Published online Dec 14, 2015. doi: 10.3748/wjg.v21.i46.13132
Peer-review started: July 4, 2015
First decision: July 19, 2015
Revised: August 4, 2015
Accepted: September 14, 2015
Article in press: September 15, 2015
Published online: December 14, 2015
Processing time: 162 Days and 6.6 Hours
AIM: To assess the diagnostic accuracy, of aminotransferase-to-platelet ratio index (APRI) alone and with antischistosomal antibody (Ab) in patients with hepatitis C virus (HCV) and schistosomiasis coinfection.
METHODS: This retrospective study included medical records of three hundred and eighty three Egyptian men patients who had undergone percutaneous liver biopsy between January 2006 to April 2014 in tertiary care hospital in Qatar for diagnosis or monitoring purpose were selected. Data of patients > 18 years of age were included in the study. The values of HCV RNA titer and antischistosomal antibody titer were also taken into consideration. Patients were excluded from the study if they had any other concomitant chronic liver disease, including; history of previous antiviral or interferon therapy, immunosuppressive, therapy, chronic hepatitis B infection, human immunodeficiency virus co-infection, autoimmune hepatitis, decompensated liver disease, hepatocellular carcinoma, prior liver transplantation, and if no data about the liver biopsy present.
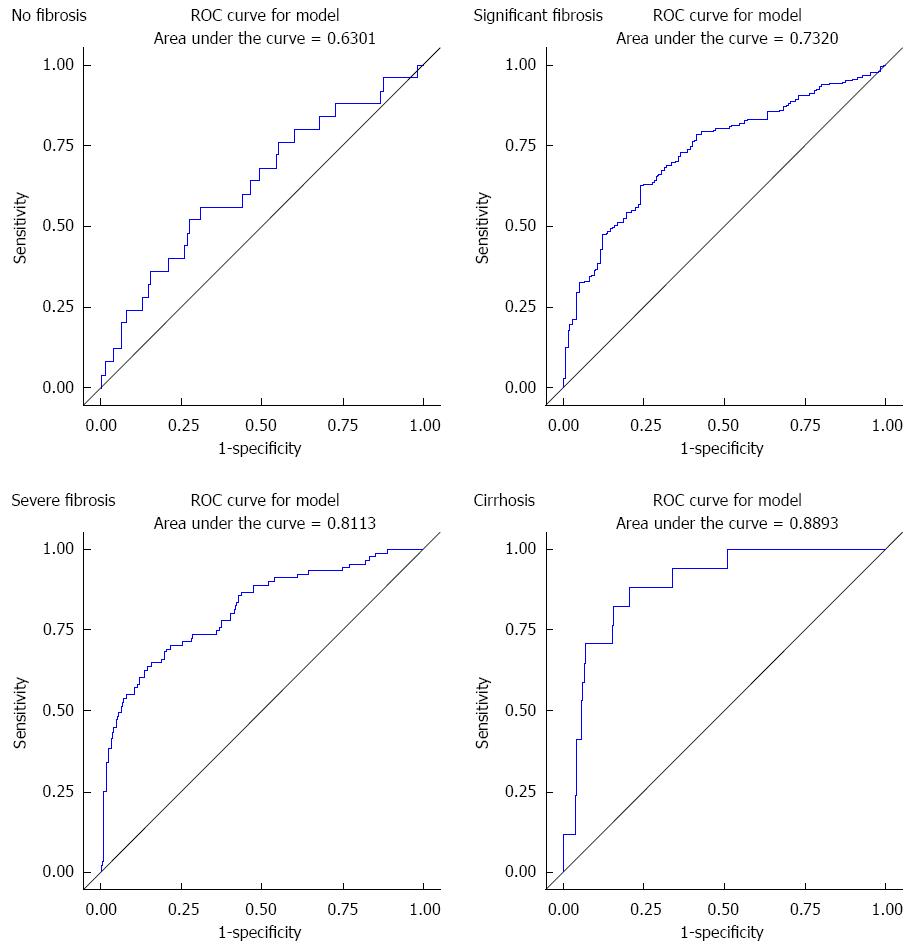
RESULTS: Median age of patients was 46 years. About 7.1% had no fibrosis, whereas 30.4%, 37.5%, 20.4%, and 4.6% had fibrosis of stage I, II, III, and IV respectively. In bivariate analysis, APRI score, levels of AST, platelet count and age of patient showed statistically significant association with liver fibrosis (P < 0.0001); whereas antischistosomal antibody titer (P = 0.52) and HCV RNA titer (P = 0.79) failed to show a significant association. The respective AUC values for no fibrosis, significant fibrosis, severe fibrosis and cirrhosis of APRI score were 63%, 73.2%, 81.1% and 88.9% respectively. This showed good sensitivity and specificity of APRI alone for grading of liver fibrosis. But the inclusion of anti-Schistosoma antibody did not improve the prediction of fibrosis stage.
CONCLUSION: The study results suggest that noninvasive biochemical markers like APRI are sensitive and specific in diagnosing the degree of fibrosis and cirrhosis in patients with coinfection of HCV and schistosomiasis as compared to biopsy. The addition of antischistosomal Ab to APRI did not improve sensitivity for predicting the degree of cirrhosis.
Core tip: In few parts of the world, in addition to hepatitis C virus (HCV) other concomitant infections play a significant role in causing liver fibrosis. This study was conducted using data from 383 patients to evaluate accuracy of noninvasive method called aspartate transaminase to platelet ratio index (APRI). Its usefulness was explored for assessment of liver fibrosis. Also the role of anti-schistosomial antibody was evaluated for improving sensitivity and specificity of APRI. The APRI when used alone showed good sensitivity and specificity for accurately evaluating liver fibrosis in patients with HCV and schistosomiasis coinfection. The addition of antischistosomal antibody or HCV-RNA Titer, did not further improve the accuracy of APRI.
-
Citation: Derbala M, Elbadri ME, Amer AM, AlKaabi S, Sultan KH, Kamel YM, Elsayed EHS, Avades TY, Chandra P, Shebl FM. Aspartate transaminase to platelet ratio index in hepatitis C virus and
Schistosomiasis coinfection. World J Gastroenterol 2015; 21(46): 13132-13139 - URL: https://www.wjgnet.com/1007-9327/full/v21/i46/13132.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i46.13132
Liver biopsy is an important investigation which plays significant role in the management and follows up of the patients with hepatitis C virus (HCV) infection. It helps in staging the degree of liver fibrosis and can also guide about the treatment response[1]. The main problem with use of liver biopsy is that it is invasive, relatively costly and there are chances of technical errors during procedure[2]. There is always a need of non-invasive and cheaper methods and all the complications associated with liver biopsy have led to the search for other “safer” modalities with comparable effectiveness.
For the assessment of liver fibrosis in hepatitis C infected patients certain noninvasive modalities are available which include serum biomarkers and imaging tools[3]. These currently available biomarkers and imaging methods can predict the degree of liver fibrosis either directly, being derived from extracellular matrix turnover[4] or indirectly by reflecting the deranged liver function[5,6].
The aspartate aminotransferases (AST) to platelet ratio index (APRI) score is an example of one of these non-invasive modalities. It constitutes of AST to platelets ratio index. This score has been studied to assess the degree of fibrosis in patients with HCV with proven efficacy[7].
In many parts of the word, HCV is not the only infection which affects chronically the liver structure and function. Other concomitant infections do contribute substantially in the pathogenesis of liver fibrosis. In Mediterranean region, especially in Egypt; patients with HCV are commonly found to be co-infected with schistosomiasis (bilharzias). Both conditions can augment portal hypertension with its consequences which includes splenomegaly, hypersplenism with pancytopenia and portal varices with and without bleeding[8]. However, in patients with schistosomiasis, portal hypertension occur secondary to periportal fibrosis rather than liver cirrhosis. It has been postulated in patients with schistosomiasis, the platelet count, which is a component of APRI score, is inversely related to the degree of periportal fibrosis and spleen size[9]. This consideration might alter the reliability of APRI score in patients with HCV infection who are also co-infected with schistosoma infection.
Significant research gaps continue to limit full understanding of the benefits and harms of screening for HCV infection and diagnostic accuracy of non-invasive tests to compare to the liver biopsy. Although liver biopsy is still regarded as the most accurate method for assessing the histological stage of HCV infection and its complications in liver, it is an invasive test with some risks for serious harms, making work-up strategies that make use of noninvasive tests with high diagnostic accuracy a potential alternative.
The relationship between platelet count and the stages of fibrosis in chronic HCV hepatitis have been examined previously, however few reports have studied this association in patients infected with Schistosoma mansoni[8]. The question, whether schistosomiasis-HCV coinfection can affect the sensitivity of APRI score in this group of patients needs attention. Also, it is important to know does antischistosomal Ab titer can modify the sensitivity of APRI score in these patients.
As per World Health Organization, in resource-limited countries, it is recommended to use noninvasive tests for the assessment of hepatic fibrosis rather than other invasive and methods requiring more resources[10].
This retrospective study was conducted to examine the diagnostic accuracy of one such noninvasive test for resource poor countries like Egypt; the APRI, in adult patients with combined schistosoma and HCV infection compared to non-shistosoma infected patients. We also tried assessing the value of adding antischistosomal Ab titer to the APRI score on its sensitivity and specificity for fibrosis staging.
The data analyzed in the study were obtained after approval from the ethics committee for human research of the Hamad Medical Corporation. The study was conducted in accordance with the Declaration of Helsinki. This study was done in the Department of Hepatology at Hamad General Hospital. A retrospective analysis of medical records of 383 men patients with HCV infection who had undergone percutaneous liver biopsy between January 2006 to April 2014 was done. Those patients were scheduled to receive treatment for HCV infection. Chronic HCV infection was defined as positive anti-HCV serology, active virus replication was manifested by the detection of HCV-RNA, a persistent increase in alanine aminotransferase (ALT), and chronic active hepatitis histological pattern. Patients 18 years or older, with confirmed diagnosis of HCV infection were included in the study. Patients were excluded from the study if they had any other concomitant chronic liver disease, including chronic hepatitis B infection, human immunodeficiency virus co-infection, autoimmune hepatitis, decompensated liver disease, hepatocellular carcinoma, history of previous antiviral or interferon therapy, immunosuppressive therapy, prior liver transplantation, and if no data about the liver biopsy present. Diagnosis of schistosomal co-infection was based on positive antischistosomal antibody titer equal to or more than 1:160 (Fumouze Diagnostics, Levallois-Perret, France). HCV-RNA detection was done using the QIAamp Viral RNA and RNeasy Mini kit (QIAGEN, Hilden, Germany) according to manufacturer’s instructions. Scheuer score was used for assessing necro-inflammatory activity and fibrosis in liver biopsies of patients with chronic viral hepatitis. APRI Score was calculated at the same time with liver biopsy.
All analyses were run using SAS 9.4. Data were presented as median with interquartile range, and frequencies with percentages. The bivariate analysis was done using χ2 test/Fisher exact test for categorical variables and Kruskal-Wallis test for continuous variables to find out the association between other factors/variables and fibrosis. Binary and multinomial logistic regressions were used to examine the predictors of fibrosis modeled as dichotomous or multi-categories fibrosis status respectively. All models were forced to retain the APRI score as predictors. P < 0.05 indicated that the associations were statistically significant. The receiver characteristic curve (ROC) analysis was used to analyze the accuracy of diagnosis of the hepatic fibrosis. An area under the ROC curve value close to one, indicated high diagnostic accuracy. Because sensitivity and specificity were considered equally important, the best cutoff points were determined using Youden’s index which maximizes sensitivity and specificity. To examine the possible role of anti-schistosomiasis in improving prediction of fibrosis stage, we compared AUC of three models predicting each fibrosis stage; namely model 1 which included APRI as the only predictor of fibrosis stage, model 2 which included anti-schistosomiasis as the only predictor of fibrosis stage, and model 3 which included both APRI and anti-schistosomiasis as predictors of fibrosis stage. The volume under the surface (VUS) method was used additionally as an extension to AUC, using a three-class fibrosis variable model)[11]. A VUS less than or equal to 16.7% is considered worthless in prediction of fibrosis. To calculate the VUS we used a non-parametric method that uses a confusion matrix approach[12]. Analysis of VUS was conducted using the SAS macro written by Kapasny and Rezac[13].
Fibrosis status were classified in several ways; two-class variables (1) any fibrosis yes (stage 1 to 4) vs no (stage 0); (2) significant fibrosis (stage 2, 3, 4) vs no/low fibrosis (stage 0, 1); (3) severe fibrosis (stage 3, 4) vs no/low/mild fibrosis (stage 0, 1, 2); and (4) cirrhosis (stage 4) vs no/low/mild/moderate fibrosis (stage 0, 1, 2, 3). A three-class fibrosis status was defined as no (stage 0), mild/moderate (stage 1, 2), severe/cirrhosis (stage 3, 4).
The study included 383 men patients. The participants were all men. Median age was 46 (38-52). Approximately 26 (7.1%) had no fibrosis, whereas 112 (30.4%), 138 (37.5%), 75 (20.4%), and 17 (4.6%) had fibrosis of stage I, II, III, and IV respectively.
In bivariate analysis, APRI score, levels of AST, platelets count and age of patient showed statistically significant association with liver fibrosis (P < 0.0001). Antischistosoma antibody titer (P = 0.53) and HCV RNA titer (P = 0.38) did not show a significant association with liver fibrosis. More specifically, there were significant difference in levels of APRI scores among the groups of no fibrosis, stage 1, 2, 3 and 4 fibrosis; median (IQR) 0.42 (0.34, 0.71), 0.44 (0.35, 0.63), 0.64 (0.41, 0.92), 1.22 (0.62, 2.22), and 2.49 (1.40, 2.80) respectively (Kruskal-Wallis test: χ2 = 98.78, P < 0.0001).The result is summarized in Table 1.
| Characteristic | Median (inter-quartile range) | Bivariate analysis1 (P value) |
| Age | 46 (38, 52) | < 0.0001 |
| APRI score | 0.63 (0.40, 1.11) | < 0.0001 |
| Anti-schistosoma antibody | 128 (0, 512) | 0.53 |
| HCV RNA titer (Log10) | 5.60 (4.98, 6.17) | 0.38 |
| AST | 42 (31, 63) | < 0.0001 |
| Platelet (× 103) | 192 (147, 232) | < 0.0001 |
| Fibrosis, n (%) | NA | |
| No | 26 (7.1) | - |
| Stage I | 112 (30.4) | - |
| Stage II | 138 (37.5) | - |
| Stage III | 75 (20.4) | - |
| Stage IV | 17 (4.6) | - |
| Grade, n (%) | < 0.00012 | |
| 0 | 2 (0.54) | - |
| I | 101 (27.4) | - |
| II | 185 (50.1) | - |
| III | 74 (20.1) | - |
| IV | 7 (1.90) | - |
In the multivariable logistic regression analysis, we further explored predictors of fibrosis status. APRI score did not significantly predict “no fibrosis” status, while age significantly predicted “no fibrosis” status, such that for a unit increase in the APRI score the odds of “no fibrosis” decreased by 16%, while for each 5-year increase in age, there was approximately 35% decrease in the odds of “no fibrosis”; OR (95%CI) 0.84 (0.45-1.57) and 0.65 (0.51-0.83) respectively. On examining predictors of “significant fibrosis” status, APRI score, older age, and severe inflammation significantly predicted “significant fibrosis” status; OR (95%CI) 2.48 (1.45-4.25), 1.23 (1.05-1.44), and 13.03 (6.90-24.60) respectively. Similarly APRI score, older age, and severe inflammation grade significantly predicted “severe fibrosis” status OR (95%CI) 3.53 (2.37-5.24), 1.29 (1.08-1.54) and 9.18 (2.44-34.60) respectively. Interestingly, for each unit increase in APRI score there was a 68% increase in the odds of “cirrhosis” status OR (95%CI) 1.68 (1.08-2.61), and for each 10000 unit increase in platelet count, there was a 15% decline in the odds of “cirrhosis” status OR (95%CI), 0.85 (0.73-0.98) (Data are not in tables).
We also ran the multinomial logistic regression model to predict a three-level, fibrosis status (no, mild/moderate, severe/cirrhosis). Compared to no fibrosis, the probability of mild/moderate fibrosis was not significantly predicted by APRI score or grade, but significantly predicted by age. On the contrary, the probability of severe fibrosis/cirrhosis was significantly higher for individuals with higher APRI score, who are older and have moderate/severe inflammation (Table 2).
| Predictor | Outcome:fibrosis | Unit | OR | 95%CI | P value |
| APRI_SCORE | Severe/cirrhosis | 1 | 2.41 | 1.12-5.18 | 0.025 |
| APRI_SCORE | Mild/moderate | 1 | 0.65 | 0.31-1.40 | 0.275 |
| AGE | Severe/cirrhosis | 5 | 1.83 | 1.37-2.46 | < 0.0001 |
| AGE | Mild/moderate | 5 | 1.46 | 1.14-1.88 | 0.003 |
| Grade moderate/severe vs no/mild | Severe/cirrhosis | NA | 11.63 | 2.41-56.12 | 0.002 |
| Grade moderate/severe vs no/mild | Mild/moderate | NA | 1.29 | 0.51-3.28 | 0.587 |
To examine the improvement in predicting fibrosis when anti-schistosoma antibodies is included as predictor of fibrosis, we ran three models. The first model included APRI score only, the second model included anti-schistosoma antibodies only, and the third model included both APRI score and anti-schistosoma antibodies.
The respective AUC values for no fibrosis, significant fibrosis, severe fibrosis and cirrhosis of APRI score were 63%, 73.2%, 81.1% and 88.9% (Figure 1).
The individual APRI has shown good sensitivity and specificity as compared to biopsy. The sensitivity and specificity increase as grades of fibrosis increases. Because sensitivity and specificity were considered equally important, the best cutoff points were determined using Youden’s index which maximizes sensitivity and specificity. APRI score did not perform well in predicting no fibrosis. The optimal cutoff point was 0.45. The cutoff for significant fibrosis, severe fibrosis and cirrhosis was 0.64, 1.06 and 1.11 respectively. The results are summarized in Table 3.
| Characteristic | AUC | Cutoff point1 | Sensitivity | Specificity |
| No fibrosis | 63% | 0.45 | 56% | 69% |
| Significant fibrosis | 73% | 0.64 | 63% | 76% |
| Severe fibrosis | 81% | 1.06 | 64% | 86% |
| Cirrhosis | 89% | 1.11 | 88% | 79% |
We investigated whether the prediction of fibrosis stage would improve if both antischistosoma antibody and APRI score were included in the prediction model. But inclusion of antischistosoma antibody did not improve the prediction of fibrosis stage (Table 4).
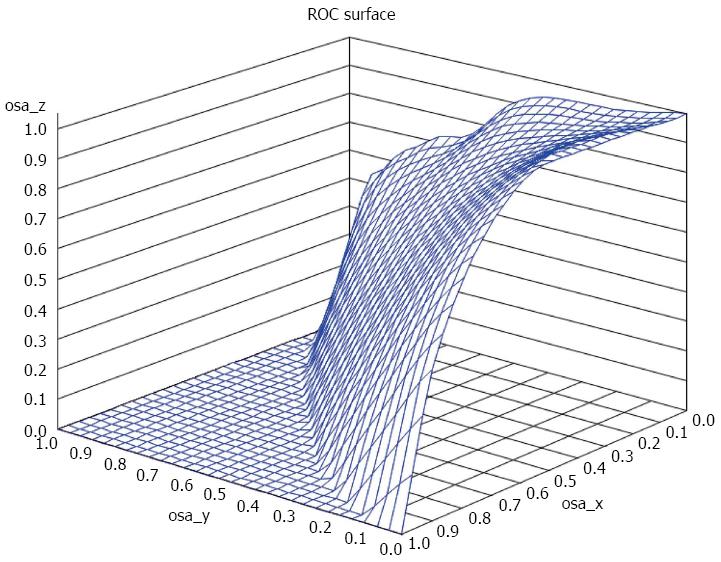
Using a three class fibrosis variable (no fibrosis, mild to moderate fibrosis and severe cirrhosis) a VUS of 43% was observed. We investigated cutoff points that minimize misclassification. Cut off points of (no fibrosis < 0.2, mild/moderate fibrosis 0.2 to < 0.7, and severe/cirrhosis > 0.7) achieved a 62.5% correctness. The correct classification was achieved in approximately 73.1% if we used cutoffs of (no fibrosis < 0.2, mild/moderate fibrosis 0.2 to < 1.0, and severe/cirrhosis > 1.0). Approximately 76.1% subjects were correctly classified using the cutoff points of (no fibrosis < 0.2, mild/moderate fibrosis 0.2 to < 1.5, and severe/cirrhosis > 1.5). Increasing the cutoff point of severe/cirrhosis to APRI score of 2 decreased the correctness of classification to 74.5% (no fibrosis < 0.2, mild/moderate fibrosis 0.2 to < 2, and severe/cirrhosis > 2). In general, the highest misclassification was in the no fibrosis class, while the lowest misclassification was in the mild/moderate fibrosis class (Figure 2).
In this study, we explore for the first time the utility of anti-schistosoma antibodies in improving the diagnostic accuracy of APRI score. World Health Organization estimates that of the world’s population, about 3% has been infected with HCV and that there are approximately more than 170 million chronic carriers who are at risk of developing liver cirrhosis and liver cancer. Egypt has one of the highest prevalence of HCV and a high morbidity and mortality from chronic liver disease, cirrhosis, and hepatocellular carcinoma. About 20% of blood donors in Egypt are anti-HCV positive. Compared to other countries in the world which share similar socioeconomic conditions and equivalent sterilization, infection control and hygienic standards for invasive medical, dental, or paramedical procedures, Egypt has considerable higher rates of HCV[14]. In Egypt, the major route of exposure is considered to be due to receiving medical injections and inefficient infection control practices. Also, due to blood and blood products transfusions prior to 1994. Of note, the reported major risk factor is a history of antischistosomal injection treatment prior to 1986. Historically, Schistosomiasis used to be a common parasitic disease in Egypt which were commonly acquired through engaging in activities such as swimming or wading in Schistosoma-contaminated irrigation channels or standing water. Because, during the 90s, only glass syringes were available for treatment of schistosomiasis, which were usually inadequately sterilized by boiling, lead to an iatrogenic HCV epidemic in Egypt. Overall, despite improvement in schistosomiasis-related morbidity during 90s, such treatment campaigns set the stage for the current large hepatitis disease burden in Egypt[15].
Considering all epidemiological significance, in this study, we tried to find out if a noninvasive method called APRI is sensitive enough to predict degree of fibrosis as accurately as liver biopsy. Also we tried to find out if addition of antischistosomal Ab titer in APRI increases the diagnostic accuracy of procedure.
In this study, we found that the degrees of liver fibrosis of patients with chronic hepatitis C were strongly associated with APRI score, levels of AST, platelets count and age of patient. There was no statistically significant association with antischistosoma antibody titer and HCV RNA titer. Various other studies tried to find out multiple factors related to liver fibrosis progression in HCV infection. Inconsistent reports were found regarding association of biomarkers. In study by Ryder et al[16], age was found to have significant association, but no association was found with AST levels. Whereas, in a study by Butt et al[17], there were positive associations between HCV RNA and duration of HCV infection with fibrosis.
The APRI is calculated as (AST/upper limit of normal range)/platelet count (109/L) × 100. The accuracy of APRI was improved with higher grade of fibrosis. It was estimated using ROC curves. This finding was similar as reported by many other studies[18-20]. But few studies concluded that APRI may not be sensitive enough to be used as a routine practice. The results may have been because of smaller sample size used in such studies[21]. APRI index has been validated as a surrogate marker of significant hepatic fibrosis in HIV/HCV-coinfected patients, and has recently been used to determine advanced fibrosis in HIV-monoinfected patients. And recently metanalysis also supported its use for assessment of liver fibrosis in patients of chronic HCV infection[22].
Addition of antischistosomal antibody to APRI was also evaluated. This addition did not improve the predictive capacity of APRI. That also did not decrease the predictive capacity of APRI suggesting that it is an independent marker and is not responsible for or may be involved with very insignificant fibrosis. There are no other published studies using antischistosomal antibody with APRI for diagnosis of liver fibrosis in chronic HCV infection.
We also tried additional method for examining three-class indicators of fibrosis status. It was an extension of the ROC analysis method, known as VUS. Using this method we found that the highest misclassification was in the no fibrosis class, while the lowest misclassification was in the mild to moderate fibrosis class.
One of the main reasons for doing this study was because of many contradicting reports about HCV-schistosoma coinfection and its impact. As per many studies, the HCV-schistosoma coinfection is very important disease in Egyptian patients. It is reported that, the prevalence of schistosomal co-infection among HCV-Egyptian patients is up to 27.3%. And this figure may increase as per the histological activity index. It is suggested that this correlation may interfere with liver fibrosis assessment of Egyptian patients[23-25]. While other studies have denied any impact of schistosomal co-infection on the outcome of HCV infection, in regard of liver pathology or HCV-specific cell-mediated immunity[26].
Of note, we included only men because of the very small number of women who have been seen in our clinic. Therefore, our results might not be generalizable to women.
A validated noninvasive marker of fibrosis is useful in patients with liver fibrosis. Such a marker could help guide clinicians in whom to treat, and serve as a longitudinal marker of treatment efficacy without the need for repeated liver biopsies. Even WHO recommends using such methods in resource poor countries for monitoring. The results of this study indicate that APRI is a useful marker in patients with coinfection of HCV and schistosomiasis. Our next study is intended to try to find a more sensitive model for schistosomal patients with HCV coninfection, using other simple noninvasive parameters to improve the sensitivity of APRI score. Further research is needed in this area to verify APRI or identify other noninvasive markers that are reliable and feasible for use in patients with HCV and other coinfection like schistosoma.
The diagnostic accuracy of aminotransferase-to-platelet ratio index (APRI) Score, non-invasive fibrosis assessment tests in adult patients with chronic hepatitis C virus (HCV)-schistosomiasis co-infection, was not reported before.
Extensive validation of currently available tools, including the investigation of their sensitivity in a special group of patients, may extend the applicability of noninvasive fibrosis markers in clinical practice.
The application of APRI score in patients with combined HCV-Schistosmiasis, may decrease the need for liver biopsy, thereby reducing its associated costs and complications. The inclusion of anti-Schistosoma antibody did not improve the prediction of fibrosis stage.
This study attempted to develop practical guidance for diagnostic purposes.
The APRI score is one of several markers that have been proposed to measure liver fibrosis; aminotransferase-to-platelet ratio index has been studied to assess the degree of fibrosis in patients with HCV with proven efficacy.
In the present study, the authors have evaluated the diagnostic accuracy of APRI alone and with antischistosomal antibody in patients with HCV and schistosomiasis coinfection. This study was well designed, but there are some issues that should be checked again.
P- Reviewer: Kayadibi H, Nair DG S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Elesawy BH, Abd El Hafez A, Dorgham LS, El-Askary A. Limited reliability of five non-invasive biomarkers in predicting hepatic fibrosis in chronic HCV mono-infected patients opposed to METAVIR scoring. Pathol Res Pract. 2014;210:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Sebastiani G, Gkouvatsos K, Pantopoulos K. Chronic hepatitis C and liver fibrosis. World J Gastroenterol. 2014;20:11033-11053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | Trifan A, Stanciu C. Checkmate to liver biopsy in chronic hepatitis C? World J Gastroenterol. 2012;18:5514-5520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012;7:105-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | El-mezayen HA, Toson el-SA, Shiha GE. Role of hyaluronic acid, its degrading enzymes, degradation products, and ferritin in the assessment of fibrosis stage in Egyptian patients with chronic hepatitis C. Eur J Gastroenterol Hepatol. 2013;25:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Attallah AM, Abdallah SO, Attallah AA, Omran MM, Farid K, Nasif WA, Shiha GE, Abdel-Aziz AA, Rasafy N, Shaker YM. Diagnostic value of fibronectin discriminant score for predicting liver fibrosis stages in chronic hepatitis C virus patients. Ann Hepatol. 2013;12:44-53. [PubMed] |
| 7. | Borsoi Viana MS, Takei K, Collarile Yamaguti DC, Guz B, Strauss E. Use of AST platelet ratio index (APRI Score) as an alternative to liver biopsy for treatment indication in chronic hepatitis C. Ann Hepatol. 2009;8:26-31. [PubMed] |
| 8. | Dai CY, Ho CK, Huang JF, Hsieh MY, Hou NJ, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY. Hepatitis C virus viremia and low platelet count: a study in a hepatitis B & C endemic area in Taiwan. J Hepatol. 2010;52:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Medeiros TB, Domingues AL, Luna CF, Lopes EP. Correlation between platelet count and both liver fibrosis and spleen diameter in patients with schistosomiasis mansoni. Arq Gastroenterol. 2014;51:34-38. [PubMed] |
| 10. | World Health Organization. Hepatitis C: Fact Sheet. Available from: http://www.who.int/mediacentre/factsheets/fs164/en/. |
| 11. | Mossman D. Three-way ROCs. Med Decis Making. 1999;19:78-89. [PubMed] |
| 12. | Kang L, Tian LL. Estimation of the volume under the ROC surface with three ordinal diagnostic categories. Comput Stat Data An. 2013;62:39-51. |
| 13. | Juraj K, Martin R. Three-way ROC analysis using SAS Soft ware. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis. 2013;LXI:2269-2275. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | World Health Organization. Hepatitis C: Global response and alert. Available from: http://www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index4.html. |
| 15. | Mohamoud YA, Mumtaz GR, Riome S, Miller D, Abu-Raddad LJ. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis. 2013;13:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 16. | Ryder SD, Irving WL, Jones DA, Neal KR, Underwood JC. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004;53:451-455. [PubMed] |
| 17. | Butt AA, Yan P, Lo Re V, Rimland D, Goetz MB, Leaf D, Freiberg MS, Klein MB, Justice AC, Sherman KE. Liver fibrosis progression in hepatitis C virus infection after seroconversion. JAMA Intern Med. 2015;175:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Li SM, Li GX, Fu DM, Wang Y, Dang LQ. Liver fibrosis evaluation by ARFI and APRI in chronic hepatitis C. World J Gastroenterol. 2014;20:9528-9533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Amorim TG, Staub GJ, Lazzarotto C, Silva AP, Manes J, Ferronato Mda G, Shiozawa MB, Narciso-Schiavon JL, Dantas-Correa EB, Schiavon Lde L. Validation and comparison of simple noninvasive models for the prediction of liver fibrosis in chronic hepatitis C. Ann Hepatol. 2012;11:855-861. [PubMed] |
| 20. | Khan DA, Fatima-Tuz-Zuhra FA, Mubarak A. Evaluation of diagnostic accuracy of APRI for prediction of fibrosis in hepatitis C patients. J Ayub Med Coll Abbottabad. 2008;20:122-126. [PubMed] |
| 21. | Karoui S, Ben Romdhane S, Serghini M, Ben Mustapha N, Boubaker J, Haouet S, Filali A. [Is APRI score a suitable tool for prediction of fibrosis in Tunisian patients with genotype 1 chronic viral hepatitis C?]. Tunis Med. 2012;90:282-285. [PubMed] |
| 22. | Baranova A, Lal P, Birerdinc A, Younossi ZM. Non-invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011;11:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 23. | Abdel-Rahman M, El-Sayed M, El Raziky M, Elsharkawy A, El-Akel W, Ghoneim H, Khattab H, Esmat G. Coinfection with hepatitis C virus and schistosomiasis: fibrosis and treatment response. World J Gastroenterol. 2013;19:2691-2696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47-52. [PubMed] |
| 25. | Bonnard P, Elsharkawy A, Zalata K, Delarocque-Astagneau E, Biard L, Le Fouler L, Hassan AB, Abdel-Hamid M, El-Daly M, Gamal ME. Comparison of liver biopsy and noninvasive techniques for liver fibrosis assessment in patients infected with HCV-genotype 4 in Egypt. J Viral Hepat. 2015;22:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Allam WR, Barakat A, Zakaria Z, Galal G, Abdel-Ghafar TS, El-Tabbakh M, Mikhail N, Waked I, Abdelwahab SF. Schistosomiasis does not affect the outcome of HCV infection in genotype 4-infected patients. Am J Trop Med Hyg. 2014;90:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |