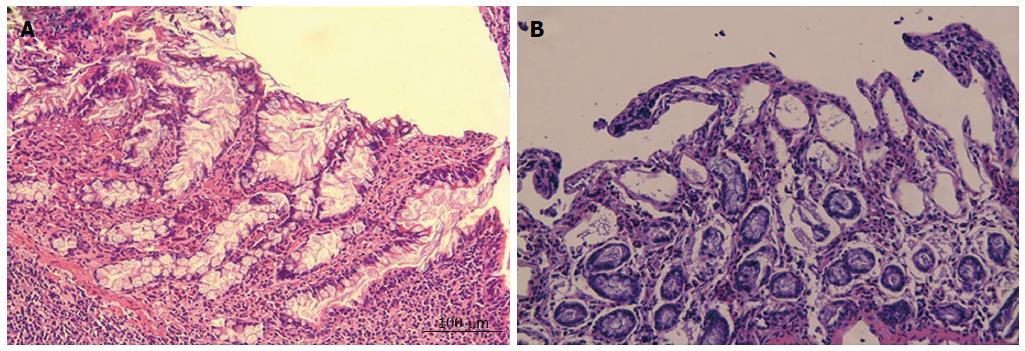
RESULTS: The intestinal mucosa of the animals that were subjected to 24 °C HCA exhibited representative ischemic reperfusion injury of grade 2 or 3 according to the Chiu score. Such intestinal mucosal injuries, with the subepithelial space and epithelial layer lifting away from the lamina propria, were accompanied by shortened and irregular villi. On the contrary, the intestinal mucosa remained normal in the sham-operated animals. In total, twenty-five miRNAs were differentially expressed between the two groups (15 upregulated and 10 downregulated in the HCA group). Among these, eight miRNAs (miR-122, miR-221-5p, miR-31, miR-421-5p, miR-4333, miR-499-3p, miR-542 and let-7d-3p) were significantly dysregulated (four higher and four lower). The expression of miR-122 was significantly (5.37-fold) increased in the HCA group vs the SO group, indicating that it may play a key role in HCA-induced mucosal injury.
CONCLUSION: Exposure to HCA caused intestinal miRNA dysregulation and barrier dysfunction in swine. These altered miRNAs might be related to the protection or destruction of the intestinal barrier.
Core tip: Swine intestine was subjected to hypothermia, cardiopulmonary bypass and ischemia/reperfusion following hypothermic circulatory arrest (HCA). These factors caused barrier dysfunction, resulting in gastrointestinal complications. Histopathological and microRNA (miRNA) array analyses were used to investigate the effects of HCA on the gut barrier. HCA was found to disturb barrier function in the small intestine and influence the miRNA levels in swine. Our results contribute to the body of research examining gut barrier function following HCA in vivo.
- Citation: Lin WB, Liang MY, Chen GX, Yang X, Qin H, Yao JP, Feng KN, Wu ZK. MicroRNA profiling of the intestine during hypothermic circulatory arrest in swine. World J Gastroenterol 2015; 21(7): 2183-2190
- URL: https://www.wjgnet.com/1007-9327/full/v21/i7/2183.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i7.2183
Since Bigelow performed the first experiments on hypothermic circulatory arrest (HCA) in 1950, HCA has become a vital technique in surgery for aortic and congenital heart disease. HCA can decrease the metabolic rate of tissues throughout the body and provides a bloodless surgical field while increasing complications such as edema formation, coagulopathy and organ dysfunction. On the grounds that the brain is the organ that is the most sensitive to ischemia, many studies have focused on neurological impairment associated with HCA, while only a few studies have focused on other organs, such as the kidney, intestines and spinal cord.
The prognosis after cardiopulmonary bypass (CPB) is closely related to the degree of gastrointestinal complications[1]. Even simple CPB will impair gastrointestinal function[2]. Although the underlying mechanism remains unclear, possible explanations for these complications include gastrointestinal hypoperfusion[3,4], gut barrier dysfunction, preoperative immune function defects, the change in the blood capillary permeability of the intestinal wall, and the systemic inflammatory response. Furthermore, HCA may increase the risk associated with gut barrier dysfunction[5]. Due to HCA, the intestines suffer from ischemic reperfusion injury (IRI).
MicroRNAs (miRNAs) are small noncoding RNAs that are capable of silencing gene expression post-transcriptionally. In recent years, research on miRNA has been mainly focused on cancer, while miRNA expression following IRI has remained poorly understood. Growing evidence indicates that some miRNAs are related to IRI, such as miRNA expression in the liver[6], kidney[7], muscle[8] and flap[9]; however, there are no reports on miRNA expression in intestinal IRI following HCA.
We postulated that miRNA is also associated with intestinal IRI during HCA. The present study aims to identify the influence of HCA on microRNA expression in the intestine by using miRNA polymerase chain reaction (PCR) arrays in swine.
A total of 8 Wuzhishan pigs (6 to 8-wk-old, weight 9.7-13 kg, average 11.66 kg) were randomly assigned into HCA 24 °C and sham operation (SO) groups. The experiment was conducted in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1996) and the Guidelines for Animal Experimentation, which were issued by the First Affiliated Hospital of Sun Yat-sen University.
The animals were sedated with intramuscular ketamine hydrochloride and maintained on IV infusions of ketamine (40 mg/kg) and fentanyl (2 μg/kg per hour) after endotracheal intubation via tracheotomy. Following this step, an arterial pressure catheter was inserted into the right femoral artery for pressure monitoring and blood sampling. Then, an 8-Fr central venous catheter was inserted via the left femoral vein for fluid administration and central venous pressure monitoring.
A median sternotomy was performed in both groups. Next, each of the piglets in the HCA group was administered heparin sodium (400 IU/kg). The ascending aorta was cannulated with a single 10F arterial cannula, and the superior and inferior vena cava were both cannulated with 12F cannulas. The CPB circuit consisted of a roller pump, a membrane oxygenator (SK3301, Medtronic Inc, Minneapolis, MN, United States), and a heat exchanger (Sarns Heater Cooler, Ann Arbor, MI, United States). The circuit was primed with electrolyte solution, blood from a donor pig and heparin (3 mg/100 mL total fluid). A mean arterial pressure of 50 to 80 mmHg was maintained by keeping the CPB flow rate between 75 mL/kg per minute and 80 mL/kg per minute in the CPB group.
After initiation of CPB, the animals were cooled to a target nasopharyngeal temperature of 30 °C by a heat exchanger (Sarns, Ann Arbor, MI, United States). After the body temperature lowered to 30 °C, the ascending aorta was cross-clamped, and the cardioplegia solution was administered. Topical ice slush was used to cool the surface of the heart throughout the HCA procedure. The heat exchanger operated until the body temperature was cooled down to 24 °C. Then, the roller pumps were turned off, and HCA was performed for the next 80 min. Next, the aorta was declamped, and the animal was rewarmed to normothermia in 60 min using a temperature gradient of 8 °C. For the hearts that encountered deleterious arrhythmia after de-clamping, direct-current defibrillation was used to restore the sinus rhythm. The animals were weaned off from CPB when the hemodynamic data became steady after partial perfusion, and the reperfusion lasted for 180 min following the removal of the cross-clamping. When the experiment was complete, midline laparotomy was performed to obtain specimens from the terminal ileum, which were preserved at -80 °C.
RNA was isolated from cryopreserved tissue using TRIzol (Invitrogen Life Technologies, Carlsbad, CA, United States) and subsequently precipitated, washed and redissolved. The RNA purity was measured using a NanoDrop ND-1000 spectrophotometer (Wilmington, DE, United States). cDNA was generated from 20 μL of RNA using the buffer and the enzymes that were provided in the Qiagen kit (Exiqon, Denmark). Pick-and-Mix custom panels (Exiqon, Denmark) containing 306 primer sets uniquely designed for microRNAs were chosen for miRNA expression profiling. The cDNA was diluted × 110 and assayed in 10-μL PCR reactions according to the protocol for miRCURY LNA TM Universal RT microRNA PCR. PCR was performed using the ABI PRISM 7900 system (Applied Biosystems, Inc., Foster City, CA, United States). The miRNA expression was normalized relative to the expression of U6 using the ΔΔCt method. Then, the fold change was calculated by using the ratio of miRNAHCA/miRNASO or miRNASO/miRNAHCA (when miRNAHCA was down-regulated).
Hematoxylin and eosin-stained sections from formalin-fixed and paraffin-embedded intestinal samples were assessed by two pathologists who were blinded to the study protocol according to Chiu’s method[10]. All samples were observed under a stereomicroscope (DM 2500B, Leica, Germany) at × 200 magnification using an objective lens with an aperture of 22 mm. Photomicrographs were obtained using the Leica Application Suite (Leica Microsystems, Wetzlar, Germany) and edited with Adobe Photoshop 12.0 (Adobe Systems Inc., San Jose, United States).
Significant differences between the two groups were tested using Student’s t-test, with a significance threshold of P < 0.05. miRNAs with expression changes of > 2-fold were considered to be differentially expressed. The statistical program SPSS 20.0 (SPSS Inc., Chicago, IL, United States) was used for statistical analysis.
No significant differences were observed in the intestinal mucosa between the two groups based on observations with the naked eye. However, histological tissue evaluations revealed that the structures of the mucosal epithelial layer and the lamina propria were well preserved in the SO group (Figure 1A). In contrast, the intestinal mucosa of the animals that were subjected to HCA demonstrated representative IRI of grade 2 or 3 according to the Chiu score (Figure 1B). This result indicates that the gut barrier function was interrupted following 80 min of ischemia and 180 min of reperfusion despite exposure to 24 °C hypothermia.
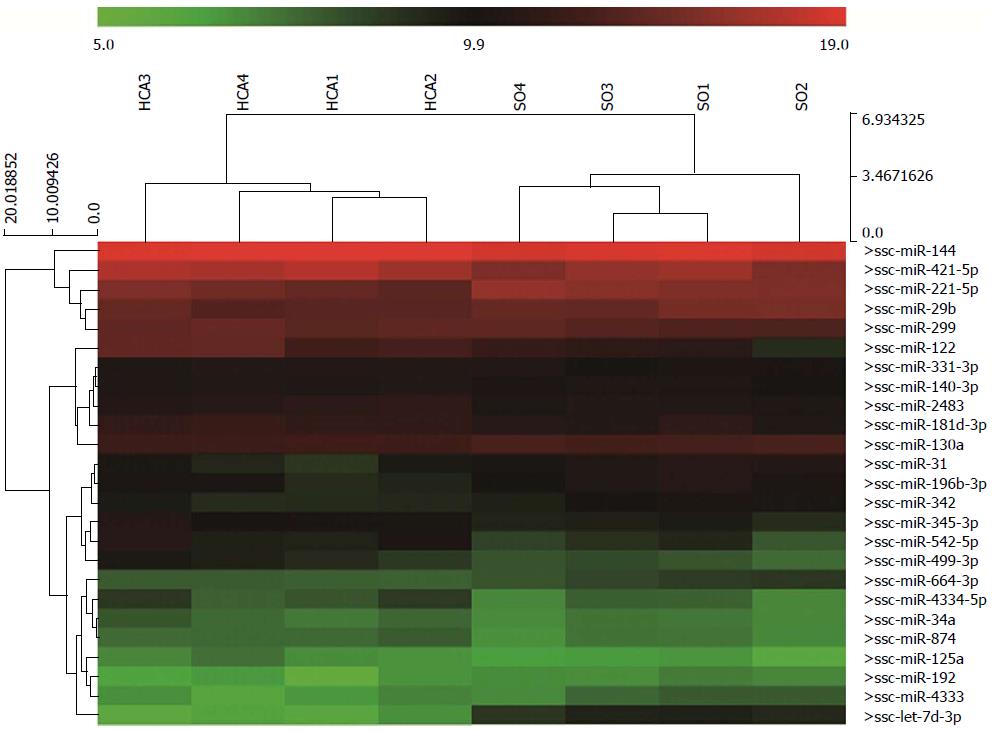
Relative to the SO group, miRNA profiling revealed that 25 miRNAs were differentially expressed (P < 0.05) in the intestine after 80 min of ischemia and 180 min of reperfusion (Figure 2 and Table 1). Of the 25 miRNAs, miR-122, -542-5p, -499-3p, -421, let-7d-3p, miR-31, -221-5p and -4333 were diversely expressed between the HCA and SO groups for P < 0.05 and a fold change > 2 levels of significance. In Table 2, we summarize six miRNAs that were previously implicated in IRI. Although these six miRNAs were not significantly expressed in the intestine, a lower miR-192 level was observed compared to the SO group (Table 2).
| Up-regulation | FC | P value | Down-regulation | FC | P value | |
| 1 | miR-122 | 5.37 | 0.0193 | let-7d-3p | 8.73 | 0.0000 |
| 2 | miR-542-5p | 2.64 | 0.0273 | miR-31 | 2.33 | 0.0141 |
| 3 | miR-499-3p | 2.47 | 0.0026 | miR-221-5p | 2.18 | 0.0248 |
| 4 | miR-421-5p | 2.41 | 0.0141 | miR-4333 | 2.01 | 0.0219 |
| 5 | miR-4334-5p | 1.95 | 0.0344 | miR-29b | 1.89 | 0.0213 |
| 6 | miR-345-3p | 1.73 | 0.0212 | miR-196b-3p | 1.79 | 0.0440 |
| 7 | miR-181d-3p | 1.63 | 0.0211 | miR-192 | 1.63 | 0.0494 |
| 8 | miR-2483 | 1.59 | 0.0074 | miR-664-3p | 1.55 | 0.0085 |
| 9 | miR-125a | 1.52 | 0.0487 | miR-342 | 1.49 | 0.0266 |
| 10 | miR-874 | 1.48 | 0.0226 | miR-130a | 1.40 | 0.0006 |
| 11 | miR-299 | 1.47 | 0.0440 | |||
| 12 | miR-34a | 1.41 | 0.0489 | |||
| 13 | miR-331-3p | 1.38 | 0.0392 | |||
| 14 | miR-140-3p | 1.36 | 0.0232 | |||
| 15 | miR-144 | 1.35 | 0.0166 |
| miRNA | miR-10a-3p | miR-10a-5p | miR-21 | miR-193a-3p | miR-210 | miR-205 |
| FC | -1.23 | -1.16 | -1.11 | -1.11 | -1.41 | -1.17 |
| P value | 0.3752 | 0.5034 | 0.6219 | 0.7919 | 0.0952 | 0.6146 |
To date, no data are available regarding the intestinal miRNA expression profile during an HCA procedure. In the present study, we detected twenty-five dysregulated miRNAs and injury to the mucosa barrier in the intestine following HCA. The factors that may have influenced miRNA expression in this study included hypothermia, CPB and circulatory arrest. Firstly, hypothermia may induce miRNA dysregulation. For example, higher miR-21 expression levels have been detected in skeletal muscle and liver at -3 °C compared to 5 °C in vivo[11]. Interestingly, in another tissue miRNA study performed in a traumatic brain injury rat model, miR-874 was markedly differentially expressed under hypothermia conditions (33 °C) compared to normothermia (37 °C)[12]. Specifically, temperature-dependent miRNA modulation was mediated by RNA-binding motif protein 3[13]. These studies were not performed at 24 °C; nevertheless, they reflected the effect of hypothermia on miRNA. Therefore, the modulated miRNAs in our study (let-7d-3p, miR-29b, miR-125a, miR-130a, miR-874) may be temperature-dependent miRNAs that are affected by HCA. Secondly, changes in the miR-499 levels in the myocardium during CPB were reported[14]; however, no change about miR-499 was reported in the intestine. miR-499 is an acute myocardial infarction marker that is overexpressed in cardiac IRI. In a study by Reddy et al[15], the authors stated that miR-499 is cardiac cell-specific in swine and cannot be detected in other organs; however, only the stomach was included in this previous study, and the intestine was not analyzed. Our data revealed differential miR-499 expression between the normal and experimental intestines, indicating that miR-499 may be a marker of IRI in the intestine, distinct from the heart. However, with the present study design, we cannot differentiate among the hypothermia-induced, CPB-induced and IRI-induced miRNA changes. Therefore, additional studies are needed to assess the individual impact of these factors. Thirdly, few studies examining alterations in the expression of miRNAs in the intestine subjected to IRI have been reported, although miRNAs were found to be altered in other organs subjected to IRI. Nevertheless, the dysregulation of several miRNAs was found in other organs subjected to IRI[9,16-18], including the liver (miR-146), kidney (miR-10a, miR-30d), and skin flap (miR-21, miR-96, miR-193-3p, miR-210). However, the miRNA expression found in our study was quite different than in other IRI studies. The decreased levels of miR-192 that we detected were consistent with a previous report on renal IRI[18]. This phenomenon also suggests that IRI is not the only factor related to intestinal miRNA changes following HCA. Consequently, this finding suggests that the superior mesenteric artery ligation model may not be suitable to mimic the intestinal pathophysiology changes in cardiac surgery.
The histological change of the intestinal mucosa is one of the key factors used to evaluate gut barrier function. Our results suggest that the gut barrier function was compromised in animals subjected to 24 °C HCA. Altered levels of several miRNAs were associated with barrier dysfunction, indicating that they may play a protective/detrimental role in HCA. Cytokines, such as IL-6 and IL-10, are key inflammation regulators of CPB-induced intestinal dysfunction mediated by the NF-kB pathway. Let-7d[19,20] and miR-421[21] were hypothesized to promote gut barrier conservation by reducing the cytokine level. However, in the present study, the let-7d expression level decreased, while the miR-421 level increased in response to HCA. Therefore, although high miR-421 levels may benefit the intestinal mucosa, reduced let-7d-3p levels are likely to negatively affect the intestinal mucosa.
Our morphological results also confirmed that IRI caused by HCA leads to barrier dysfunction. Hypoxia inducible factor (HIF) is activated by IRI[22], resulting in intestinal mucosa barrier dysfunction[23]. HIF is down-regulated by factor inhibiting HIF (FIH-1)[24], a target gene of miR-31[25], suggesting that the decreased miR-31 expression observed in our study may protect the intestinal barrier. The CPB procedure activates several pathways, including oxidative stress, which dysregulates senescence-associated miR-542 expression[26] and inhibits cell proliferation in vitro[27]. Ultimately, high miR-542 levels might injure the mucosa in our model. miR-122 showed significantly (5.37-fold) increased expression in the HCA group vs the SO group. During myocardial IRI, the activity of myocardial p38-MAPK may lead to the down-regulation of miR-122[28,29], suggesting that miR-122 may also show similar changes during IRI in other organs. On the contrary, miR-122 was shown to be up-regulated in intestinal IRI in our study, which may be due to a different activated sub-family of p38-MAPK or organ differences. In a recent study by Ye et al[30], it was shown that TNF-alpha may lead to increased miR-122 levels in the intestine, suggesting that miR-122 may be involved in TNF-alpha induced intestinal barrier dysfunction. However, NOD2 and TNF-alpha are two gut function-associated target genes of miR-122. Lower NOD2 levels protect intestinal epithelial cells by increasing anti-inflammatory cytokines[31], and reduced TNF-alpha levels alleviate intestinal IRI[32,33]. In contrast, elevated miR-122 levels are postulated to protect barrier function in our HCA model.
IRI can lead to increased miR-221 expression through the high-mobility group box 1 protein pathway[34,35]; however, the miR-221 level was decreased in HCA, suggesting that hypothermia or CPB influence the expression of miR-221 in HCA. Low miR-221 expression is related to high TNF mRNA expression[36,37]; therefore, miR-221 down-regulation may be detrimental to intestinal epithelial cells via activation of the NF-kB pathway[38].
There are several advantages of the swine model, such as steady hemodynamics, tolerance of long duration circulatory arrest and similarity to human beings. Furthermore, the age of six to eight weeks in swine likely corresponds to the relevant age in human infants for congenital heart disease. This model more closely resembles the real disease state in human beings, while animals such as rats, canines and rabbits have failed to match closely with real disease states. Therefore, we selected the swine as a model of HCA. Little is known about mucosa injuries caused by circulatory arrest. Karhausen reported that 45 min of circulatory arrest can lead to obvious rat intestinal mucosa IRI[39]. Although the duration of ischemia was longer in our study, minor microscopic changes were found. A possible explanation might be that the rat intestine is more sensitive to ischemia or temperature changes than it is to the duration of the ischemia.
One limitation of this study is that we did not set up groups of cardiopulmonary hypothermia without circulatory arrest or circulatory arrest at normal temperatures. Therefore, although intestinal miRNA could be influenced by HCA, we were not able to detect the most significant factor among hypothermia, CPB and circulatory arrest during HCA. However, the animal model that we aim to establish can be used to simulate the clinical surgical process. Therefore, circulatory arrest following atmospheric temperature and superior mesenteric artery ligation without CPB may not provide useful information. Another limitation of our study is that we did not link the dysregulated miRNAs with significant changes in pathological characteristics. Although many candidate genes are potentially targeted by the 7 identified miRNAs, only a few of these target genes were validated in other studies. We assessed the mutual target genes of these miRNAs, but the results we obtained are not ideal for determining the connection between miRNAs and the gut barrier, suggesting that the direct relationship between the genes and the pathological characteristics shown in our figures is difficult to identify. We hope that the initial data provided by our study may inspire other researchers to determine the connection between these miRNAs and the gut barrier.
In summary, intestinal barrier dysfunction and miRNA deregulation occurred when the swine were subjected to 24 °C HCA. Among these miRNAs, altered miR-122, miR-31 and miR-421-5p levels may protect barrier function, while altered miR-542-5p, let-7d-3p and miR-221-5p levels may negatively affect barrier function. Furthermore, miR-499 may be a marker of IRI in the intestine.
We would like to acknowledge the Key Laboratory on Assisted Circulation and KangChen Bio-tech Inc. for experimental assistance.
Although the incidence of gastrointestinal complications is low following cardiopulmonary bypass (CPB) during cardiac surgery, the complications may be serious. Hypothermic circulatory arrest (HCA), a special technique in CPB, may increase the risk associated with gut barrier dysfunction.
Due to HCA, ischemic reperfusion injury (IRI) occurs in the intestine. The study aimed to determine the influence of reperfusion injury during HCA on microRNA expression in the intestine.
The injury to the mucosal barrier in the intestine following HCA was validated in swine, which are large animals. Furthermore, miRNA dysregulation was observed following the HCA procedure.
A better understanding of the miRNAs involved in HCA related to intestinal mucosal injury may be helpful in developing strategies to protect organ function.
CPB is a technique that enables blood circulation throughout the body when the heart is stopped during cardiac surgery. HCA is a brief period during CPB in which the systemic circulation is shut down while maintaining heart and brain perfusion.
This manuscript concerns HCA-regulated miRNA expression in swine intestine. By conducting a miRNA array experiment, the authors targeted some miRNAs which might be significant in HCA or I/R-induced tissue injury. Overall, the research design is very decent with adequate animal experiment, miRNA sample preparation, and array profiling.
P- Reviewer: Hwang KC, Nasir O, Tsai YH S- Editor: Ma YJ L- Editor: Logan S E- Editor: Zhang DN
| 1. | Mangi AA, Christison-Lagay ER, Torchiana DF, Warshaw AL, Berger DL. Gastrointestinal complications in patients undergoing heart operation: an analysis of 8709 consecutive cardiac surgical patients. Ann Surg. 2005;241:895-901; discussion 901-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Haverich A, Hagl C. Organ protection during hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2003;125:460-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Croome KP, Kiaii B, Fox S, Quantz M, McKenzie N, Novick RJ. Comparison of gastrointestinal complications in on-pump versus off-pump coronary artery bypass grafting. Can J Surg. 2009;52:125-128. [PubMed] |
| 4. | Dong GH, Wang CT, Li Y, Xu B, Qian JJ, Wu HW, Jing H. Cardiopulmonary bypass induced microcirculatory injury of the small bowel in rats. World J Gastroenterol. 2009;15:3166-3172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | McMonagle MP, Halpenny M, McCarthy A, Mortell A, Manning F, Kilty C, Mannion D, Wood AE, Corbally MT. Alpha glutathione S-transferase: a potential marker of ischemia-reperfusion injury of the intestine after cardiac surgery? J Pediatr Surg. 2006;41:1526-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Chen Q, Kong L, Xu X, Geng Q, Tang W, Jiang W. Down-regulation of microRNA-146a in the early stage of liver ischemia-reperfusion injury. Transplant Proc. 2013;45:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Wang N, Zhou Y, Jiang L, Li D, Yang J, Zhang CY, Zen K. Urinary microRNA-10a and microRNA-30d serve as novel, sensitive and specific biomarkers for kidney injury. PLoS One. 2012;7:e51140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Hsieh CH, Jeng JC, Jeng SF, Wu CJ, Lu TH, Liliang PC, Rau CS, Chen YC, Lin CJ. MicroRNA profiling in ischemic injury of the gracilis muscle in rats. BMC Musculoskelet Disord. 2010;11:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Chang KP, Lai CS. Micro-RNA profiling as biomarkers in flap ischemia-reperfusion injury. Microsurgery. 2012;32:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1426] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 11. | Biggar KK, Dubuc A, Storey K. MicroRNA regulation below zero: differential expression of miRNA-21 and miRNA-16 during freezing in wood frogs. Cryobiology. 2009;59:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Truettner JS, Alonso OF, Bramlett HM, Dietrich WD. Therapeutic hypothermia alters microRNA responses to traumatic brain injury in rats. J Cereb Blood Flow Metab. 2011;31:1897-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Pilotte J, Dupont-Versteegden EE, Vanderklish PW. Widespread regulation of miRNA biogenesis at the Dicer step by the cold-inducible RNA-binding protein, RBM3. PLoS One. 2011;6:e28446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Qin H, Chen GX, Liang MY, Rong J, Yao JP, Liu H, Wu ZK. The altered expression profile of microRNAs in cardiopulmonary bypass canine models and the effects of mir-499 on myocardial ischemic reperfusion injury. J Transl Med. 2013;11:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Reddy AM, Zheng Y, Jagadeeswaran G, Macmil SL, Graham WB, Roe BA, Desilva U, Zhang W, Sunkar R. Cloning, characterization and expression analysis of porcine microRNAs. BMC Genomics. 2009;10:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Liu F, Lou YL, Wu J, Ruan QF, Xie A, Guo F, Cui SP, Deng ZF, Wang Y. Upregulation of microRNA-210 regulates renal angiogenesis mediated by activation of VEGF signaling pathway under ischemia/perfusion injury in vivo and in vitro. Kidney Blood Press Res. 2012;35:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Chang KP, Lee HC, Huang SH, Lee SS, Lai CS, Lin SD. MicroRNA signatures in ischemia-reperfusion injury. Ann Plast Surg. 2012;69:668-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci USA. 2010;107:14339-14344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 313] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 19. | Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 713] [Cited by in RCA: 714] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 20. | Jiang L, Cheng Z, Qiu S, Que Z, Bao W, Jiang C, Zou F, Liu P, Liu J. Altered let-7 expression in Myasthenia gravis and let-7c mediated regulation of IL-10 by directly targeting IL-10 in Jurkat cells. Int Immunopharmacol. 2012;14:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Spinelli SV, Diaz A, D’Attilio L, Marchesini MM, Bogue C, Bay ML, Bottasso OA. Altered microRNA expression levels in mononuclear cells of patients with pulmonary and pleural tuberculosis and their relation with components of the immune response. Mol Immunol. 2013;53:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1923] [Cited by in RCA: 1817] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 23. | Feinman R, Deitch EA, Watkins AC, Abungu B, Colorado I, Kannan KB, Sheth SU, Caputo FJ, Lu Q, Ramanathan M. HIF-1 mediates pathogenic inflammatory responses to intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2010;299:G833-G843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 503] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 25. | Olaru AV, Selaru FM, Mori Y, Vazquez C, David S, Paun B, Cheng Y, Jin Z, Yang J, Agarwal R. Dynamic changes in the expression of MicroRNA-31 during inflammatory bowel disease-associated neoplastic transformation. Inflamm Bowel Dis. 2011;17:221-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Faraonio R, Salerno P, Passaro F, Sedia C, Iaccio A, Bellelli R, Nappi TC, Comegna M, Romano S, Salvatore G. A set of miRNAs participates in the cellular senescence program in human diploid fibroblasts. Cell Death Differ. 2012;19:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Yoon S, Choi YC, Lee S, Jeong Y, Yoon J, Baek K. Induction of growth arrest by miR-542-3p that targets survivin. FEBS Lett. 2010;584:4048-4052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Thomas CJ, Ng DC, Patsikatheodorou N, Limengka Y, Lee MW, Darby IA, Woodman OL, May CN. Cardioprotection from ischaemia-reperfusion injury by a novel flavonol that reduces activation of p38 MAPK. Eur J Pharmacol. 2011;658:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Chen YJ, Chang LS. Hydroquinone-induced miR-122 down-regulation elicits ADAM17 up-regulation, leading to increased soluble TNF-α production in human leukemia cells with expressed Bcr/Abl. Biochem Pharmacol. 2013;86:620-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 31. | Chen Y, Wang C, Liu Y, Tang L, Zheng M, Xu C, Song J, Meng X. miR-122 targets NOD2 to decrease intestinal epithelial cell injury in Crohn’s disease. Biochem Biophys Res Commun. 2013;438:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Esposito E, Mazzon E, Muià C, Meli R, Sessa E, Cuzzocrea S. Splanchnic ischemia and reperfusion injury is reduced by genetic or pharmacological inhibition of TNF-alpha. J Leukoc Biol. 2007;81:1032-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Chen LW, Chang WJ, Chen PH, Liu WC, Hsu CM. TLR ligand decreases mesenteric ischemia and reperfusion injury-induced gut damage through TNF-alpha signaling. Shock. 2008;30:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Tetteh HA. The role of HMGB1 in ischemia-reperfusion injury in the rat small intestine. J Surg Res. 2013;183:96-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Galardi S, Mercatelli N, Farace MG, Ciafrè SA. NF-kB and c-Jun induce the expression of the oncogenic miR-221 and miR-222 in prostate carcinoma and glioblastoma cells. Nucleic Acids Res. 2011;39:3892-3902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Chou WW, Wang YT, Liao YC, Chuang SC, Wang SN, Juo SH. Decreased microRNA-221 is associated with high levels of TNF-α in human adipose tissue-derived mesenchymal stem cells from obese woman. Cell Physiol Biochem. 2013;32:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | El Gazzar M, McCall CE. MicroRNAs distinguish translational from transcriptional silencing during endotoxin tolerance. J Biol Chem. 2010;285:20940-20951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 38. | Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359-1377. [PubMed] |
| 39. | Karhausen J, Qing M, Gibson A, Moeser AJ, Griefingholt H, Hale LP, Abraham SN, Mackensen GB. Intestinal mast cells mediate gut injury and systemic inflammation in a rat model of deep hypothermic circulatory arrest. Crit Care Med. 2013;41:e200-e210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |