Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1838
Peer-review started: April 18, 2014
First decision: May 29, 2014
Revised: August 4, 2014
Accepted: September 5, 2014
Article in press: September 5, 2014
Published online: February 14, 2015
Processing time: 300 Days and 2.8 Hours
AIM: To investigate fibroblast growth factor receptor 4 (FGFR4) protein expression in Chinese patients with resectable gastric cancer (GC) and the association with clinicopathological characteristics and survival.
METHODS: One hundred and seventy-five GC patients who underwent curative surgical procedures were enrolled in this study. The protein expression of FGFR4 in formalin-fixed, paraffin-embedded (FFPE) GC tissues was determined by immunohistochemical (IHC) analysis. Patient clinicopathological data and survival information were also collected and χ2 statistical analysis was performed to analyze FGFR4 protein expression in the subgroups with differing clinicopathological characteristics including; gender, age, tumor location, differentiation, tumor-node-metastasis stage, macroscopic type, depth of invasion, lymph node metastases, distant metastasis, neural invasion and vascular invasion. Furthermore, some common molecular markers of GC in our cancer center, including p53, p27, topoisomerase IIα (Topo IIα) were also determined by IHC and their association with FGFR4 protein expression evaluated. The probability of survival for different subgroups with different clinicopathological characteristics was calculated using the Kaplan-Meier method and survival curves plotted using the log rank test.
RESULTS: Seventy seven cases (44%) were found to have high expression of FGFR4 protein. Significantly different FGFR4 expression was observed between gastric cancers with differing expression of Topo IIα (log rank χ2 = 9.4760, P = 0.0236). No significant differences were observed between subgroups defined by any of the other clinicopathological characteristics. The median survival time of the FGFR4 high expression (77 cases) and low expression groups (98 cases) was 27 mo and 39 mo, respectively. The five-year survival rates and median survival times of gastric cancers with high FGFR4 expression were worse than those with low expression (30.8% vs 39.2%, 27 mo vs 39 mo), respectively, however, no significant difference was observed in survival time (log rank χ2 = 1.0477, P = 0.3060). Survival analysis revealed that high expression of FGFR4 was a predictor of poor outcome in GC patients if the tumor was small (less than or equal to 3 cm in size) (log rank χ2 = 5.5033, P = 0.0190), well differentiated (log rank χ2 = 7.9757, P = 0.0047), and of T1 or T2 stage invasion depth (log rank χ2 = 4.8827, P = 0.0271).
CONCLUSION: Our results suggest that high tumor expression of FGFR4 protein is not an independent risk factor for GC cancer initiation, but is a useful prognostic marker for GC patients when the tumor is relatively small, well differentiated, or in the early stages of invasion.
Core tip: This study investigated the possible contributions of fibroblast growth factor receptor 4 (FGFR4) protein expression as a risk factor for gastric cancer (GC), and the associations between protein expression and clinicopathological parameters. The results suggested that FGFR4 protein expression may correlate with the expression of Topo IIα. Furthermore, we demonstrated that FGFR4 protein expression is not a risk factor for GC initiation, but may be a useful prognostic marker for GC patients with tumors which are relatively small, well differentiated, or in the early stages of invasion.
- Citation: Chen H, Shen DP, Zhang ZZ, Liu JH, Shen YY, Ni XZ. Fibroblast growth factor receptor 4 protein expression and clinicopathological features in gastric cancer. World J Gastroenterol 2015; 21(6): 1838-1844
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1838.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1838
The overall survival of patients with gastric cancer (GC) continues to improve due to the introduction of multidisciplinary treatment approaches and the identification of novel targeted agents; however, GC remains the fourth most commonly diagnosed cancer and the second leading cause of cancer-related deaths worldwide[1,2]. It remains of great clinical importance to identify new biomarkers for early diagnosis, targeted treatment and prognostic evaluation in gastric cancer.
The human fibroblast growth factor receptor (FGFR) 4 protein belongs to the FGFR family of receptor tyrosine kinases, which are involved in the regulation of diverse cellular processes including cell growth, differentiation, survival, and migration[3]. Targeting of such receptors with novel drugs is a proven therapeutic strategy, as exemplified by the clinical success of trastuzumab in treating patients with HER2 amplified breast cancer[4]. The upregulation of FGFR4 protein expression occurs in prostate[5], breast[6], pancreatic[7], renal[8] and ovarian cancers[9], and has been associated with resistance to chemotherapy in breast cancer[10]. A growing body of research indicates that inhibition of the FGF pathway may present an effective therapeutic option for cancer. Moreover, activation of the FGFR pathway may, in some cases, provide a mechanism of resistance against current targeted and antiangiogenic drugs[11].
A recent report showed that high expression of FGFR4 protein accelerated the progression of advanced GC and might be associated with poor disease prognosis in GC patients[12]. To our knowledge, this is the only report on the association between FGFR4 protein expression and GC progression in Chinese patients, and therefore requires further confirmation. Importantly, to date no studies have been conducted on the correlation between FGFR4 protein expression and the risk of GC. In this study, we investigated the expression of FGFR4 protein in the context of clinicopathological features and patient prognosis, using an expanded population of 175 Chinese patients with resectable GC.
A retrospective cohort study was conducted and included 175 GC patients who underwent curative surgery at Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, from August 2006 to March 2009. We reviewed the medical charts and pathological records for clinicopathological parameters such as age, gender, histological subtype and pathological stage. Formalin-fixed, paraffin-embedded samples of tumors were evaluated for FGFR4 protein using immunohistochemical (IHC) analysis. None of the patients had undergone preoperative chemotherapy, radiation or targeted therapy. The study included 50 women and 125 men aged 28 to 85 years. The median age was 62 years. The tumor sample characteristics of all 175 cases are shown in Table 1. Of all the tumors examined, 32 (18.28%) were located in the cardiac region, 71 (40.58%) in the body, and 72 (41.14%) in the pylorus. 76 (43.43%) cases were poorly differentiated (grades I and II), and 99 (56.57%) cases were well differentiated (grades III and IV). Tumor-node-metastasis (TNM) classification revealed that 37 cases were stage I (21.14%), 45 were stage II (25.71%), 69 were stage III (39.43%) and 24 were stage IV (13.71%). Clinical stage was determined according to the Union for International Cancer Control TNM staging system, and tumor grade was based on the World Health Organization classification. Postoperative follow-up ended in March, 2014.
| Clinicopathological characteristics | n | FGFR4 positive | FGFR4 negative | χ2 | P value |
| 175 | 77 (44.00) | 98 (56.00) | |||
| Gender | 0.1140 | 0.8663 | |||
| Male | 125 | 56 (44.8) | 69 (55.20) | ||
| Female | 50 | 21 (42.00) | 29 (58.00) | ||
| Age (yr) | 0.9211 | 1.0000 | |||
| < 60 | 72 | 32 (44.44) | 40 (55.56) | ||
| ≥ 60 | 103 | 45 (43.69) | 58 (56.31) | ||
| Tumor size | 0.1400 | 0.1518 | |||
| ≤ 3 cm | 60 | 31 (51.67) | 29 (48.33) | ||
| > 3 cm | 115 | 46 (40.00) | 69 (60.00) | ||
| Tumor location | 1.3942 | 0.4980 | |||
| U | 32 | 17 (53.13) | 15 (46.88) | ||
| M | 71 | 29 (40.85) | 42 (59.15) | ||
| L | 72 | 31 (43.06) | 41 (56.94) | ||
| Tumor differentiation | 0.2191 | 0.1129 | |||
| Poor | 76 | 29 (38.16) | 47 (61.84) | ||
| Well | 99 | 48 (48.48) | 51 (51.52) | ||
| Macroscopic type | |||||
| EGC | 21 | 8 (38.10) | 13 (61.90) | 9.3842 | 0.0522 |
| I | 9 | 3 (33.33) | 6 (66.67) | ||
| II | 5 | 3 (60.00) | 2 (40.00) | ||
| III | 127 | 62 (48.82) | 65 (51.18) | ||
| IV | 13 | 1 (7.69) | 12 (92.31) | ||
| TNM stages | 0.3499 | 0.9504 | |||
| I | 37 | 15 (40.54) | 22 (59.46) | ||
| II | 45 | 21 (46.67) | 24 (53.33) | ||
| III | 69 | 30 (43.48) | 39 (56.52) | ||
| IV | 24 | 11 (45.83) | 13 (54.17) | ||
| T | 0.9523 | 0.8128 | |||
| 1 | 21 | 8 (38.10) | 13 (61.90) | ||
| 2 | 29 | 14 (48.28) | 15 (51.72) | ||
| 3 | 64 | 30 (46.88) | 34 (53.13) | ||
| 4 | 61 | 25 (40.98) | 36 (59.02) | ||
| N | 1.8160 | 0.6115 | |||
| 0 | 55 | 27 (49.09) | 28 (50.91) | ||
| 1 | 32 | 11 (34.38) | 21 (65.63) | ||
| 2 | 24 | 11 (45.83) | 13 (54.17) | ||
| 3 | 64 | 28 (43.75) | 36 (56.25) | ||
| M | 0.0379 | 1.0000 | |||
| 0 | 151 | 66 (43.71) | 85 (56.29) | ||
| 1 | 24 | 11 (45.83) | 13 (54.17) | ||
| Neural invasion | 0.7576 | 0.5146 | |||
| Yes | 25 | 9 (36.00) | 16 (64.00) | ||
| No | 150 | 68 (45.33) | 82 (54.67) | ||
| Vascular invasion | 0.4473 | 0.2356 | |||
| Yes | 35 | 13 (37.14) | 22 (62.86) | ||
| No | 140 | 64 (45.71) | 76 (54.29) | ||
| P53 | 3.0941 | 0.3773 | |||
| 0 | 72 | 37 (51.39) | 35 (48.61) | ||
| 1 | 43 | 16 (37.21) | 27 (62.79) | ||
| 2 | 20 | 7 (35.00) | 13 (65.00) | ||
| 3 | 40 | 17 (42.50) | 23 (57.50) | ||
| P27 | 0.9924 | 0.8031 | |||
| 0 | 87 | 37 (42.53) | 50 (57.47) | ||
| 1 | 72 | 34 (47.22) | 38 (52.78) | ||
| 2 | 12 | 4 (33.33) | 8 (66.67) | ||
| 3 | 4 | 2 (50.00) | 2 (50.00) | ||
| Topo IIα | 9.4760 | 0.0236 | |||
| 0 | 83 | 29 (34.94) | 54 (65.06) | ||
| 1 | 66 | 31 (46.97) | 35 (53.03) | ||
| 2 | 25 | 14 (60.87) | 9 (39.13) | ||
| 3 | 3 | 3 (100) | 0 (0) |
Tissue sections of paraffin-embedded formalin-fixed tissue blocks were deparaffinized with xylene for 5 min, followed by two washes with 100% ethanol for 10 min each. The slides were then incubated in 95% ethanol for 10 min and washed twice in dH2O for 5 min. Antigen retrieval was performed by placing the slides in 10 mmol/L citrate buffer (pH 6.0) and microwave treatment for 15 min. Tissue sections were cooled to room temperature (RT), and washed with phosphate-buffered saline (PBS) and distilled water. IHC was carried out on 4-μm sections using specific antibodies against FGFR4 (sc-124, Santa Cruz), p53 (sc-126, Santa Cruz), p27 (sc-393380, Santa Cruz), and Topo IIα (sc-65743, Santa Cruz). IHC samples were examined by two pathologists who were experienced in gastrointestinal cancers and unaware of the clinical information. Immunostains were standardized using appropriate positive and negative controls for each antibody.
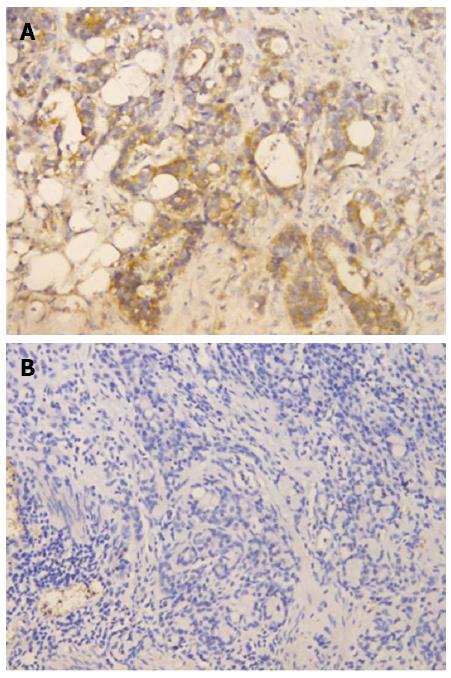
The FGFR4 was evaluated according to both the signal intensity and the percentage of stained cells. The signal intensity was scored as negative (0), weak (1), moderate (2) or strong (3). When the percentage of FGFR4 immune-positive tumor cells was considered, a score of 1 was given when < 10% of cells were positive; 2 when 10%-50% of cells were positive and 3 when > 50% of cells were positive. Both scores were multiplied and the resulting score was used to categorize FGFR4 expression as low expression (< 3) or high expression (> 3).
The expression of p53, p27 and Topo IIα were assessed by determining the number of positively stained nuclei, with less than 10% of stained cells indicating a negative result. A score of 1 was given when 10%-30% of the cells stained positively. Scores of 2 or 3 were given when 30%-50% or > 50% of the cells stained positively, respectively.
Pearson χ2 statistical analysis was performed to assess FGFR4 protein expression in the subgroups with differing clinicopathological characteristics. The probability of survival for different subgroups was calculated using the Kaplan-Meier method and the survival curves were plotted using the log rank test. All statistics were performed using 2-sided analysis, with a significance level of P < 0.05, using the “SPSS 19.0” statistical software package.
According to the criteria described previously, among 175 cases, 77 (44%) had high FGFR4 protein expression (Figure 1A) and 98 (56%) had low expression (Figure 1B).
A significant correlation was observed between FGFR4 protein expression and Topo IIα expression in gastric cancers (log rank χ2 = 9.4760, P = 0.0236). No relationships were observed between FGFR4 expression and gender, age, tumor size, tumor location, tumor differentiation, macroscopic type, p53 status, p27 status or TNM GC classification (P > 0.05; Table 1). Furthermore, within the subgroups, no relationships were observed between FGFR4 protein expression and depth of invasion, lymph node metastasis, distant metastasis, neural invasion or vascular invasion (Table 1).
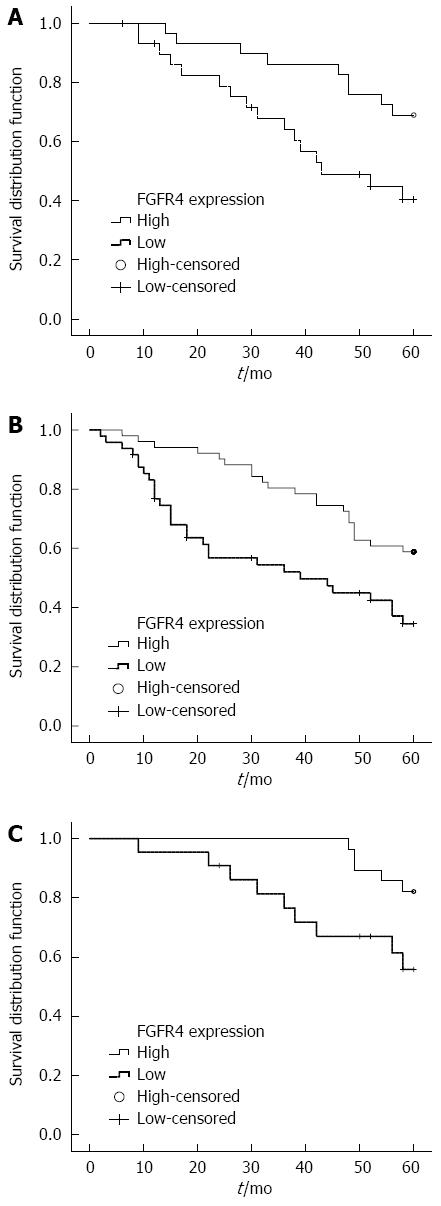
The five-year survival rate for patients with tumors showing low expression of FGFR4 was 39.2%, and the median survival time was 39 mo. The five-year survival rate for patients with tumors showing high FGFR4 expression was 30.8%, and median survival time was 27 mo. However, analysis of the entire patient cohort using Kaplan-Meier survival analysis showed no difference in survival between patients with high and low FGFR4 expressing tumors (log rank χ2 = 1.0477, P = 0.3060). When the patient population was stratified by clinicopathological parameters, such as age at diagnosis, gender, tumor size, differentiation, pathological stage, neural or vascular invasion, we found that the expression of FGFR4 protein was associated with a shorter survival time in GC patients if the tumor was small (less than or equal to 3 cm in size) (log rank χ2 = 5.5033, P = 0.0190, Figure 2A), well differentiated (log rank χ2 = 7.9757, P = 0.0047, Figure 2B), or of T1 or T2 stage (log rank χ2= 4.8827, P = 0.0271, Figure 2C). No survival differences were observed in any of the other subgroups (Table 2).
| Clinicopathological characteristics | FGFR4 positive | FGFR4 negative | χ2 | P value | ||
| Median survival time (mo) | 5-yr survival rate | Median survival time (mo) | 5-yr survival rate | |||
| Gender | ||||||
| Male | 27 | 27.4 | 47 | 36.7 | 1.1198 | 0.2900 |
| Female | 43 | 42.2 | 46 | 44.8 | 0.0000 | 0.9967 |
| Age (yr) | ||||||
| < 60 | 31 | 23.9 | 35 | 30.0 | 0.4080 | 0.5230 |
| ≥ 60 | 44 | 36.1 | 52 | 46.0 | 0.6206 | 0.4308 |
| Tumor size | ||||||
| ≤ 3 cm | 42 | 40.4 | 60 | 69.0 | 5.5033 | 0.0190 |
| > 3 cm | 22 | 24.8 | 24 | 26.2 | 0.0746 | 0.7848 |
| Tumor location | ||||||
| U | 18 | 31.1 | 38 | 31.4 | 0.7164 | 0.3973 |
| M | 43 | 31.5 | 46 | 38.1 | 0.0225 | 0.8807 |
| L | 35 | 29.1 | 52 | 42.5 | 1.1274 | 0.2883 |
| Tumor differentiation | ||||||
| Poor | 17 | 23.4 | 35 | 20.1 | 2.2622 | 0.1326 |
| Well | 31 | 47.0 | 56 | 62.1 | 7.9757 | 0.0047 |
| TNM stages | ||||||
| I + II | 56 | 57.7 | 60 | 69.6 | 1.3128 | 0.2519 |
| III + IV | 15 | 3.5 | 19 | 10.6 | 0.5328 | 0.4654 |
| T | ||||||
| T1 + T2 | 54 | 55.8 | 60 | 82.1 | 4.8827 | 0.0271 |
| T3 + T4 | 22 | 19.4 | 18 | 21.3 | 0.0479 | 0.8268 |
| N | ||||||
| N0 | 58 | 67.4 | 60 | 75.0 | 0.4856 | 0.4859 |
| N1 + N2 + N3 | 18 | 10.1 | 25 | 24.3 | 2.5385 | 0.1111 |
| M | ||||||
| 0 | 43 | 35.3 | 52 | 45.4 | 1.2078 | 0.2718 |
| 1 | 9 | 0.0 | 12 | 0.0 | 0.4042 | 0.5249 |
| Neural I invasion | ||||||
| Yes | 19 | 38.9 | 56 | 18.8 | 2.1949 | 0.1385 |
| No | 36 | 30.2 | 49 | 43.3 | 3.1118 | 0.0777 |
| Vascular I invasion | ||||||
| Yes | 21 | 13.8 | 22 | 14.3 | 0.0507 | 0.8219 |
| No | 42 | 34.0 | 54 | 46.2 | 1.7283 | 0.1886 |
In this single-center study, we investigated the FGFR4 protein expression status of 175 resectable GC specimens using IHC analysis. Herein, we focused on the role of FGFR4 as a prognostic marker for predicting cancer behavior and clinical outcome in GC patients undergoing curative surgery. To our knowledge, this is the largest study conducted to date.
Our data showed that 44% of cases (77) exhibited high FGFR4 protein expression, a result which is in keeping with a similarly high expression rate of around 38%, documented in a previous study which also reported that GC tissues have higher FGFR4 protein expression than normal tissues[13]. Overexpression of FGFR4 protein has been described in various malignancies and has been shown to play an important biological role. Roidl et al[14] in 2009 demonstrated that FGFR4 expression is up-regulated in response to doxorubicin treatment in apoptosis-resistant cancer cell clones. Turkington et al[15] in 2014 demonstrated that FGFR4 has an important role in resistance to oxaliplatin and 5-FU treatment in a range of colorectal cancer cell line models, whilst Zaid et al[9] in 2013 demonstrated that gene silencing of FGFR4 and inhibition of ligand-receptor binding both significantly decreased ovarian tumor growth both in vitro and in vivo. Recently, a study using a combination of the FGFR4 inhibitor, PD173074, and 5-fluorouracil showed an anti-proliferative and pro-apoptotic effect in GC cells in vitro[16]. Targeting gastric cancers with high levels of FGFR4 protein expression may represent a new therapeutic modality.
In our 175 patient cohort, no relationships were observed between FGFR4 protein expression and age, gender, tumor location, tumor differentiation, macroscopic type, TNM classification or other clinicopathological characteristics (P > 0.05). This is in keeping with similar data from several published studies on GC, hepatocellular carcinoma[17] and other tumor types. Results from earlier studies also showed that FGFR4 expression correlated significantly with the expression of human epidermal receptor 2 (HER-2), p21, and proliferating cell nuclear antigen (PCNA)[13]. In our study, we found that FGFR4 expression correlated positively with Topo IIα expression, but not with p53 or p27.
Topo IIα is a nuclear enzyme which modulates the topology of chromosomal DNA by causing transient double-stranded DNA breaks. This enzyme plays a key role in a number of DNA-related processes[18], is essential for cell growth and is typically expressed at high levels in rapidly growing cancer cells[19]. Notably, the fact that specific enzymatic inhibition of Topo IIα results in significant antitumor activity confirms that Topo IIα is an important target for anticancer agents[20]. Furthermore, reports have also shown that Topo IIα is involved in multiple mechanisms of drug resistance in primary gastric cardiac adenocarcinoma[21]. Hence, we suggest that the correlation of high FGFR4 and Topo IIα protein expression may, in part, explain the relatively poor prognosis for GC patients. Clearly, the underlying molecular mechanisms involved are complex and require further investigation.
In our study, the median survival time and 5-year survival rate for patients with high FGFR4 protein expression were both worse than those with low expression. However, no statistically significant differences were observed (log rank χ2 = 1.0477, P = 0.3060). These findings agree with the analysis of 94 GC patients performed by Ye et al[12] in 2012. We postulate that the up-regulation of FGFR4 may contribute to an antiapoptotic effect in GC cells[13], with similar data reported in hepatocellular carcinoma[22] and colorectal cancer[23].
Notably, we observed significant statistical differences in FGFR protein expression following stratification of tumors by size, differentiation, and invasion depth (P < 0.05). The high expression of FGFR4 appears to play an important role in the prognosis of GC with fewer other risk factors including small tumor size, degree of differentiation and early stage invasive depth. Our results demonstrate that FGFR4 protein expression is a prognostic factor in relatively small (less than 3 cm), well-differentiated (grades I and II) and early stage invasive (stages I and II) GC tumors.
The role of FGFR4 as a cancer prognostic factor, however, still remains controversial. Li et al[24] in 2014 investigated 316 colorectal cancer cases and concluded that FGFR4 positivity was significantly correlated with shorter disease-free survival (DFS) and overall survival (OS). A further study by Brito et al[25] in 2012 demonstrated that FGFR4 protein overexpression and gene amplification were predictors of poor outcome in adult patients with adrenocortical tumors. In contrast, Dutra et al[26] in 2012 showed that low FGFR4 protein expression was related to lymph node positivity and premature relapse of disease, as well as disease-related death after analyzing 75 patients with squamous cell carcinoma of the mouth and oropharynx. Similar disagreement also occurs in GC. Ye et al[12] in 2012 analyzed 94 GC cases and subgroup analysis illustrated that in GC patients with III/IV stage, the prognosis of patients with high expression of FGFR4 was much poorer. This is in contrast to the data presented here. We suggest that the smaller sample size in the study by Ye et al[12] may explain these conflicting results. Our study included a higher proportion of patients with large tumors and late-stage disease compared to the study by Ye et al[12]. A further possible explanation for this may be underestimation of the biomarker heterogeneity of GC. Clearly, further research with larger sample sizes is required to explain the full impact of FGFR4 on the development and prognosis of GC.
In conclusion, to date, this is the largest study focusing on the expression of FGFR4 protein in GC. Notably, our data show that high FGFR4 protein expression is related to the expression of Topo IIα and poor overall survival in patients harboring relatively small (≤ 3 cm), well-differentiated tumors with early stage invasive depth. Overall, this study suggests that FGFR4 may represent an attractive therapeutic target in a subgroup of gastric cancers.
The fibroblast growth factor (FGF) pathway may represent an effective therapeutic option for cancer as FGF receptor 4 (FGFR4) protein expression is upregulated in several cancers. However, there are few studies on the role of FGFR4 in gastric cancer (GC).
The authors investigated FGFR4 protein expression in Chinese patients with resectable GC and the association with clinicopathological characteristics and survival.
To date, this is the largest study focusing on the expression of FGFR4 protein in GC.
FGFR4 may be an effective therapeutic biomarker for GC. More studies should be performed to investigate the role of FGFR4 in GC.
This is an interesting article focusing on FGFR4 protein expression GC. They concluded that the FGFR4 high expression is not an independent risk factor for GC cancer initiation but that it is a useful prognostic marker for GC patients when the tumor is relatively small, well differentiated or in early depth invasion. More studies on the role of FGFR4 in GC should be performed.
P- Reviewer: Abbott DE, Uraoka T, Yahya RS S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10453] [Article Influence: 696.9] [Reference Citation Analysis (0)] |
| 2. | Dikken JL, Jansen EP, Cats A, Bakker B, Hartgrink HH, Kranenbarg EM, Boot H, Putter H, Peeters KC, van de Velde CJ. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol. 2010;28:2430-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Haugsten EM, Wiedlocha A, Olsnes S, Wesche J. Roles of fibroblast growth factor receptors in carcinogenesis. Mol Cancer Res. 2010;8:1439-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Jain VK, Turner NC. Challenges and opportunities in the targeting of fibroblast growth factor receptors in breast cancer. Breast Cancer Res. 2012;14:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Gowardhan B, Douglas DA, Mathers ME, McKie AB, McCracken SR, Robson CN, Leung HY. Evaluation of the fibroblast growth factor system as a potential target for therapy in human prostate cancer. Br J Cancer. 2005;92:320-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Tenhagen M, van Diest PJ, Ivanova IA, van der Wall E, van der Groep P. Fibroblast growth factor receptors in breast cancer: expression, downstream effects, and possible drug targets. Endocr Relat Cancer. 2012;19:R115-R129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Leung HY, Gullick WJ, Lemoine NR. Expression and functional activity of fibroblast growth factors and their receptors in human pancreatic cancer. Int J Cancer. 1994;59:667-675. [PubMed] |
| 8. | Takahashi A, Sasaki H, Kim SJ, Kakizoe T, Miyao N, Sugimura T, Terada M, Tsukamoto T. Identification of receptor genes in renal cell carcinoma associated with angiogenesis by differential hybridization technique. Biochem Biophys Res Commun. 1999;257:855-859. [PubMed] |
| 9. | Zaid TM, Yeung TL, Thompson MS, Leung CS, Harding T, Co NN, Schmandt RS, Kwan SY, Rodriguez-Aguay C, Lopez-Berestein G. Identification of FGFR4 as a potential therapeutic target for advanced-stage, high-grade serous ovarian cancer. Clin Cancer Res. 2013;19:809-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Sugiyama N, Varjosalo M, Meller P, Lohi J, Hyytiäinen M, Kilpinen S, Kallioniemi O, Ingvarsen S, Engelholm LH, Taipale J. Fibroblast growth factor receptor 4 regulates tumor invasion by coupling fibroblast growth factor signaling to extracellular matrix degradation. Cancer Res. 2010;70:7851-7861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Kumar SB, Narasu L, Gundla R, Dayam R, J A R P S. Fibroblast growth factor receptor inhibitors. Curr Pharm Des. 2013;19:687-701. [PubMed] |
| 12. | Ye YW, Zhang X, Zhou Y, Wu J, Zhao C, Yuan L, Wang G, Du C, Wang C, Shi Y. The correlations between the expression of FGFR4 protein and clinicopathological parameters as well as prognosis of gastric cancer patients. J Surg Oncol. 2012;106:872-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Ye YW, Zhou Y, Yuan L, Wang CM, Du CY, Zhou XY, Zheng BQ, Cao X, Sun MH, Fu H. Fibroblast growth factor receptor 4 regulates proliferation and antiapoptosis during gastric cancer progression. Cancer. 2011;117:5304-5313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Roidl A, Berger HJ, Kumar S, Bange J, Knyazev P, Ullrich A. Resistance to chemotherapy is associated with fibroblast growth factor receptor 4 up-regulation. Clin Cancer Res. 2009;15:2058-2066. [PubMed] |
| 15. | Turkington RC, Longley DB, Allen WL, Stevenson L, McLaughlin K, Dunne PD, Blayney JK, Salto-Tellez M, Van Schaeybroeck S, Johnston PG. Fibroblast growth factor receptor 4 (FGFR4): a targetable regulator of drug resistance in colorectal cancer. Cell Death Dis. 2014;5:e1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Ye YW, Hu S, Shi YQ, Zhang XF, Zhou Y, Zhao CL, Wang GJ, Wen JG, Zong H. Combination of the FGFR4 inhibitor PD173074 and 5-fluorouracil reduces proliferation and promotes apoptosis in gastric cancer. Oncol Rep. 2013;30:2777-2784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Chen Z, Xie B, Zhu Q, Xia Q, Jiang S, Cao R, Shi L, Qi D, Li X, Cai L. FGFR4 and TGF-β1 expression in hepatocellular carcinoma: correlation with clinicopathological features and prognosis. Int J Med Sci. 2013;10:1868-1875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Giles GI, Sharma RP. Topoisomerase enzymes as therapeutic targets for cancer chemotherapy. Med Chem. 2005;1:383-394. [PubMed] |
| 19. | Chen W, Qiu J, Shen YM. Topoisomerase IIα, rather than IIβ, is a promising target in development of anti-cancer drugs. Drug Discov Ther. 2012;6:230-237. [PubMed] |
| 20. | Azarova AM, Lyu YL, Lin CP, Tsai YC, Lau JY, Wang JC, Liu LF. Roles of DNA topoisomerase II isozymes in chemotherapy and secondary malignancies. Proc Natl Acad Sci USA. 2007;104:11014-11019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 21. | Shi H, Lu D, Shu Y, Shi W, Lu S, Wang K. Expression of multidrug-resistance-related proteins P-glycoprotein, glutathione-S-transferases, topoisomerase-II and lung resistance protein in primary gastric cardiac adenocarcinoma. Cancer Invest. 2008;26:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Mellor HR. Targeted inhibition of the FGF19-FGFR4 pathway in hepatocellular carcinoma; translational safety considerations. Liver Int. 2014;34:e1-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Liu R, Li J, Xie K, Zhang T, Lei Y, Chen Y, Zhang L, Huang K, Wang K, Wu H. FGFR4 promotes stroma-induced epithelial-to-mesenchymal transition in colorectal cancer. Cancer Res. 2013;73:5926-5935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Li CS, Zhang SX, Liu HJ, Shi YL, Li LP, Guo XB, Zhang ZH. Fibroblast growth factor receptor 4 as a potential prognostic and therapeutic marker in colorectal cancer. Biomarkers. 2014;19:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Brito LP, Ribeiro TC, Almeida MQ, Jorge AA, Soares IC, Latronico AC, Mendonca BB, Fragoso MC, Lerario AM. The role of fibroblast growth factor receptor 4 overexpression and gene amplification as prognostic markers in pediatric and adult adrenocortical tumors. Endocr Relat Cancer. 2012;19:L11-L13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Dutra RL, de Carvalho MB, Dos Santos M, Mercante AM, Gazito D, de Cicco R, Group G, Tajara EH, Louro ID, da Silva AM. FGFR4 profile as a prognostic marker in squamous cell carcinoma of the mouth and oropharynx. PLoS One. 2012;7:e50747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |