Published online Dec 14, 2015. doi: 10.3748/wjg.v21.i46.13095
Peer-review started: February 1, 2015
First decision: March 16, 2015
Revised: August 4, 2015
Accepted: August 28, 2015
Article in press: August 31, 2015
Published online: December 14, 2015
Processing time: 311 Days and 6.3 Hours
AIM: To evaluate the prevalence and characteristics of colorectal adenoma and carcinoma in an inner city Hispanic population.
METHODS: We reviewed the reports of 1628 Hispanic patients who underwent colonoscopy at Howard University from 2000 to 2010. Advanced adenoma was defined as adenoma ≥ 1 cm in size, adenomas with villous histology, high grade dysplasia and/or invasive cancer. Statistical analysis was performed using χ2 statistics and t-test.
RESULTS: The median age of the patients was 54 years, 64.2% were females. Polyps were observed in 489 (30.0%) of patients. Adenoma prevalence was 16.8% (n = 273), advanced adenoma 2.4% (n = 39), and colorectal cancer 0.4% (n = 7). Hyperplastic polyps were seen in 6.6% of the cohort (n = 107). Adenomas predominantly exhibited a proximal colonic distribution (53.7%, n = 144); while hyperplastic polyps were mostly located in the distal colon (70%, n = 75). Among 11.7% (n = 191) patients who underwent screening colonoscopy, the prevalence of colorectal lesions was 21.4% adenoma, 2.6% advanced adenoma; and 8.3% hyperplastic polyps.
CONCLUSION: Our data showed low colorectal cancer prevalence among Hispanics in the Washington DC area. However, the pre-neoplastic pattern of colonic lesions in Hispanics likely points toward a shift in this population that needs to be monitored closely through large epidemiological studies.
Core tip: Hispanics are becoming a sizable portion of the United States population and are one of the fastest growing demographic groups. Like other minorities, their adherence to screening colonoscopy is low. With changes in their diet and lifestyle, they seem to be presenting more colorectal lesions of neoplastic nature than their counterparts in their home countries. Increased attention and awareness within this population is needed to preempt an increase in colorectal cancer incidence.
- Citation: Ashktorab H, Laiyemo AO, Lee E, Cruz-Correa M, Ghuman A, Nouraie M, Brim H. Prevalence and features of colorectal lesions among Hispanics: A hospital-based study. World J Gastroenterol 2015; 21(46): 13095-13100
- URL: https://www.wjgnet.com/1007-9327/full/v21/i46/13095.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i46.13095
Hispanics/Latinos are the largest and fastest growing ethnic group in the United States. In 2010 there were 50.5 million Hispanics in the United States, comprising 16% of the total population[1]. It is projected that there will be 132.8 million Hispanics by 2050 representing 30% of the nation’s population by that time[1]. As such, public health issues in this population will have major effects on the population at large.
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths among men and women in the United States. It is estimated that 141210 patients will be diagnosed with colorectal cancer and about 49380 will die of the disease in the United States[2]. Among all ethnic groups in the United States, Hispanics have the lowest burden of the disease[3]. CRC is the second most commonly diagnosed cancer among Hispanic men and women. It is the second leading cause of cancer deaths in Hispanic men and third leading cause of cancer deaths among Hispanic women[2]. In 2009, 10400 new cases and 3100 deaths from colorectal cancer were expected among Hispanic men and women.
Although Hispanics have lower rates of CRC incidence and mortality than other ethnic groups, the rates among Hispanics born and raised in the continental United States are higher than those among residents of Puerto Rico and Spanish-speaking countries in South and Central America[4,5]. It was thought that diets which are higher in fat, refined carbohydrates, and animal proteins and lower levels of physical activity contributed to this increased CRC incidence.
Hispanics are more likely to be diagnosed with advanced stage colorectal cancer than non-Hispanic whites and have a lower probability of survival after diagnosis after accounting for differences in age and stage[6]. Five years survival after diagnosis with CRC in Hispanics is 58.6% compared to 65.1% in non-Hispanic whites[3].
Factors that may contribute to survival disparities include less access to and lower use of colorectal cancer screening tests and less access to timely and high-quality treatments. Generally, Hispanics have low income and low education levels[7]. Lack of insurance has also been found to be associated with lower cancer screening rates in this population[8].
Hispanics have lower colorectal cancer screening rates than any other minority group in the United States. In adults over 50 years, the rates of Hispanics reporting sigmoidoscopy within the past five years or colonoscopy within the past ten years was only 34.6%, the lowest of all the ethnic groups to which they were compared [non-Hispanic/Latino whites (52.7%), African Americans/blacks (47.3%), Asian Americans (42.6%)].
Colorectal carcinoma usually arises from an adenomatous polyp and observational studies suggest that the adenoma-to-carcinoma sequence takes approximately 10 to 15 years[9]. The hypothesis that invasive colorectal carcinoma develops from intermediate precancerous precursors is supported by pathologic, epidemiologic and observational clinical data, both in humans and in animal models. As such, the early detection of precancerous lesions will have a major impact on CRC incidence in this population.
Here, we analyzed colon lesions’ prevalence in an inner city Hispanic community in Washington DC to determine trends of colorectal neoplasia in this population.
We reviewed the medical records of 21201 patients who had undergone colonoscopy from January 2000 to December 2010 at Howard University Hospital, Washington, DC. The Howard University Institutional Review Board has approved the present study. Demographic information was collected from medical coding and billing section based on ICD-9 classification. Only self-identified Hispanic patients (n = 1628) were included in the study. All included subjects were outpatients. This included 583 (35.8%) males and 1045 (64.2%) females. Pathology reports of patients who had either biopsy or polypectomy were obtained from the pathology department. The number and location of polyps were recorded during the colonoscopy. The histology and sizes of the polyps were obtained from pathology records. Adenomas 1 cm or greater in size, those with villous histology, with high grade dysplasia and/or invasive cancer were considered advanced adenomas. Polyps located from cecum to splenic flexure were considered proximal while those from other parts of the colon including the rectum were classified as distal.
Distribution of variables was explored by table of frequency or median (IQR). Categorical variables including sex, indication for colonoscopy, and diagnosis in histopathology and polyp location were explored and compared using χ2 test. A logistic model was developed to assess independent predictors of finding a polyp by colonoscopy. P values less than 0.05 were considered significant. We used STATA 12.0 (StataCorp. College Station, TX) for all data analyses.
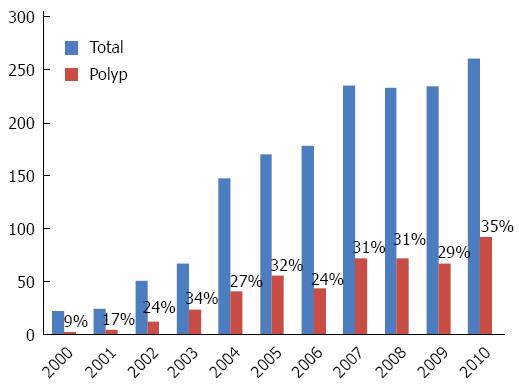
One thousand six hundred twenty eight colonoscopies were reported in Hispanics from 2000 to 2010. The number of colonoscopies increased from 22 in 2000 to 261 in 2010 (P < 0.0001). The frequency of polyp diagnosis was 489 (30%). This frequency was 9% in 2000 and increased to 35% in 2010 (Figure 1).
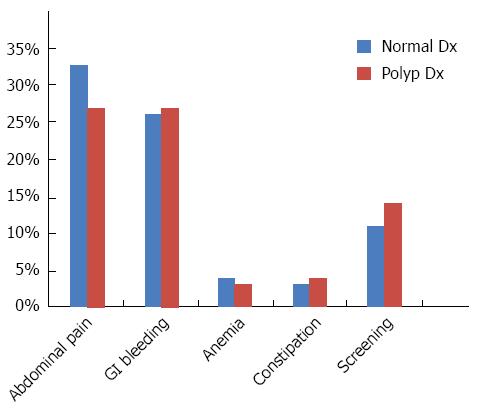
The most common indications for colonoscopy were abdominal pain and blood in stool/GI bleeding. A sizable portion of colonoscopies were performed for screening in patients without symptoms (Figure 2). Few patients displayed more than one symptom with some abdominal pain associated with constipation and GI bleeding associated with anemia. Abdominal pain was the primary indication in patients whose colonoscopy led to polyps’ detection (Figure 2).
Males represented 36% of the study group. Males made up 41% of the patients with polyps and were found to be statistically more prone to develop polyps than females (P = 0.005). Patients with polyps were older than those without polyps (median age 56 years vs 52 years, P < 0.001).
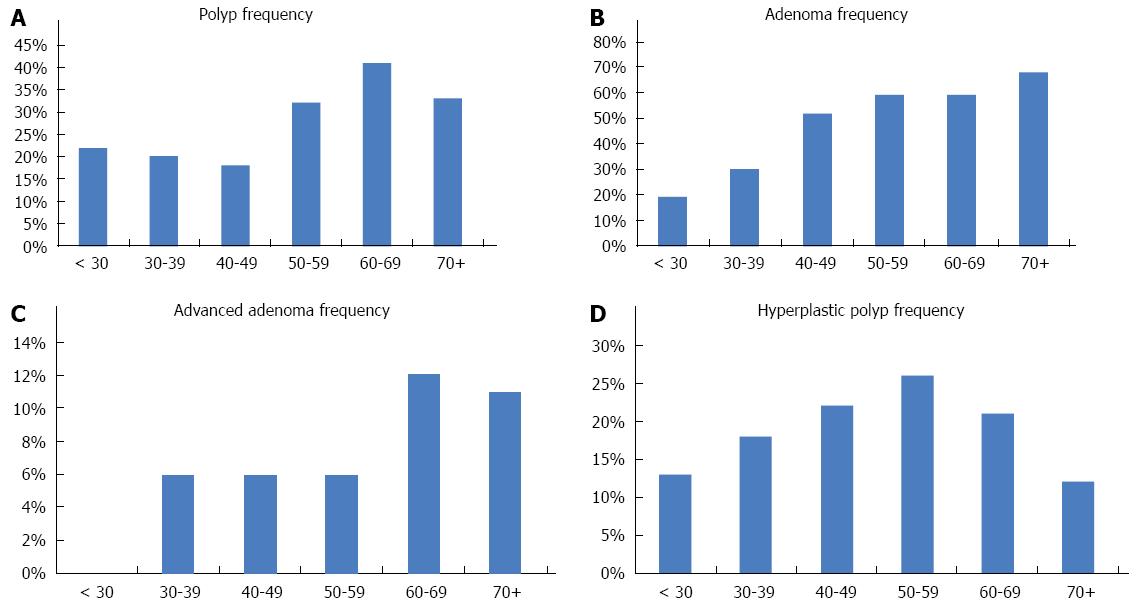
The most frequent type of polyps were tubular adenoma (n = 236, 48%), hyperplastic polyps (n = 81, 17%), mixed polyps (n = 29, 6%), inflammatory polyps (n = 18, 4%) and cancers (n = 7, 0.4%). Regarding polyp location, 45% were distal, 38% were proximal while in 17% of patients, polyps were located on both sides of the colon. The polyps’ presence was found to be strongly associated with patients’ age with a peak at 60-69 years (P < 0.0001) (Figure 3A).
Adenoma: Two hundred and seventy three (56%) polyps were diagnosed as adenomas. Median (IQR) adenoma size was 0.4 (0.3-0.6). Table 1 shows demographic and clinical variables for patients with and without adenomas. There were no differences between these two groups regarding symptoms’ distribution (P = 0.3). Adenomas were located in distal (34%), proximal (41%) and multiple colon locations (24%) (Table 1).
| No adenomatous polyps | Adenomatous polyps | P value | |
| n = 216 | n = 273 | ||
| Male gender | 93 (43) | 107 (39) | 0.4 |
| Age, median (IQR) | 54 (47-62) | 57 (52-64) | < 0.0001 |
| Number of polyps, median (range) | 1 (0-5) | 1 (0-8) | 0.0001 |
| Location of polyps1 | < 0.0001 | ||
| Distal | 124 (58) | 88 (34) | |
| Proximal | 70 (33) | 107 (41) | |
| Both | 18 (8) | 63 (24) |
In multiple logistic regression analysis, each year was associated with an OR = 1.03 (95%CI: 1.02-1.05, P < 0.0001, Figure 3B), proximal location was associated with an OR = 2.05 (95%CI: 1.36-3.10, P < 0.0001) while multiple locations was associated with an OR = 4.76 (95%CI: 2.61-8.67, P < 0.0001) for prevalence of adenoma.
Advanced adenoma: There were 39 (8%) advanced adenomas among the patients with polyps. Median (IQR) of adenoma size was 1.1 (1.0-1.3) for advanced adenoma. Table 2 shows demographic and clinical variables for patients with and without advanced adenomas. Figure 3C shows that advanced adenomas are more prevalent in patients older than 60 years.
| No advanced adenoma | Advanced adenoma | P value | |
| n = 450 | n = 39 | ||
| Male sex | 185 (41) | 15 (38) | 0.7 |
| Age, median (IQR) | 56 (51-62) | 62 (53-66) | 0.010 |
| Number of polyp, median (range) | 1 (0-5) | 2 (0-8) | 0.0001 |
| Location of polyps1 | < 0.0001 | ||
| Distal | 203 (46) | 9 (29) | |
| Proximal | 169 (39) | 8 (26) | |
| Both | 67 (15) | 14 (45) |
There was no statistically significant difference between these two groups regarding symptoms’ distribution (P = 0.1). Advanced adenomas were located in distal colon (29%), proximal colon (26%) and multiple locations (45%). In multiple logistic regression analysis, multiple location was associated with an OR = 4.71 (95%CI: 1.95-11.38, P = 0.001) for risk of advanced adenoma.
Hyperplastic polyps: One hundred seven (22%) polyps were hyperplastic polyps (HP). Median (IQR) of HP size was 0.4 (0.3-0.5). Table 3 shows demographic and clinical variables of patients with and without HP polyps. These polyps were more frequent at 50-59 years of age (Figure 3D), much earlier than adenomas and advanced adenomas’ age of onset.
| No HP | HP | P value | |
| n =382 | n = 107 | ||
| Male gender | 149 (39) | 51 (48) | 0.1 |
| Age, median (IQR) | 57 (51-64) | 55 (51-62) | 0.7 |
| Number of polyp, median (range) | 1 (0-8) | 1 (0-5) | 0.1 |
| Location of polyps1 | < 0.0001 | ||
| Distal | 154 (42) | 58 (57) | |
| Proximal | 162 (44) | 15 (15) | |
| Both | 53 (14) | 28 (28) |
There were no differences between HP and non-HP groups regarding symptoms’ distribution (P = 0.2). Hyperplastic polyps were located in distal (57%), proximal (15%) and multiple colon locations (28%). In a logistic regression, proximal location of polyps was associated with an OR = 0.25 (95%CI: 0.13-0.45, P < 0.0001) for risk of HP.
Subgroup analysis of more recent patients (2006-2010): Among 1144 patients who underwent colonoscopy after 2005, the prevalence of polyps was 31% (n = 349). Among patients with polyps in colonoscopy, the prevalence of adenoma, advanced adenoma and HPP were 55%, 7% and 24%, respectively. Restricting the analysis to recent colonoscopies doesn’t affect the predictor of adenoma or HPP. With this restriction, older age will be added to significant predictors of advanced adenoma (OR = 1.05, 95%CI: 1.01-1.09, P = 0.023).
It is well known that CRC is a major public health problem in the United States and worldwide. The rate of sporadic CRC is about 85% of all recorded cases[10]. These sporadic cases are primarily triggered by environmental exposures[11,12]. Indeed most ethnic groups in the United States have higher CRC rates than their counterparts in their countries of origin. This is true for African Americans and Asians and most likely will apply in the future to other populations including Hispanics which constitute the fastest growing component of the United States population. Studies of CRC in this population are scarce, which is likely due to the fact that Hispanics show a low CRC incidence.
We conducted the present study in an inner city Hispanic population from the Washington DC area. The colonoscopy reports for the 2000-2010 decade at Howard University Hospital in DC were reviewed and pathology reports were analyzed to shed light on specific characteristics of colonic lesions in Hispanics. The number of colonoscopies rose every year of the analyzed decade and along with it the number of detected polyps. This trend is similar to other populations as colonoscopy has been popularized widely since the year 2000[10]. The advertisement campaign has been leading to increases in CRC screening in the United States population at large, including in minorities.
The rate of polyps was relatively moderate in the years up to 2005, but increased to more than 30% in the years 2006 to 2010. The primary symptoms that led to most of the colonoscopies were abdominal pain and blood in the stools, similar to other populations as well[13,14]. Other symptoms included anemia and constipation. It is worth noting, that a sizable portion of colonoscopies were performed for screening, a trend that needs to be encouraged especially in minority populations. The detected polyps were predominantly found in patients older than 50 years with the group of patients between 60 and 69 years with the highest yield of colorectal neoplasia. However, many polyps were detected at much younger age pointing, probably, to increased occurrence of colonic lesions in this population (Figure 3A). While most of these lesions are of benign nature (hyperplastic, non adenomatous polyps), they might be pointing to a potential increase in colon oncogenic transformation in this population in the future.
Among the 489 colonic lesions detected, 273 (56%) were of adenomatous nature. The mean age of patients with adenoma was 57 years and their location was primarily proximal. However, distally located adenomas were also detected in the analyzed population. Of these 273 adenomas, 39 were of advanced nature, as defined by their large size, numbers and villous histology. The characteristics of patients with advanced adenomas included a higher median age (62 years) and multiple polyps distributed all over the colon. These findings are consistent with the fact that age is a risk factor for colon cancer development and also the presence of multiple polyps at different locations pointing to pancolonic neoplastic predisposition[15,16].
Hyperpalstic polyps were found in 107 (22%) patients. The median age of HP patients was 55 years. The lesions were predominantly distal (58%). These findings are in correlation with the preferential proximal location of adenoma and advanced adenomas. Indeed the proximal colon is the seat of high bacterial activity that is thought to participate in the creation of stressful conditions for the colon mucosa[11,17,18]. It is also important to note that the proximal colon’s lesions are generally highly targeted by DNA methylation than distal lesions.
We only found 7 cancers in Hispanics seen at Howard University from 2000 to 2010. This low number points still to a low incidence of the disease in this population. However, the pattern of pre-neoplastic lesions seems to be following that of the general population although this needs to be confirmed in large follow-up studies. Would that mean that Hispanics will acquire the same CRC rate as the general population? That is a possibility, especially in light of changes in cancer patterns in other populations upon their migration to the United States. Another possibility might be that Hispanics’ pre-neoplastic lesions lack the cumulative effect of genetic and epigenetic alterations to drive benign lesions to malignancy. Thorough exome, whole genome methylation and other high throughput studies are required to address this issue. Nationwide multicenter studies are also required to confirm the findings of our study to assess possible future impacts of observed Hispanic colonic lesions on general public health.
Hispanic Americans are the fastest growing segment of the United States population. Western diet and lifestyle expose them to an increased risk of colorectal neoplasia.
Minority groups are at higher risk of colorectal neoplasia, a treatable disease if caught at early stages.
This is one of the first studies that are addressing colorectal neoplasia prevalence in Hispanic Americans, a group that is thought to have a low prevalence of the disease.
The authors demonstrated that at our institution, the rate of colonoscopy have increased in the study period (2000 to 2010) and with it the rate of detected colorectal lesions.
The above manuscript aims to characterise adenoma and cancer prevalence in an inner city Hispanic population with respect to other ethnic groups.
P- Reviewer: Park JH, Pinsky PF S- Editor: Ji FF L- Editor: A E- Editor: Liu XM
| 1. | US Census Bureau. US Interim Projections by Age, Sex, Race, and Hispanic Origin. 2010 [2014, October 2]. Available from: http://www.census.gov/newsroom/releases/archives/population/cb08-123.html. |
| 2. | American Cancer Society. Cancer Facts & Figures, 2011-13. 2013 [2014, October 3]. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-028312.pdf. |
| 3. | SEER - National Cancer Institute. SEER Incidence, Age-Adjusted Rates and Trendsa By Race/Ethnicity and Sex. 2014 [2014, October 3]. Available from: http://seer.cancer.gov/csr/ 1975_2008/results_single/ sect_01_table.01.pdf. |
| 4. | Cancer incidence in five continents. Volume VIII. IARC Sci Publ. 2002;1-781. |
| 5. | Soto-Salgado M, Suárez E, Calo W, Cruz-Correa M, Figueroa-Vallés NR, Ortiz AP. Incidence and mortality rates for colorectal cancer in Puerto Rico and among Hispanics, non-Hispanic whites, and non-Hispanic blacks in the United States, 1998-2002. Cancer. 2009;115:3016-3023. [PubMed] |
| 6. | Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004;101:3-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 774] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 7. | Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 567] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 8. | Institute of Medicine (U.S.). Committee on the Consequences of Uninsurance Care without coverage: too little, too late 2002. Washington, DC: National Academy Press 2002; xvi 193. |
| 9. | American Cancer Society. Surveillance Research. 2009; Available from: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2009/acs/groups/content/@research/documents/document/acspc-038893.ppt. |
| 10. | Nouraie M, Hosseinkhah F, Brim H, Zamanifekri B, Smoot DT, Ashktorab H. Clinicopathological features of colon polyps from African-Americans. Dig Dis Sci. 2010;55:1442-1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Brim H, Kumar K, Nazarian J, Hathout Y, Jafarian A, Lee E, Green W, Smoot D, Park J, Nouraie M. SLC5A8 gene, a transporter of butyrate: a gut flora metabolite, is frequently methylated in African American colon adenomas. PLoS One. 2011;6:e20216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Sears CAH, Brim H. Do the Colonic Microbial Flora Serve to Trigger Colon Cancer? In AGA Perspectives. Bethesda, MD: AGA Institute 2010; 18-19. |
| 13. | Vega-Villaamil P, Salve-Bouzo M, Cubiella J, Valentín-Gómez F, Sánchez-Hernández E, Gómez-Fernández I, Fernández-Seara J. Evaluation of the implementation of Galician Health Service indications and priority levels for colonoscopy in symptomatic patients: prospective, cross-sectional study. Rev Esp Enferm Dig. 2013;105:600-608. [PubMed] |
| 14. | Olokoba AB, Obateru OA, Bojuwoye MO, Olatoke SA, Bolarinwa OA, Olokoba LB. Indications and findings at colonoscopy in Ilorin, Nigeria. Niger Med J. 2013;54:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 16. | Nguyen SP, Bent S, Chen YH, Terdiman JP. Gender as a risk factor for advanced neoplasia and colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:676-681.e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Brim H, Zahaf M, Laiyemo AO, Nouraie M, Pérez-Pérez GI, Smoot DT, Lee E, Razjouyan H, Ashktorab H. Gastric Helicobacter pylori infection associates with an increased risk of colorectal polyps in African Americans. BMC Cancer. 2014;14:296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Brim H, Yooseph S, Zoetendal EG, Lee E, Torralbo M, Laiyemo AO, Shokrani B, Nelson K, Ashktorab H. Microbiome analysis of stool samples from African Americans with colon polyps. PLoS One. 2013;8:e81352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |