Published online Dec 28, 2015. doi: 10.3748/wjg.v21.i48.13466
Peer-review started: June 6, 2015
First decision: July 10, 2015
Revised: July 24, 2015
Accepted: August 28, 2015
Article in press: August 29, 2015
Published online: December 28, 2015
Processing time: 203 Days and 19.4 Hours
AIM: To investigate the effect of gingerol on colonic motility and the action of L-type calcium channel currents in this process.
METHODS: The distal colon was cut along the mesenteric border and cleaned with Ca2+-free physiological saline solution. Muscle strips were removed and placed in Ca2+-free physiological saline solution, which was oxygenated continuously. Longitudinal smooth muscle samples were prepared by cutting along the muscle strips and were then placed in a chamber. Mechanical contractile activities of isolated colonic segments in rats were recorded by a 4-channel physiograph. Colon smooth muscle cells were dissociated by enzymatic digestion. L-type calcium currents were recorded using the conventional whole-cell patch-clamp technique.
RESULTS: Gingerol inhibited the spontaneous contraction of colonic longitudinal smooth muscle in a dose-dependent manner with inhibition percentages of 13.3% ± 4.1%, 43.4% ± 3.9%, 78.2% ± 3.6% and 80.5% ± 4.5% at 25 μmol/L, 50 μmol/L, 75 μmol/L and 100 μmol/L, respectively (P < 0.01). Nifedipine, an L-type calcium channel blocker, diminished the inhibition of colonic motility by gingerol. Gingerol inhibited L-type calcium channel currents in colonic longitudinal myocytes of rats. At a 75 μmol/L concentration of gingerol, the percentage of gingerol-induced inhibition was diminished by nifedipine from 77.1% ± 4.2% to 42.6% ± 3.6% (P < 0.01). Gingerol suppressed IBa in a dose-dependent manner, and the inhibition rates were 22.7% ± 2.38%, 35.77% ± 3.14%, 49.78% ± 3.48% and 53.78% ± 4.16% of control at 0 mV, respectively, at concentrations of 25 μmol/L, 50 μmol/L, 75 μmol/L and 100 μmol/L (P < 0.01). The steady-state activation curve was shifted to the right by treatment with gingerol. The value of half activation was -14.23 ± 1.12 mV in the control group and -10.56 ± 1.04 mV in the 75 μmol/L group (P < 0.05) with slope factors, Ks, of 7.16 ± 0.84 and 7.02 ± 0.93 (P < 0.05) in the control and 75 μmol/L groups, respectively. However, the steady-state inactivation curve was not changed, with a half-inactivation voltage, 0.5 V, of -27.43 ± 1.26 mV in the control group and -26.56 ± 1.53 mV in the 75 μmol/L gingerol group (P > 0.05), and a slope factor, K, of 13.24 ± 1.62 in the control group and 13.45 ± 1.68 (P > 0.05) in the 75 μmol/L gingerol group.
CONCLUSION: Gingerol inhibits colonic motility by preventing Ca2+ influx through L-type calcium channels.
Core tip: Gingerol, a non-pungent molecule, has an inhibitory effect on colonic motility. There are many ion channels and second messengers involved in this process; however, no reports have described the effects of gingerol on L-type calcium channel currents. In the present study, we found that 6-gingerol obviously inhibited spontaneous contraction of longitudinal smooth muscle by preventing Ca2+ influx through L-type calcium channels.
- Citation: Cai ZX, Tang XD, Wang FY, Duan ZJ, Li YC, Qiu JJ, Guo HS. Effect of gingerol on colonic motility via inhibition of calcium channel currents in rats. World J Gastroenterol 2015; 21(48): 13466-13472
- URL: https://www.wjgnet.com/1007-9327/full/v21/i48/13466.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i48.13466
Ginger, the rhizome of Zingiber officinale Roscoe, and its components have various pharmacological actions, including anti-inflammatory, anti-cancer, anti-oxidant, anti-platelet, anti-aggregation, anti-fungal, anti-constipation and anti-diarrheal activities[1-6]. The chemical constituents of ginger rhizomes are volatile and non-volatile pungent phytochemicals and include the biologically active components, gingerols, shogaols, paradols and zingerone[7,8].
Gingerol, a non-pungent component, has an inhibitory effect on colonic motility. Iwami et al[9] reported that gingerol can inhibit colonic motility without adverse effects on small intestinal motility and the cardiovascular system. The non-pungent property of gingerol makes it useful as an oral or suppository medicine for treating diarrhea and other gastrointestinal disorders[10,11]. Abnormal facilitation of gastrointestinal motility and excessive fluid secretion of the gastrointestinal tract cause diarrhea. There are many ion channels and second messengers involved in this process; however, no reports have described the effects of gingerol on L-type calcium channel currents. The purpose of the present study was to clarify the effect of gingerol on colonic motility and the role of L-type calcium channel currents in this process.
Male Wistar rats (weighing 250 ± 50 g) were purchased from the Experimental Animal Center of Dalian Medical University. They were maintained in plastic cages at 23 ± 2 °C and a relative humidity of 55% ± 2% under standard conditions with a 12-h light/dark cycle (light: 7:00 AM to 7:00 PM) for 1 wk prior to performing the experiments. The experiments were approved by the Animal Care and Use Committee of Dalian Medical University.
Male Wistar rats bred at the Experimental Animal Center of Dalian Medical University (Dalian, China) were anesthetized with ethyl ether prior to cervical exsanguination. The distal colon was cut along the mesenteric border and cleaned with Ca2+-free physiological saline solution (PSS). Muscle strips (about 3 mm × 10 mm) were removed and placed in Ca2+-free PSS, which was oxygenated continuously. Following the careful removal of the mucosa and submucosa by dissection, smooth muscle strips were obtained. Longitudinal smooth muscle samples were prepared by cutting along the muscle strips and were then placed in a chamber. One end of the strip was fixed on the lid of the chamber by a glass claw, and the other end was attached to an isometric force transducer (TD-112S, Japan) to record contraction. The chamber (5 mL volume) was constantly perfused with pre-Tyrode’s solution at 1 mL/min. The temperature of the chamber was maintained at 37.0 ± 0.5 °C by a water bath thermostat (WC/09-05, Chongqing, China). The muscle strips were incubated for at least 40 min before beginning the experiments.
Colon smooth muscle cells (SMCs) were dissociated by enzymatic digestion. The colon tissue was pinned to the Sylgard surface of a Petri dish, and the mucosa was carefully dissected away under an anatomical microscope. The smooth muscle strips were then cut into small strips (2 mm × 2 mm), placed in 2 mL calcium-free PSS supplemented with 0.12% (w/v) collagenase (type II), 0.2% soybean trypsin inhibitor and 0.2% bovine serum albumin, and incubated for 20-30 min at 37 °C. The tissue pieces were rinsed in Ca2+ free PSS solution five times to remove the collagenase enzymes and were maintained at 4 °C for 6 h until use. Single SMCs were isolated by several gentle triturations through the tip of a free-polished Pasteur pipette.
L-type calcium currents were recorded using the conventional whole-cell patch-clamp technique. Patch-clamp pipettes were manufactured from borosilicate glass capillaries (GC 150T-7.5, Clark Electromedical Instruments, London, United Kingdom) using a 2-stage puller (PP-83, Narishige, Tokyo, Japan). When filled with pipette solution, the resistance of the patch pipette was 3-5 MΩ. The isolated myocytes were transferred to a small chamber on the stage of an inverted microscope (IX-71 Olympus, Japan) for 10-15 min and were well-attached to the bottom of the chamber. Then, the chamber was continuously superfused with PSS. An 8-channel perfusion system (L/M-sps-8, List Electronics, Germany) was used to change the perfusate. Whole-cell currents were recorded with an EPC-10 amplifier (EPC, Germany). All experiments were performed at room temperature (20-25 °C).
Tyrode’s solution contained the following (in mmol/L): 147 NaCl, 4 KCl, 1.05 MgCl2·6H2O, 0.42 CaCl2·2H2O, 1.81 Na2PO4·2H2O and 5.5 glucose. The Ca2+-free PSS contained the following (in mmol/L): 134.8 NaCl, 4.5 KCl, 5 glucose and 10 HEPES; the pH was adjusted to 7.4 with Tris (hydroxymethyl) aminomethane. The modified K-B solution contained the following (in mmol/L): 50 L-glutamate, 50 KCl, 20 taurine, 20 KH2PO4, 3 MgCl2·6H2O, 10 glucose, 10 HEPES, and 0.5 egtazic acid (pH adjusted to 7.40 with KOH). The extracellular solution contained the following (in mmol/L): 127 NaCl, 5 TEA, 4 NaHCO3, 0.33 NaH2PO4, 10 HEPES, 1.8 MgCl2, 2 CaCl2, 10 glucose, and 5 Na2-pyruvate (pH adjusted to 7.4 with NaOH). The pipette solution contained the following (in mmol/L): 126 CsCl, 1 MgCl2, 10 HEPES, 3.1 Mg·ATP, 5 Na2-phosphocreatine, and 0.42 Li2·GTP; the pH was adjusted to 7.2 with CsOH. Nifedipine and gingerol were purchased from Sigma Chemical Co. (United States).
All data are expressed as mean ± SD. The Duncan’s multiple range test was used. Differences were considered to be significant at P-values of less than 0.05.
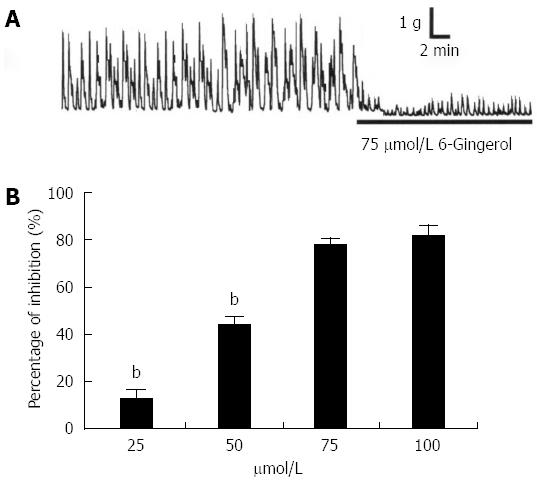
Spontaneous contraction of the muscle strips usually appeared after approximately 40 min of incubation in Tyrode’s solution. The effects of 75 μmol/L gingerol on the spontaneous contraction of colonic longitudinal smooth muscle were observed. After administering gingerol, spontaneous contraction was significantly inhibited (Figure 1A, n = 8). Different concentrations of gingerol obviously inhibited the spontaneous contraction of the muscle strips in a dose-dependent manner, with inhibition percentages of 13.3% ± 4.1%, 43.4% ± 3.9%, 78.2% ± 3.6% and 80.5% ± 4.5% at 25 μmol/L, 50 μmol/L, 75 μmol/L and 100 μmol/L, respectively (Figure 1B, n = 8).
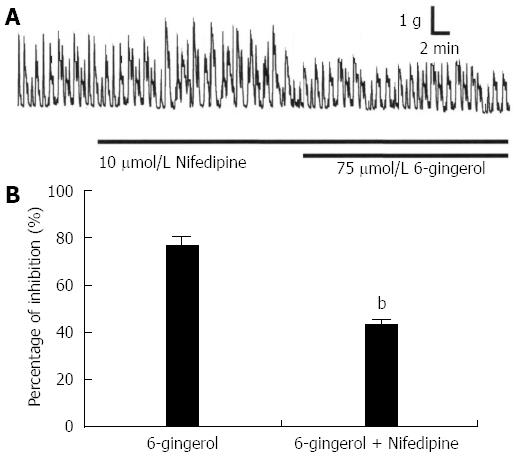
To further investigate the mechanism of the gingerol-induced inhibition of spontaneous contraction, the effect of gingerol on gastric motility was observed in the presence of nifedipine (10 μmol/L), an L-type calcium channel blocker. Nifedipine was found to diminish the gingerol-induced colonic motility inhibition. At a 75 μmol/L concentration of gingerol, the percentage of gingerol-induced inhibition was diminished by nifedipine from 77.1% ± 4.2% to 42.6% ± 3.6% (P < 0.01) (Figure 2, n = 6).
The membrane potential was clamped at -80 mV, and an ICa was elicited by a step voltage pulse from -60 mV to +60 mV for 400 ms at 10 s intervals. With an external solution containing 2 mmol/L CaCl2, an L-type calcium current could be conducted under whole-cell configuration. The peak value of the L-type calcium current appeared at 0 mV, and turnover potential appeared between +50 mV and +60 mV. The amplitude of ICa was relatively small. After replacing 2 mmol/L Ca2+ with 10 mmol/L Ba2+ in the external solution, an IBa was elicited under the same stimulus modality. The shapes of the ICa and IBa I-V curves were the same. However, the amplitude of IBa was much larger than that of ICa. This indicates that IBa is a better carrier of Ca2+ than ICa.
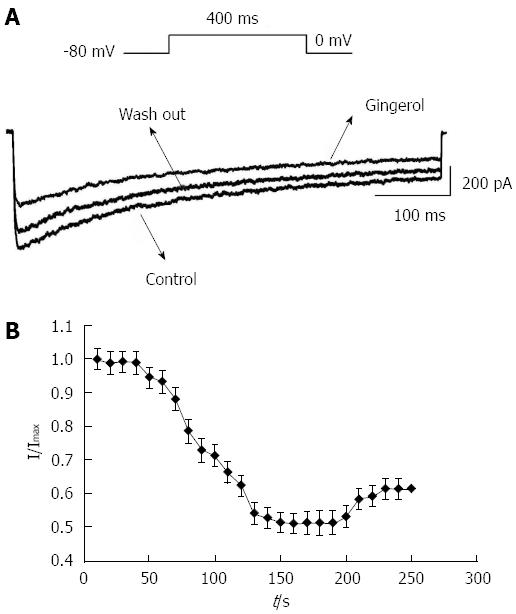
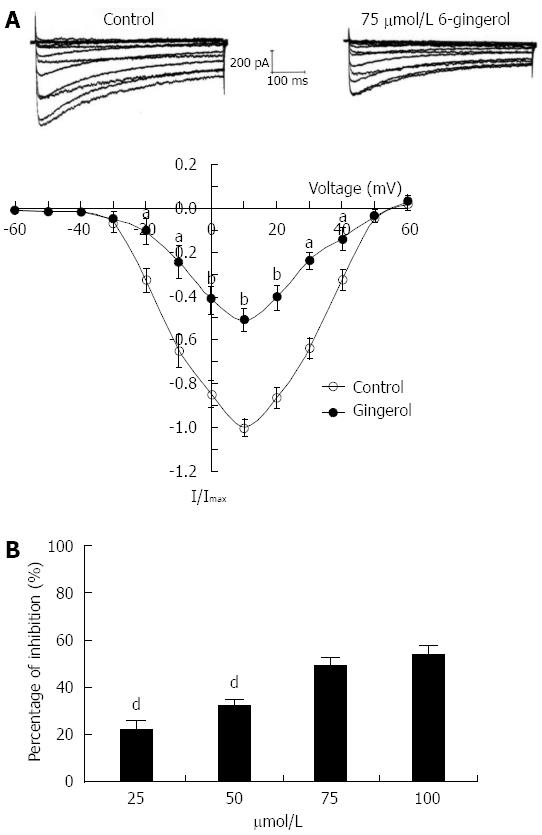
Under whole-cell configuration, the membrane potential was clamped at -80 mV, and an IBa was elicited by a single step command pulse from -80 to 0 mV for 400 ms at 10 s intervals. The time-course showed that IBa was immediately inhibited by the addition of gingerol (50 mmol/L), and within approximately 150 s this inhibitory effect had stabilized. The inhibitory percentage was 51.28% ± 2.12%, and IBa recovered partially after washout with normal control superfusing solution (Figure 3). Gingerol significantly decreased IBa in the I-V relation curve at every depolarized command step potential from -20 to +40 mV. Gingerol suppressed IBa in a dose-dependent manner, and the inhibition rates were 22.7% ± 2.38%, 35.77% ± 3.14%, 49.78% ± 3.48% and 53.78% ± 4.16% of control at 0 mV, respectively, at concentrations of 25 μmol/L, 50 μmol/L, 75 μmol/L and 100 μmol/L (Figure 4).
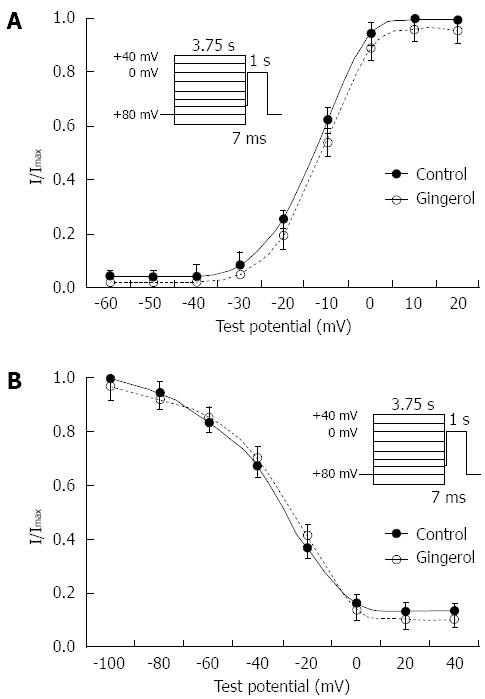
A double-pulse protocol was used to measure the steady state inactivation of IBa as a function of membrane potential. Prepulse potentials ranging from -100 to +40 mV were applied for a duration of 3.75 s. Following a 7 ms interpulse interval at a potential of -60 mV, the membrane potential was raised to a test potential of 0 mV for 1 s. The currents were then normalized to the current obtained at -100 mV (I/Imax) and plotted against each prepulse potential. The plotted data were well fitted by the Boltzmann equation, with a half-inactivation voltage, 0.5 V, of -27.43 ± 1.26 mV in the control group and -26.56 ± 1.53 mV in the 75 μmol/L gingerol group (P > 0.05, n = 6), and a slope factor, K, of 13.24 ± 1.62 in the control group and 13.45 ± 1.68 (P > 0.05) in the 75 μmol/L gingerol group (Figure 5A). The steady-state activation curves were estimated from the peak conductance at each potential using the following equation: IBa = gBa (V - Vrev), where gBa, V and Vrev are peak conductance, test potential and observed reversal potential, respectively. The value of half activation was -14.23 ± 1.12 mV in the control group and -10.56 ± 1.04 mV in the 75 μmol/L group (P < 0.05, n = 6) with slope factors, Ks, of 7.16 ± 0.84 and 7.02 ± 0.93 (P < 0.05) in the control and 75 μmol/L groups, respectively (Figure 5B).
In the present study, gingerol inhibited the spontaneous contraction of colonic longitudinal smooth muscle in a dose-dependent manner. Nifedipine (10 μmol/L), an L-type calcium channel blocker, diminished the inhibition of colonic motility by gingerol. Furthermore, gingerol suppressed IBa in a dose-dependent manner. Gingerol treatment of colonic longitudinal myocytes of rats resulted in the activation curve being shifted to the right, while the inactivation curve did not change.
Ginger has many uses in many of the world’s medicinal systems[1,12,13]. More commonly, ginger has been traditionally used in disorders of the gastrointestinal tract, as a stomachic, laxative, sialagogue, antiemetic and anti-dyspeptic agent, gastric emptying enhancer and appetizer, and as an antidiarrheal and anti-colic agent[14]. Gingerol, which is not a natural component of ginger, is a reduced analogue of gingerone. Most previous studies of gingerol have focused on its anti-cancer, anti-oxidant and anti-inflammatory properties[1]. Nigam et al[15] reported that the anti-cancer properties of 6-gingerol are mediated by its induction of apoptosis. A previous study indicated that 6-gingerol significantly reduced the DNA strand breaks and micronuclei formation caused by patulin (PAT). Moreover, 6-gingerol effectively suppressed PAT-induced intracellular ROS formation and decreased 8-OHdG level. GSH depletion induced by PAT in HepG2 cells was also attenuated by 6-gingerol pretreatment. These findings suggest that 6-gingerol has a strong protective ability against the genotoxicity caused by PAT and that the antioxidant activity of 6-gingerol may play an important role in attenuating the genotoxicity of PAT[16]. Nonn et al[17] reported that 6-gingerol can up-regulate MKP5 and decrease cytokine-induced p38-dependent pro-inflammatory changes.
Recent studies have examined the effect of a component of ginger on diarrhea. It has been demonstrated that zingerone inhibits enterotoxin-induced fluid secretion in the ileum in mice[18]. Because excessive fluid secretion by the gastrointestinal tract causes diarrhea, zingerone is likely the active constituent of ginger that is responsible for its antidiarrheal activity. In addition to excessive secretion, abnormal facilitation of gastrointestinal motility is another cause of diarrhea. It has been reported that ginger also has suppressive effects on gut motility. Crude ginger extract inhibits rat ileal motility via the inhibition of enteric neural excitatory transmission and smooth muscle mechanical activity in vitro[19]. Ghayur and Gilani reported inhibitory effects of ginger crude extract on high K+-induced contractions in isolated guinea pig colons[14]. Furthermore, herbal medicines that include ginger extracts inhibit colonic motility in rats[10,20].
In order to investigate the effect of 6-gingerol on colonic motility and the role of L-type calcium channel in the process, in the present study, we observed the effect of 6-gingerol on spontaneous contraction of colonic longitudinal smooth muscle at different concentrations. The results indicated that spontaneous contraction was significantly inhibited by gingerol. It is in agreement with that of David Banji[21]. Furthermore, 6-gingerol obviously inhibited spontaneous contraction in a dose dependent manner. The inhibition percentage was not significantly different between the 75 μmol/L and 100 μmol/L gingerol treatments, indicating that the gingerol concentration of 100 μmol/L is near to its highest effective dose. As we know, there is a close relationship between calcium and muscle contraction. We therefore utilized nifedipine (10 μmol/L), an L-type calcium channel blocker. At a gingerol concentration of 75 μmol/L, after administering nifedipine, the percentage of gingerol-induced inhibition was diminished from 77.1% ± 4.2% to 42.6% ± 3.6%. This indicates that gingerol inhibited spontaneous contraction by inhibiting calcium influx via L-type calcium channels, at least in part. This was also shown at a cellular level. These results indicate that the calcium influx via L-type calcium channels was diminished by gingerol to inhibit the spontaneous contraction of the colon in rats. Townsend et al[22] reported that 6-gingerol prevented Ca2+ influx through L-type Ca2+ channels to promote airway smooth muscle relaxation. Furthermore, pretreatment with 20 mmol/L ruthenium red (a ryanodine receptor antagonist) significantly diminished the initial increase of calcium caused by 6-gingerol, suggesting a mechanism of action of 6-gingerol that involves intracellular calcium store depletion. However, we do not know whether intracellular calcium stores participate in the 6-gingerol-induced inhibition of colonic contraction in rats.
Taken together, these findings indicate that 6-gingerol inhibits spontaneous contraction by preventing Ca2+ influx through L-type calcium channels. Future studies will investigate if intracellular calcium stores participate in this process.
Ginger, an herbal medicine, has been traditionally used to treat various kinds of diseases including gastrointestinal symptoms. Gingerol, a non-pungent analogue of gingerone, which is an active constituent of ginger, can effect on diarrhea via inhibiting colonic motility. However, the mechanism of gingerol to inhibit colonic motility is not clear.
Gingerol has been reported to possess a variety of biological properties including anti-cancer, anti-oxidant, anti-inflammation, anti-aggregation, antifungal and anti-diarrhea. But the molecular mechanisms underlying the effects of gingerol on gene expression, the signaling pathway and effectual protein involved are required to elucidate.
Recent reports have highlighted that gingerol will be useful as an oral or suppository medicine for treating diarrhea and other gastrointestinal disorders. Abnormal facilitation of gastrointestinal motility and excessive fluid secretion of the gastrointestinal tract cause diarrhea. There are many ion channels and second messengers involved in this process. However, there is no report in which the effect of gingerol on L-type calcium channel current is described. The purpose of the present study is to clarify the effect of gingerol on colonic motility and the role of L-type calcium channel current in the process.
By understanding the effect of gingerol on colonic motility and the role of L-type calcium channel current in the process, this study may help us to understand the mechanism of gingerol to treat diarrhea.
The L-type calcium channel (also known as the dihydropyridine channel, or DHP channel) is part of the high-voltage activated family of voltage-dependent calcium channel. “L” stands for long-lasting referring to the length of activation. This channel has four subunits (Cav1.1, Cav1.2, Cav1.3, and Cav1.4).
This is a good, solid study with some flaws in data presentation. Part of the discussion, which should refer to newly obtained data, repeats paragraphs from the results section.
P- Reviewer: Fichna J S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Wang S, Zhang C, Yang G, Yang Y. Biological properties of 6-gingerol: a brief review. Nat Prod Commun. 2014;9:1027-1030. [PubMed] |
| 2. | Haniadka R, Saldanha E, Sunita V, Palatty PL, Fayad R, Baliga MS. A review of the gastroprotective effects of ginger (Zingiber officinale Roscoe). Food Funct. 2013;4:845-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Baliga MS, Haniadka R, Pereira MM, Thilakchand KR, Rao S, Arora R. Radioprotective effects of Zingiber officinale Roscoe (ginger): past, present and future. Food Funct. 2012;3:714-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Nicoll R, Henein MY. Ginger (Zingiber officinale Roscoe): a hot remedy for cardiovascular disease? Int J Cardiol. 2009;131:408-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Liao YR, Leu YL, Chan YY, Kuo PC, Wu TS. Anti-platelet aggregation and vasorelaxing effects of the constituents of the rhizomes of Zingiber officinale. Molecules. 2012;17:8928-8937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Touba EP, Zakaria M, Tahereh E. Anti-fungal activity of cold and hot water extracts of spices against fungal pathogens of Roselle (Hibiscus sabdariffa) in vitro. Microb Pathog. 2012;52:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Govindarajan VS. Ginger--chemistry, technology, and quality evaluation: part 1. Crit Rev Food Sci Nutr. 1982;17:1-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 145] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Govindarajan VS. Ginger-chemistry, technology, and quality evaluation: part 2. Crit Rev Food Sci Nutr. 1982;17:189-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 71] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Iwami M, Shiina T, Hirayama H, Shima T, Takewaki T, Shimizu Y. Inhibitory effects of zingerone, a pungent component of Zingiber officinale Roscoe, on colonic motility in rats. J Nat Med. 2011;65:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Iwami M, Shiina T, Hirayama H, Shimizu Y. Intraluminal administration of zingerol, a non-pungent analogue of zingerone, inhibits colonic motility in rats. Biomed Res. 2011;32:181-185. [PubMed] |
| 11. | Rahman S, Parvez AK, Islam R, Khan MH. Antibacterial activity of natural spices on multiple drug resistant Escherichia coli isolated from drinking water, Bangladesh. Ann Clin Microbiol Antimicrob. 2011;10:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 740] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 13. | Haniadka R, Rajeev AG, Palatty PL, Arora R, Baliga MS. Zingiber officinale (ginger) as an anti-emetic in cancer chemotherapy: a review. J Altern Complement Med. 2012;18:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci. 2005;50:1889-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Nigam N, George J, Srivastava S, Roy P, Bhui K, Singh M, Shukla Y. Induction of apoptosis by [6]-gingerol associated with the modulation of p53 and involvement of mitochondrial signaling pathway in B[a]P-induced mouse skin tumorigenesis. Cancer Chemother Pharmacol. 2010;65:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Yang G, Zhong L, Jiang L, Geng C, Cao J, Sun X, Liu X, Chen M, Ma Y. 6-gingerol prevents patulin-induced genotoxicity in HepG2 cells. Phytother Res. 2011;25:1480-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Nonn L, Duong D, Peehl DM. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis. 2007;28:1188-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Chen JC, Huang LJ, Wu SL, Kuo SC, Ho TY, Hsiang CY. Ginger and its bioactive component inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea in mice. J Agric Food Chem. 2007;55:8390-8397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Borrelli F, Capasso R, Pinto A, Izzo AA. Inhibitory effect of ginger (Zingiber officinale) on rat ileal motility in vitro. Life Sci. 2004;74:2889-2896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Yang Z, Pan A, Zuo W, Guo J, Zhou W. Relaxant effect of flavonoid naringenin on contractile activity of rat colonic smooth muscle. J Ethnopharmacol. 2014;155:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Banji D, Banji OJ, Pavani B, Kranthi Kumar Ch, Annamalai AR. Zingerone regulates intestinal transit, attenuates behavioral and oxidative perturbations in irritable bowel disorder in rats. Phytomedicine. 2014;21:423-429. [PubMed] [DOI] [Full Text] |
| 22. | Townsend EA, Siviski ME, Zhang Y, Xu C, Hoonjan B, Emala CW. Effects of ginger and its constituents on airway smooth muscle relaxation and calcium regulation. Am J Respir Cell Mol Biol. 2013;48:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |