Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1989
Peer-review started: June 26, 2014
First decision: August 6, 2014
Revised: September 11, 2014
Accepted: October 14, 2014
Article in press: October 15, 2014
Published online: February 14, 2015
Processing time: 230 Days and 7.7 Hours
We present a rare case of intrahepatic cholangiocarcinoma (ICC) with multiple skeletal muscle metastases. The patient was a 55-year-old Asian woman presenting with abdominal pain; abdominal and pelvic computed tomography and magnetic resonance cholangiopancreatography revealed an unresectable ICC with hepatic metastasis and metastastatic lymphadenopathy in the porto-caval area. After 3 mo of treatment with palliative radiotherapy and chemotherapy, magnetic resonance imaging of the thoracolumbar spine detected right psoas muscle and paraspinous muscle metastases. We performed an ultrasound-guided percutaneous fine-needle biopsy that confirmed a similar pattern of poorly differentiated adenocarcinoma. The patient treated with palliative chemotherapy and achieved 10 mo of survival. Here we report the first case quickly spread to multiple sites of muscle even though the three-month treatment, compare to the other cases reported muscle metastases at diagnosis.
Core tip: The case presented with multiple skeletal muscle metastases from an intrahepatic cholangiocarcinoma which only 5 cases have been reported so far.
- Citation: Lee J, Lee SW, Han SY, Baek YH, Kim SY, Rhyou HI. Rapidly aggravated skeletal muscle metastases from an intrahepatic cholangiocarcinoma. World J Gastroenterol 2015; 21(6): 1989-1993
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1989.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1989
An intrahepatic cholangiocarcinoma (ICC) is a malignant tumor that originates in the small bile ducts. The risk factors for cholangiocarcinoma are primary sclerosing cholangitis, biliary-duct cysts, hepatolithiasis, liver fluke infections, and conditions such as hepatitis B infection, hepatitis C infection, obesity, and cirrhosis[1]. Surgical resection is the only treatment modality providing a chance for cure; the reported median survival, after surgical resection, ranges from 16 to 46 mo, with a 5-year survival rate of 21%-25%[2]. If the tumor is unresectable, the median survival is less than 1 year. In this setting, patients may need palliative therapies such as surgical or endoscopic biliary drainage, chemotherapy, radiation therapy, radiofrequency ablation, hepatic intraarterial chemotherapy, photodynamic therapy or stenting[3].
Common metastatic sites for ICC are the regional lymph nodes (hilar, peripancreatic, and periaortic lymph nodes) and adjacent organs. Hematogenous ICC metastases commonly occur to distant organs, such as the lungs, bones, and adrenal glands, but skeletal muscle metastasis is unusual for any malignancy. Here, we describe an unusual case of distant skeletal muscle metastasis from an ICC.
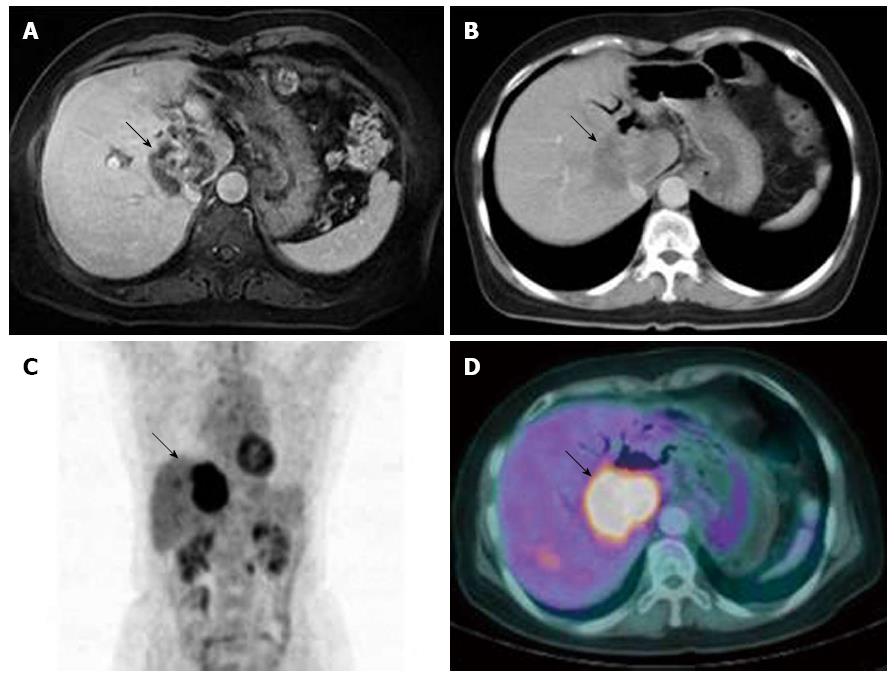
A 55-year-old Asian woman presented to our gastroenterology department with abdominal pain. Ultrasound and contrast-enhanced computed tomography (CT) examinations of her abdomen and pelvis, reported by the referring hospital, showed an ill defined, low-density lesion in segments S1, S5, and S8 of liver (Figure 1A). The patient’s past medical history included cholecystectomy with Roux-en-Y hepaticojejunostomy because of an intrahepatic duct stone, and common hepatic duct stones in 1995. In 2009, she had undergone coil-embolization for an unruptured aneurysm(12 mm × 9 mm × 10 mm) of her left internal carotid artery communicating segment. She denied tobacco or alcohol use, and her family medical history was unremarkable.
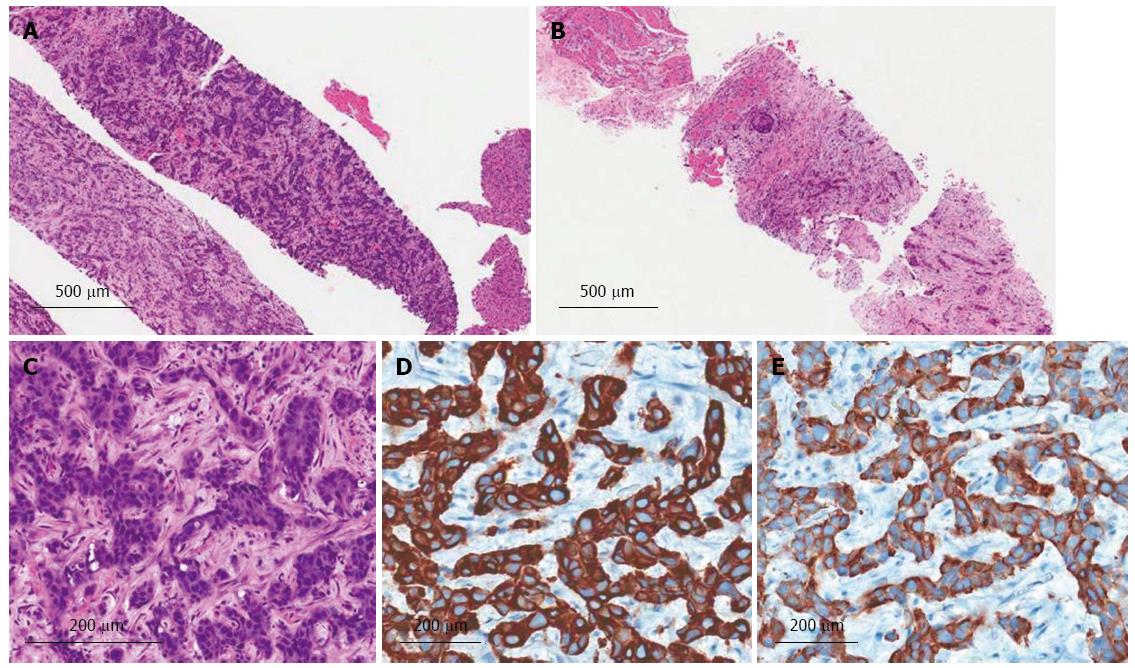
Upon admission, a physical examination determined her blood pressure (100/60 mmHg), pulse (70/min), respiration rate (20/min), and temperature (36.6 °C). Upon examination, the patient was not icteric but had right upper quadrant abdominal tenderness. Laboratory tests revealed her white blood cell 6330/μL (3000-9300/μL), platelet 214000/μL (140000-360000/μL), hemoglobin 11.9 g/dL (11-15 g/dL), blood urea nitrogen 24 mg/dL (8-26 mg/dL), creatinine 0.8 mg/dL (0.7-1.0 mg/dL), aspartate aminotransferase 32 IU/L (0-35 IU/L), alanine aminotransferase 29 IU/L (10-35 IU/L), alkaline phosphatase 589 IU/L (104-338 IU/L), total bilirubin 0.6 mg/dL (0.2-1.2 mg/dL), alpha-fetoprotein 4.54 ng/mL (0-15 ng/mL), carcinoembryonic antigen 9.45 ng/mL (0-8 ng/mL), and carbohydrate antigen 19-9 3.01 U/mL(0-37 U/mL). All hepatic viral markers were negative, as was her chest radiograph. Magnetic resonance cholangiopancreatography (MRCP) showed a 5.7 cm, heterogeneous hepatic mass in S1, S5, and S8, and multiple hepatic metastases in the right lobe (Figure 1B). An ultrasound-guided percutaneous needle biopsy of the low-echoic liver mass was performed; histopathology confirmed a poorly differentiated adenocarcinoma (Figure 2A and C), which was positive for cytokeratins 7 and 19 (Figure 2D and E). Positron-emission tomography-computed tomography (PET-CT) detected a 6 cm, hypermetabolic, intrahepatic malignancy involving S1, adjacent to the right lobe of the liver (maximum standardized uptake value, 10.41), multiple hepatic metastases in the right lobe, and metastatic lymphadenopathy in the porto-caval, common hepatic, and left para-aortic areas (Figure 1C and D).
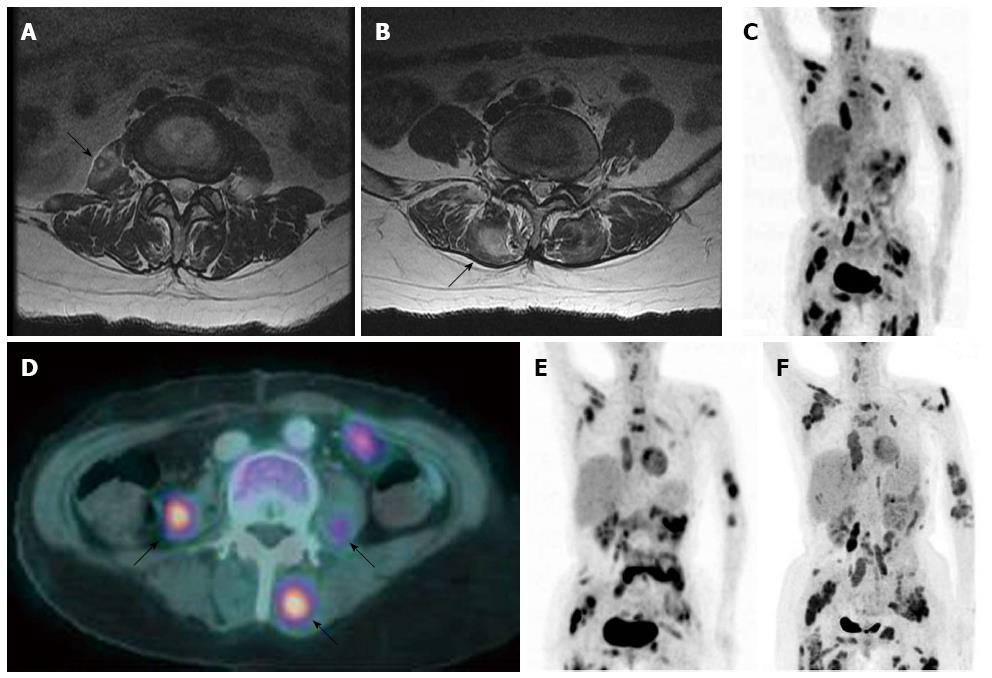
The patient received palliative three-dimensional conformal radiotherapy, with a total dose of 4750 cGy in 19, 250 cGy fractions and concurrent chemotherapy with monthly intra-arterial floxuridine (0.3 mg/kg per day, days 1-14). After 3 mo of treatment, she complained of low back pain; magnetic resonance imaging (MRI) of her thoracolumbar spine revealed metastatic masses in both the paraspinous and right psoas muscles (Figure 3A and B). An ultrasound-guided percutaneous needle biopsy of the low-echoic, paraspinous muscle confirmed a metastatic poorly differentiated adenocarcinoma (Figure 2B). PET-CT showed multiple, distant, metastatic hypermetabolic masses in her paravertebral area, left upper arm, bilateral periscapular area, anterior and posterior chest walls, bilateral psoas muscles, lower anterior abdominal wall, bilateral gluteal muscles, and bilateral proximal thigh muscles (Figure 3C-F). The patient was then subjected to chemotherapy with once every 3 wk gemcitabine (1000 mg/m2 per day) and cisplatin (100 mg/m2 per day) but died 10 mo after diagnosis of this progressive disease.
Distant metastases to skeletal muscle are rare, despite the skeletal muscle accounting for approximately 50% of the total body mass and its abundant vascularization[4]. The reason for this low metastatic rate is not well understood. The effects of lactic acid on tumor cell production[5], and the influence of variable and turbulent blood flow, β-adrenergic stimulation, tissue oxygen levels, and host immune responses may provide protection to the skeletal muscle[6]. Moreover, protease inhibitors in the extracellular matrix of the muscle may also help prevent cancer cell invasion[7].
However, recent improvements in radiological procedures may facilitate a more frequent diagnosis of skeletal muscle metastasis. CT, MRI, ultrasound, 67 Ga scintigraphy, and PET-CT are useful for establishing a diagnosis of intramuscular metastasis. CT provides an accurate means of evaluating and defining the extent of skeletal muscle tumors and their relationship to adjacent bones, fascial planes, vessels, and fat. MRI is the gold standard for imaging of skeletal muscle disease and is a valuable modality in the detection of skeletal muscle metastasis[8]. T1-weighted images of skeletal muscle metastasis have a low- to iso-intense signal, and there is high signal intensity in T2-weighted images[9,10]. Since these findings are not specific for soft tissue metastasis of carcinomas, pathological examinations of biopsy specimens provide the most useful findings for differentiating soft tissue metastasis of a carcinoma from soft tissue sarcomas and abscesses.
Treatment guidelines have not been established for patients with carcinomas and distant skeletal muscle metastases. External beam radiation, chemotherapy, surgical resection of localized disease, and combination therapies have been considered for their potential improvement of patient symptoms, without any significant prolongation of survival. Hence, the prognosis for carcinoma patients with distant metastases to the skeletal muscle is poor, with a median survival of less than 12 mo[11-13].
To our knowledge, only 5 other cases of metastases to skeletal muscle from cholangiocarcinoma have been reported in the world literature (Table 1).
| Patient No. (Ref.) | Age (yr)/sex | Ethnicities | Primary origin CC | Metastatic sites | Treatment | Follow-up (mo) | Outcome |
| 1[14] | 48/F | NA | Extrahepatic- | Rectus femoris | Resection | 8 | Dead |
| 2[15] | 45/M | Asian | Intrahepatic | Paraspinal muscle in thorax, buttock | Chemotherapy, Radiation | 4 | Dead |
| 3[4] | 44/M | Asian | Intrahepatic- | Bilateral psoas and spinal erector muscles | NA | NA | NA |
| 4[16] | 69/W | NA | Extrahepatic- | Psoas and iliacus muscles | Radiation | 2 | Dead |
| 5[17] | 72/M | NA | NA | Calf muscle | Resection | NA | NA |
In conclusion, we describe the first patient rapidly metastasized multiple sites of muscle from ICC even though the three-month palliative radiochemotherapy, compare to the other cases reported muscle metastases at diagnosis.
A 55-year-old Asian woman with a history of cholecystectomy with roux-en-Y hepaticojejunostomy because of IHD stones presented with abdominal pain.
On physical examination, the patient had tenderness over right upper quadrant of abdomen.
Hepatocellularcarcinoma, cholangiocarcinoma, metastatic carcinoma
WBC 6330/uL, AFP 4.54 ng/mL, CEA 9.45 ng/mL, and CA 19-9 3.01 U/mL; liver function test were within normal limits.
Computed tomography (CT)/positron-emission tomography (PET) scan showed 5.7 cm, heterogeneous hepatic mass in S1, S5, and S8 with SUV of 10.4, and follow-up PET-CT showed multiple, distant, metastatic hypermetabolic masses in her paravertebral area, left upper arm, bilateral periscapular area, anterior and posterior chest walls, bilateral psoas muscles, lower anterior abdominal wall, bilateral gluteal muscles, and bilateral proximal thigh muscles.
Ultrasound guided liver biopsy and muscle biopsy revealed a poorly differentiated adenocarcinoma of cytokeratins 7 and 19 positive.
The patient was treated with concurrent chemotherapy(intra-arterial floxuridine) and radiation therapy (4750 cGy in 19, 250 cGy fractions) and then palliative Gemcitabine-cisplatin after progression.
Only 5 other cases of metastatic carcinoma to muscle have been reported and the pathogenesis and treatment has not been established.
An intrahepatic cholangiocarcinoma (ICC) is a malignant tumor that originates in the small bile ducts and rarely metastasized to skeletal muscle.
This case report represents rapidly progressed to multiple muscle metastasis from ICC, compare to the other cases reported muscle metastases at diagnosis.
The case report of a “rapidly aggravated skeletal muscle metastases from an intrahepatic cholangiocarcinoma” is very exciting data to the reader and may provide the novel important information of this cancer. However, the manuscript writing didn’t focus and summarize the results to support the conclusion. Moreover, many points should be concerned before consideration to publish.
P- Reviewer: Pinlaor S, Plentz RR S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 690] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 2. | Seyama Y, Kubota K, Sano K, Noie T, Takayama T, Kosuge T, Makuuchi M. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 246] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 3. | Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45:856-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Kwon OS, Jun DW, Kim SH, Chung MY, Kim NI, Song MH, Lee HH, Kim SH, Jo YJ, Park YS. Distant skeletal muscle metastasis from intrahepatic cholangiocarcinoma presenting as Budd-Chiari syndrome. World J Gastroenterol. 2007;13:3141-3143. [PubMed] |
| 5. | Seely S. Possible reasons for the high resistance of muscle to cancer. Med Hypotheses. 1980;6:133-137. [PubMed] |
| 6. | Sridhar KS, Rao RK, Kunhardt B. Skeletal muscle metastases from lung cancer. Cancer. 1987;59:1530-1534. [PubMed] |
| 7. | Pauli BU, Schwartz DE, Thonar EJ, Kuettner KE. Tumor invasion and host extracellular matrix. Cancer Metastasis Rev. 1983;2:129-152. [PubMed] |
| 8. | Pretorius ES, Fishman EK. Helical CT of skeletal muscle metastases from primary carcinomas. AJR Am J Roentgenol. 2000;174:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Amano Y, Kumazaki T. Gastric carcinoma metastasis to calf muscles: MR findings. Radiat Med. 1996;14:35-36. [PubMed] |
| 10. | Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168:555-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Porile JL, Olopade OI, Hoffman PC. Gastric adenocarcinoma presenting with soft tissue masses. Am J Gastroenterol. 1990;85:76-77. [PubMed] |
| 12. | Kondo S, Onodera H, Kan S, Uchida S, Toguchida J, Imamura M. Intramuscular metastasis from gastric cancer. Gastric Cancer. 2002;5:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop Relat Res. 1993;213-217. [PubMed] |
| 14. | Ding GH, Yang JH, Cheng SQ, Gong H, Liu K, Dai BH, Gong B, Zhao LH, Cong WM, Wu MC. Hilar Cholangiocarcinoma with Synchronous Metastases to Breast and Skeletal Muscle: A Case Report and Literature Review. Zhongde Linchuang Zhongliuxue Zazhi. 2006;5:P216-P218. |
| 15. | Park SK, Kim YS, Kim SG, Jang JY, Moon JH, Lee MS, Kim BS, Koh ES, Park JM. Detection of distant metastasis to skeletal muscle by 18F-FDG-PET in a case of intrahepatic cholangiocarcinoma. Korean J Hepatol. 2010;16:325-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Li J, Henry MR, Roberts LR. Rare distant skeletal muscle metastasis from hilar cholangiocarcinoma: report of a case. J Gastrointest Cancer. 2011;42:171-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Yoshimura Y, Isobe K, Koike T, Arai H, Aoki K, Kato H. Metastatic carcinoma to subcutaneous tissue and skeletal muscle: clinicopathological features in 11 cases. Jpn J Clin Oncol. 2011;41:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |