Published online Dec 7, 2015. doi: 10.3748/wjg.v21.i45.12954
Peer-review started: May 12, 2015
First decision: July 19, 2015
Revised: August 7, 2015
Accepted: September 30, 2015
Article in press: September 30, 2015
Published online: December 7, 2015
Processing time: 221 Days and 10.1 Hours
AIM: To compare the effectiveness of hybrid therapy with other recommended regimens using meta-analysis.
METHODS: Bibliographical searches for randomized trials comparing hybrid and other therapies were performed in PubMed, the Cochrane Library and relevant congresses up to February 2015 using the following keywords (all fields and/or MeSH): (“Helicobacter pylori” or “H. pylori”) and (“hybrid therapy” or “sequential-concomitant therapy”). Meta-analyses were performed with Cochrane Review Manager 5.1. The random effect model proposed by DerSimonian and Laird and the Mantel-Haenszel method were used to estimate the pooled relative risk and 95%CI of the efficacy outcomes between hybrid therapy and other eradication therapies.
RESULTS: Eight studies (2516 subjects) met entry criteria. The antimicrobial resistance in the study groups ranged from 6.9% to 23.5%. The mean cure rates of hybrid therapy by intention-to-treat (ITT) and per-protocol analyses were 88.5% (n = 1207; range: 80.0% to 97.4%) and 93.3% (n = 1109; range: 85.7% to 99.1%), respectively. Meta-analysis showed there was no significant difference in ITT eradication rate between hybrid and sequential therapy (relative risk: 1.01; 95%CI: 0.92-1.11). Subgroup analysis revealed hybrid therapy was more effective than sequential therapy in the non-Italian populations (95%CI: 1.01-1.18) and was only less effective in one, Italian population (95%CI: 0.83-0.98). There was no significant difference in eradication rate between hybrid therapy and concomitant therapy (95%CI: 0.93-1.02). No head-to-head comparisons of hybrid therapy and standard triple therapy or bismuth quadruple therapy were found. However, a multicenter, randomized trial showed that reverse hybrid therapy was superior to standard triple therapy (95.5% vs 88.6% ITT; P = 0.011).
CONCLUSION: Hybrid therapy appears to be an effective, safe, and well-tolerated treatment for H. pylori infection in the era of increasing antibiotic resistance.
Core tip: This article is aimed to review current evidences of hybrid therapy for Helicobacter pylori infection. The mean cure rates of hybrid therapy by intention-to-treat and per-protocol analyses were 88.5% and 93.3%, respectively. Meta-analysis showed that hybrid therapy was more effective than sequential therapy in the non-Italian population. In contrast, it was less effective than sequential therapy in the Italian population. There was no significant difference in eradication rate between hybrid therapy and concomitant therapy. Reverse hybrid therapy is a new one-step tow-phase treatment, achieving a higher eradication rate than standard triple therapy with similar tolerability and less pharmaceutical cost.
-
Citation: Hsu PI, Lin PC, Graham DY. Hybrid therapy for
Helicobacter pylori infection: A systemic review and meta-analysis. World J Gastroenterol 2015; 21(45): 12954-12962 - URL: https://www.wjgnet.com/1007-9327/full/v21/i45/12954.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i45.12954
Helicobacter pylori (H. pylori) treatment continues to be a challenge for physicians as antimicrobial resistance has continued to increase worldwide in part due to overuse of antibiotics in medicine and agriculture[1]. While many international guidelines still recommend standard triple therapy as a first-line therapy they often include a caveat about the problem of increasing clarithromycin resistance[2-4]. Several recent large clinical trials and meta-analyses have shown that the eradication rate of standard triple therapy has generally declined to unacceptable levels (i.e., 80% or less) with some European studies reporting failure rates of 25%-60%[5-7]. Several strategies including bismuth-containing and non-bismuth-containing quadruple therapies (including sequential, concomitant and hybrid therapies) have been shown to produce acceptable cure rates in the presence of clarithromycin resistance[8-10].
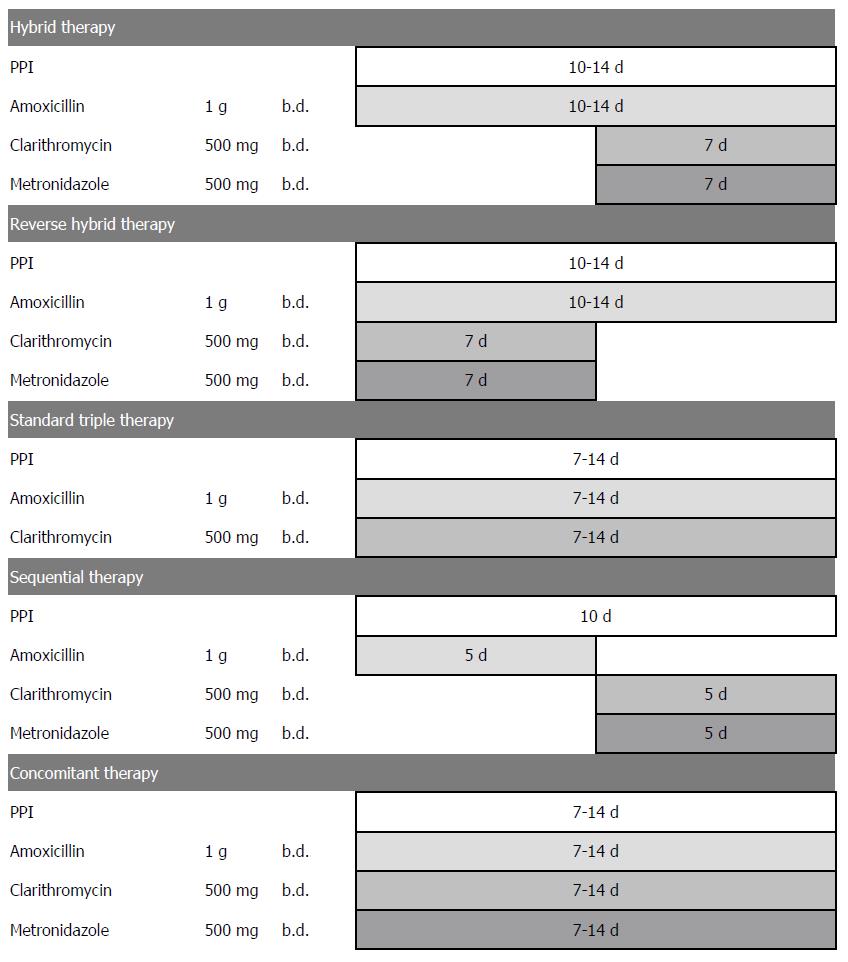
The standard hybrid regimen is functionally a combination of sequential therapy and concomitant therapy as it consists of a proton pump inhibitor (PPI) and amoxicillin for 10-14 d with the addition of clarithromycin and metronidazole for the final 7 d (i.e., a 2 + 4 regimen) (Figure 1). The original study[11] produced an eradication rate of 99.1% (95%CI: 97.3%-100.9%) by per-protocol (PP) analysis and 97.4% (95%CI: 94.5%-100.3%) by intention-to-treat (ITT) analysis. Several subsequent randomized trials confirmed that hybrid regimens were either comparable with or more effective than sequential therapy[12-15]. A recent large multicenter randomized trial in areas with high clarithromycin and metronidazole resistance confirmed that both 14-d hybrid and concomitant therapies cured more than 90% of H. pylori infections[16]. Additionally, a recent clinical trial from Taiwan tested the efficacy of a 12-d reverse hybrid therapy (a 4 + 2 regimen) (Figure 1) and achieved grade A success (≥ 95% cure rate) in an area with moderately high clarithromycin resistance (13%) and high metronidazole resistance (31%)[17]. Recent expert recommendations have proposed hybrid therapy as a treatment option for H. pylori in areas with moderate or high clarithromycin resistance[1,18,19].
This article reviewed the efficacy of hybrid therapy in the treatment of H. pylori infection and compared the treatment success of hybrid and other recommended regimens.
Bibliographical searches were performed in PubMed and the Cochrane Library up to February 2015 using the following keywords (all fields and/or MeSH): (“Helicobacter pylori” or “H. pylori”) and (“hybrid therapy” or “sequential-concomitant therapy”). Articles published in any language were included. Reference lists from the trials selected in the electronic search were hand-searched to identify further relevant trials. We also conducted a manual search of abstract from the scientific meetings of the International Workshop of the American Digestive Disease Week, the United European Gastroenterology Week, the European Helicobacter Study Group and the Asian Pacific Digestive Week. Abstracts of the articles selected in each of these multiple searches were assessed by two reviewers. In case of duplicate reports or studies obviously reporting results from the same study population, only the published results with largest numbers of cases were retrieved. All articles included were randomized controlled trials. All patients were H. pylori treatment naïve and had not used antibiotics or bismuth citrate in the preceding month. Nonrandomized studies were excluded, as were case reports, letters, editorials, commentaries, reviews, and abstracts with insufficient details to meet the inclusion criteria.
Meta-analyses were performed with Cochrane Review Manager 5.1. [Review Manager (RevMan) (Computer program), Version 5.1, Copenhagen: the Nordic Cochrane Centre, the Cochrane Collaboration, 2011]. The random effect model proposed by DerSimonian and Laird[20] and the Mantel-Haenszel method[21] were used to estimate the pooled relative risk and 95%CI of the efficacy outcomes between hybrid therapy and other eradication therapies from eligible studies. The Mantel-Haenszel method was used for within-study variance and the DerSimonian and Laird method was used for incorporating both within-study and between study variances. Heterogeneity was assessed using Cochran’s Q statistics and quantified using the I2 statistics[22]. I2 statistic represented the percentage of total variation attributable to between-study heterogeneity rather than sampling error. I2 values of approximately 25%, 50%, and 75% were considered as representing low, moderate, and high heterogeneity, respectively.
In populations with low to absent clarithromycin resistance, all modern clarithromycin regimens containing either 3 or 4 drugs reliably achieve cure rates of 90% or greater if given for 7 or more days. However, clarithromycin resistance compromises the efficacy of standard triple therapy and a number of studies have shown that sequential therapy (i.e., a PPI plus amoxicillin for 5 d followed by a PPI plus clarithromycin and metronidazole or a 2 + 3 regimen) was more effective than standard triple therapy in areas of high clarithromycin resistance[23]. However in regions with high levels of metronidazole resistance the success rate with sequential therapy falls off significantly[24-26]. As there was no clear rationale for dropping the amoxicillin during the final portion of sequential therapy, a group of investigators from Taiwan and the United States examined whether continuing the amoxicillin through the second phase (i.e., making a 2 + 4 regimen from sequential’s 2 + 3 regimen) would increase the cure rate[11]. The initial study showed that 14-d hybrid therapy (a PPI plus amoxicillin for the first 7 d followed by a quadruple regimen with a PPI plus amoxicillin, clarithromycin and metronidazole for the final 7 d) achieved > 95% H. pylori eradication in a population with a low rate of clarithromycin resistance (7.0%). They then evaluated whether the duration of hybrid therapy could be reduced while maintaining a high eradication rate[27]. They randomized 220 H. pylori-infected subjects to 10-d, 12-d, or 14-d hybrid therapies consisting of esomeprazole 40 mg and amoxicillin 1 gm twice daily for 10, 12, or 14 d plus clarithromycin 500 mg, and metronidazole 500 mg twice daily for the final 7 d. The population studied had a clarithromycin resistance of 6.9% and metronidazole resistance rate of 31.0%. The eradication rates with PP analyses were similar: 95.0% for 10-d, 95.1% for 12-d, and 93.4% for 14-d hybrid therapies[27]. These results suggested that in contrast to sequential therapy, hybrid therapy was highly successful despite a high prevalence of metronidazole resistance.
Switching drugs halfway through the course increases the complexity of hybrid therapy. Reversing the sequence of drug administration of hybrid therapy simplifies the treatment and makes it become a one-step two-phase therapy (Figure 1). The study group therefore tested the efficacy of 12-d reverse hybrid therapy (i.e., 4 drugs followed by 2 drugs)[17]. The 12-d reverse hybrid therapy achieved an eradication rate of 95.5% (191/200) and 95.9% (186/194) by intention-to-treat and per-protocol analysis, respectively in a population with 13% clarithromycin resistance and 32% metronidazole resistance.
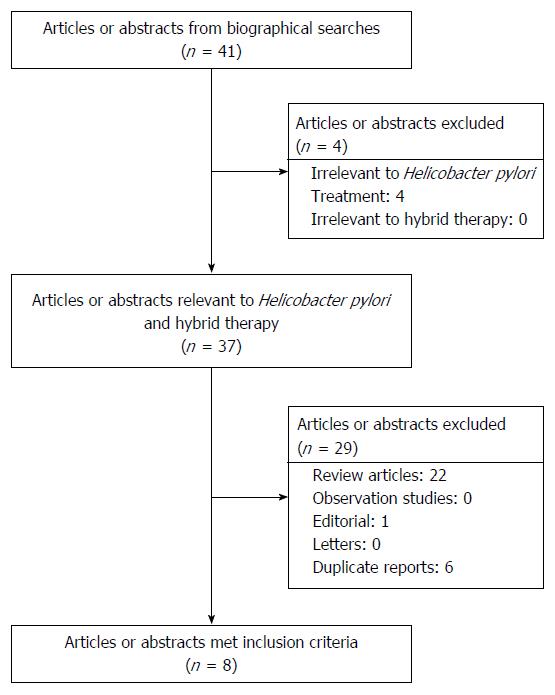
Our initial search yielded 41 citations (Figure 2). Of these, four were excluded because they were irrelevant to H. pylori infection. Twenty-two review articles, one editorial and six duplicate reports were also excluded. Eight randomized controlled trials[11-17,27] were eligible for analysis. These eight studies were performed in Asia and Europe with clarithromycin, amoxicillin and metronidazole resistances ranging from 6.9% to 23.5%, 0% to 1.8% and 20.7% to 56.1%, respectively. Similar hybrid regimens were prescribed, with minor modifications, namely, the PPI used (esomeprazole, pantoprazole, and omeprazole), the nitroimidazole (metronidazole or tinidazole), the duration (between 10 and 14 d) and the sequence of drug administration (standard hybrid or reverse hybrid). The eradication rates in different geographic areas ranged from 85.7% to 99.1% by per-protocol (PP) analysis with of mean H. pylori cure rate of 93.3% (n = 1109; 95%CI: 91.8%-94.8%). The mean cure rate ITT was 88.5% (n = 1207; range: 80.0% to 97.4%; 95%CI: 86.7%-90.3%).
The prevalence of adverse effects of hybrid therapy ranged from 9.0% to 47.1%[11-17,27]. Overall, the mean prevalence of adverse effects was 24.7% (95%CI: 22.3%-27.1%). The profiles and frequencies of adverse events are listed in Table 1. The most frequent adverse effects were abdominal pain (1.4%-12.8%), diarrhea (0.5%-11.6%), taste perversion (1.0%-18.1%) and headache (0.5%-12.9%). Most adverse events were mild to moderate in severity. The frequency of adverse effects required the interruption of therapy was 3.6% (95%CI: 2.5%-4.6%; range: 2.0%-6.7%). In general, hybrid therapy was well tolerated. The overall good compliance rate was 96.2% (95%CI: 95.1%-97.3%; range 93.8%-98.8%).
| Adverse events | Frequency |
| Abdominal pain | 1.4%-12.8% |
| Diarrhea | 0.5%-11.6% |
| Constipation | 0%-8.6% |
| Taste perversion | 1.0%-18.1% |
| Headache | 0.5%-12.9% |
| Dizziness | 1.9%-5.1% |
| Nausea | 1.8%-7.5% |
| Vomiting | 0.5%-7.1% |
| Skin rash | 0%-2.9% |
Antimicrobial resistance is a key factor determining H. pylori eradication rates[25,28-32]. Clarithromycin resistance negates the effect of clarithromycin reducing triple therapy to dual PPI amoxicillin therapy. A meta-analysis showed that with standard triple therapy clarithromycin resistance was associated with an average decline in eradication rate of approximately 60%[29]. Clarithromycin resistance also reduces the efficacy of sequential therapy but markedly less than with standard triple therapy likely because of the addition of a forth antimicrobial, metronidazole. A recent meta-analysis showed the overall eradication rate of sequential therapy in patients harboring strains resistant to clarithromycin was 72.8% (95%CI: 61.1%-82.8%)[32]. With regard to hybrid therapy, four recent prospective studies[11,16,17,27] reported that clarithromycin resistance had less effect on eradication rate of the new therapy [i.e., susceptible = 99.1% (105/106) and resistant = 85.7% (12/14)], respectively. This difference did not achieve statistical significance but the number of strains with resistance was small. Importantly, the effect of metronidazole resistance on the efficacy of hybrid therapy also appeared minor 100% (68/68) and 94.2% (49/52) for clarithromycin-sensitive and resistant strains, respectively.
Sequential therapy is ineffective in patients with dual resistance (clarithromycin and metronidazole)[23]. Recently, Wu et al[33] investigated the effect of dual resistance on the efficacy of sequential and concomitant therapies and reported that it did not significantly influence the effectiveness of concomitant therapy. Overall, the number of clarithromycin- and metronidazole-dual resistant strains exposed to 14-d hybrid therapy was low (only 14 in the four studies with 10 being successfully eradicated). An accurate determination of the efficacy of hybrid therapy in dual resistant strains will require additional studies.
As with all antimicrobial therapies, good adherence to the regimen has also proven to be a significant predictor of successful eradication with hybrid therapy with cure rate of 91% and 50% in patients with compliance ≥ 80% and < 80%, respectively[16]. The cure rate achieved by hybrid therapy was also not significantly affected by the type of gastrointestinal disease (peptic ulcer vs non-ulcer dyspepsia), smoking, and CYP2C19 genotype[11,17,26].
Head-to-head comparisons of hybrid therapy and sequential therapy has been performed in 5 randomized trials[11-15,34]. A prospective randomized trial from Iran (a country with a high rate of clarithromycin and metronidazole resistance) demonstrated that 14-d hybrid therapy was superior to 10-d sequential therapy either by ITT (89.5% vs 76.7%, P = 0.001) or PP analyses (92.9% vs 79.9%, P = 0.001)[12]. The other four randomized controlled trials were done in populations with lower frequencies of resistance and reported comparable cure rates with hybrid and sequential therapies[11,13-15].
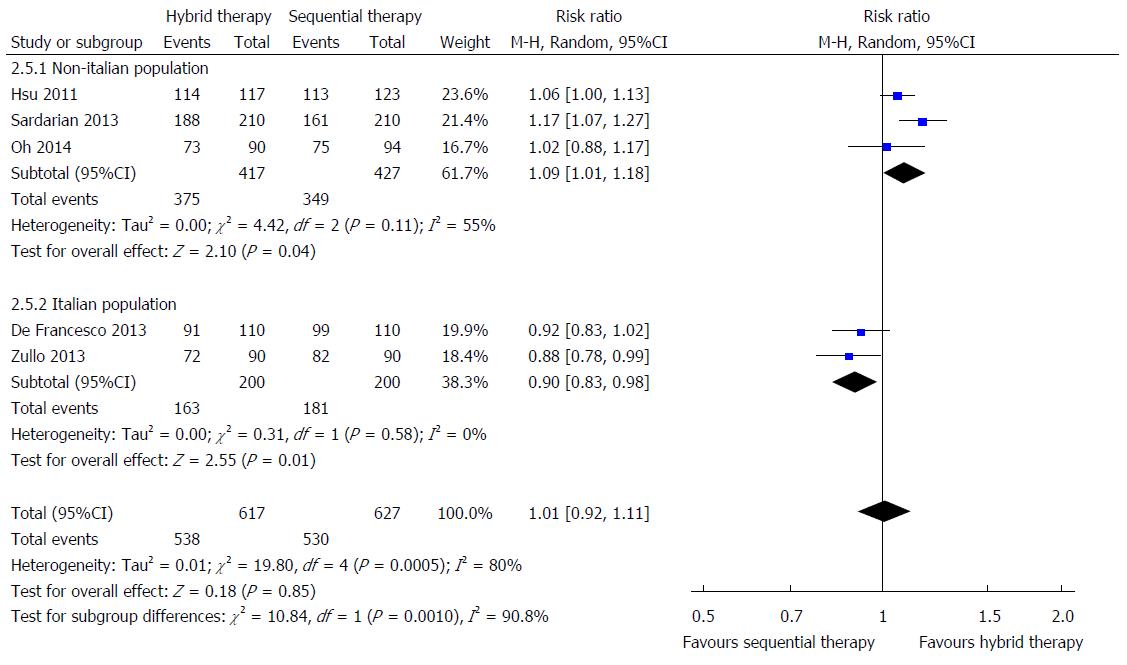
In all, 627 patients were treated 14-d hybrid therapy compared with 617 patients treated with sequential therapy lasting 10 or 14 d. Meta-analysis shows no significant differences in ITT eradication rate between 14-d hybrid and 10- to 14-d sequential therapy (Figure 3; pooled relative risk: 1.01; 95%CI: 0.92-1.11). Because high heterogeneity was noted between studies (I2 = 80%, P = 0.0005), we further performed subgroup analyses according to different geographic regions and treatment duration. The relative risk of eradication rate with ITT analysis was significantly different between the Italian and non-Italian populations (subgroup interaction test, P = 0.0010). The hybrid therapy was more effective than sequential therapy in the non-Italian population (mean results: 89.9% vs 81.7%; relative risk = 1.09; 95%CI: 1.01-1.18; Figure 3). In contrast, hybrid therapy was less effective than sequential therapy in the Italian population (mean results: 81.5% vs 90.5%; relative risk = 0.90; 95%CI: 0.83-0.98). The PP analysis yielded similar results in the comparison of hybrid and sequential therapy for non-Italian populations (relative risk = 1.09; 95%CI: 1.01-1.18) with no significant difference in eradication rates between the two treatments in the Italian population (relative risk = 0.98; 95%CI: 0.90-1.08) by PP analysis. Possible explanations for the discrepancies included different antibiotic resistances of H. pylori strains, different treatment durations of sequential therapies, or variable adherence to the protocols.
With regard to the analysis by different treatment durations, there was a borderline significant trend toward higher ITT eradication rate with 14-d hybrid therapy (90.3%, 187/207) compared with 14-d sequential therapy (86.6%, 188/217) with the relative risk of 1.05 (95%CI: 1.00-1.11). However, there was no significant difference in eradication rate between 14-d hybrid therapy (83.7%, 343/410) and 10-d sequential therapy (83.4%, 342/410) (95%CI: 0.94-1.17).
Regarding tolerance, hybrid and sequential therapies exhibited comparable frequencies of adverse events (24.8% vs 24.4%, respectively) and drug compliance (95.4% vs 96.3%, respectively).
Concomitant therapy is a non-bismuth quadruple therapy proven successful in the presence of clarithromycin resistance[9,25]. It is a 4-drug regimen containing a PPI, clarithromycin, amoxicillin and metronidazole which are all given for the entire duration of therapy. A meta-analysis of 15 studies showed that a significant higher eradication rate was achieved with concomitant therapy compared to standard triple therapy in areas with high clarithromycin resistance[35]. A head-to-head non-inferiority trial of 10-d sequential and 10-d concomitant therapy showed they were equivalent (eradication rate: 92.3% vs 93.0%, respectively)[33]. From the perspective of clinical practice, the concomitant regimen is less complex than sequential or hybrid regimen, which is a two-step therapy.
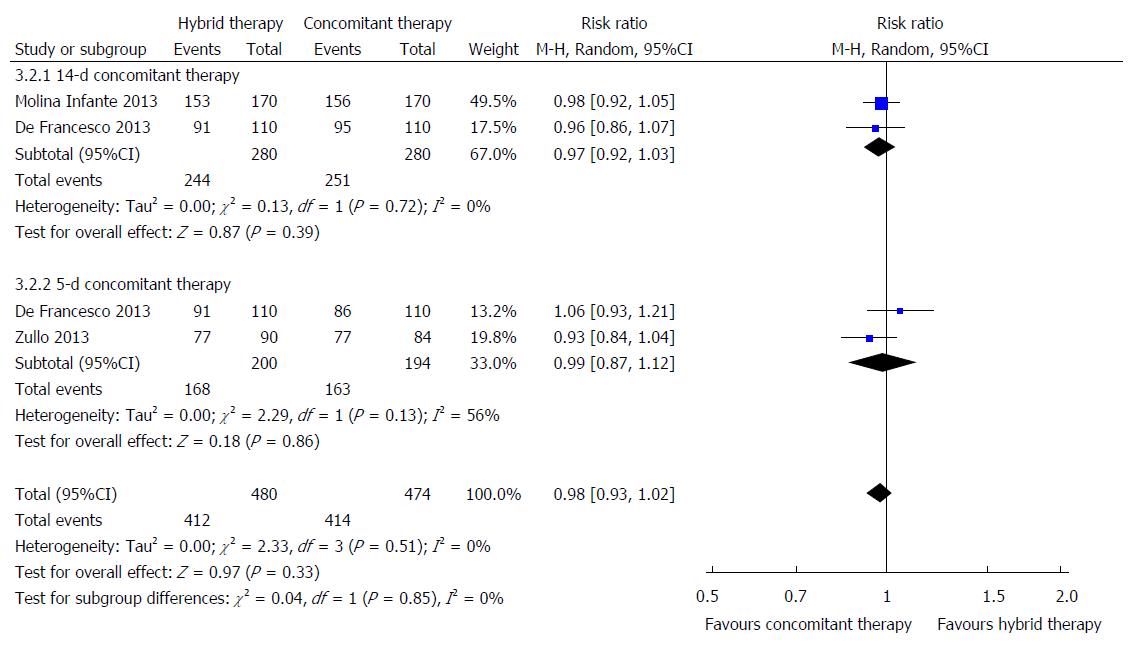
All three randomized trials comparing the efficacies of hybrid therapy and concomitant therapy[13,14,16] showed that no differences in eradication rates, either by ITT or PP analyses, between 14-d hybrid therapy and 14-d concomitant therapy. A study from Italy[14] which compared markedly different durations of therapy (14-d hybrid therapy vs 5-d concomitant therapy) showed that 14-d hybrid therapy was superior by PP analysis (95.7% vs 85.1%). However, two meta-analyses of concomitant therapy reported a significant effect of duration on treatment success with concomitant therapy[9,34] such that the study had a significant bias.
In all, 370 patients were treated with 14-d hybrid therapy compared to 480 patients treated with concomitant therapy lasting 5 or 14 d. According to ITT analysis, the eradication rate was 85.4% (316/370; 95%CI: 81.8%-90.0%) for hybrid therapy and 86.3% (414/480; 95%CI: 83.2%-89.4%) for concomitant therapy lasting 5 or 14 d. As shown in Figure 4, there was no significant difference in eradication rate between 14-d hybrid therapy and 5 to 14-d concomitant therapy with the pooled relative risk is 0.98 (95%CI: 0.93-1.02). When the analysis excluded the 5-d concomitant studies, results with 14-d hybrid and 14-d concomitant therapy were still similar (relative risk = 0.97; 95%CI: 0.92-1.03).
Recently, a large multicenter randomized controlled trial showed that there was a borderline significant trend (P = 0.05) toward higher compliance with 14-d hybrid therapy compared with 14-d concomitant therapy (98.8% vs 95.2%)[16]. In all, 14-d hybrid therapy and 5- to 14-d concomitant therapy had comparable compliance rates (95.9% vs 94.4%, respectively) and frequencies of adverse events (34.4% vs 37.1%, respectively).
Currently, there are no head-to-head studies comparing the eradication rate of hybrid therapy and standard triple therapy for H. pylori infection. However, the current data are consistent with the hypothesis that in regions with high clarithromycin resistance hybrid therapy should be superior to triple therapy. Hsu et al. recently conducted a multi-center, randomized controlled trial to compare the efficacies of 12-d reverse hybrid therapy and 12-d standard triple therapy in an area of moderate clarithromycin resistance (10%)[17]. The 12-d reverse hybrid therapy achieved a higher eradication rate than 12-d standard triple therapy (95.5% vs 88.6%) by ITT analysis. PP analysis showed similar results (95.9% vs 88.5%). The rates of resistance to clarithromycin, amoxicillin, and metronidazole in that study were 10%, 0% and 25%, respectively. As expected in the standard triple therapy group, the patients with clarithromycin-resistant strains had a lower eradication rate than those with clarithromycin-susceptible strains (28.6% vs 90.0%). In contrast, there were no significant differences in eradication rates between patients with clarithromycin-resistant and -susceptible strains (98.4% vs 100.0%) in the hybrid therapy group.
The ideal first-line treatment for H. pylori infection would be cheap and highly effective. The cost of the drugs for 12-d reverse hybrid therapy in Taiwan is nearly 6.8 dollars less than that for 12-d triple therapy (i.e., $37.2 vs $44.0). The economic advantage is further strengthened by the consideration that 12-d reverse hybrid regimen is 6.9% more effective than 12-d standard triple therapy.
Recently, the eradication rates of standard triple therapy have declined to less than 80% in many countries, largely owing to antimicrobial resistance. Several strategies including bismuth-containing quadruple therapy and non-bismuth-containing quadruple therapy (sequential, concomitant therapy or hybrid therapy) have been proposed to increase the eradication rates. Hybrid therapy is a novel therapeutic approach based on a combination of sequential therapy and concomitant therapy. More than 1200 patients have been treated with this regimen. The meta-analysis in this study showed that hybrid therapy was more effective than sequential therapy except in one Italian population. That conflicting result may be due to differences in antimicrobial resistances or in adherence with the regimens by the patients. Both hybrid therapy and concomitant therapy appear similarly highly effective. In conclusion, hybrid therapy appears to be an effective, safe, and well-tolerated treatment for H. pylori infection despite increasing antibiotic resistance.
Recently, the eradication rates of standard triple therapy have declined to less than 80% in many countries, owing to emerging organism resistances. Several strategies including sequential, concomitant therapy and hybrid therapy have been proposed to increase the eradication rate.
The pilot study of hybrid therapy showed that the novel treatment achieved an excellent eradication rate (99.1% by per-protocol and 97.4% by intention-to-treat analyses). It has therefore become a hot topic to compare the efficacy of hybrid therapy with other recommended regimens for Helicobacter pylori (H. pylori) eradication therapy.
Meta-analysis showed there was no significant difference in eradication rate between hybrid and sequential therapy. Subgroup analysis revealed that hybrid therapy was more effective than sequential therapy in the non-Italian population. In contrast, it was less effective than sequential therapy in the Italian population. There was no significant difference in eradication rate between hybrid therapy and concomitant therapy. Reverse hybrid therapy is a new one-step tow-phase treatment, achieving a higher eradication rate than standard triple therapy with similar tolerability and less pharmaceutical cost.
Hybrid and reverse hybrid therapies appear to be an effective, safe, and well-tolerated treatment for H. pylori infection in the era of increasing antibiotic resistance.
Hybrid therapy consists of a proton pump inhibitor and amoxicillin for 10-14 d, with addition of clarithromycin and metronidazole for the final 7 d. Reverse hybrid therapy consists of a proton pump inhibitor and amoxicillin for 10-14 d, with addition of clarithromycin and metronidazole for the initial 7 d.
The authors performed a comprehensive review with meta-analysis for hybrid therapy. The results indicate that hybrid therapy can be recommended as a treatment option for the first-line therapy of H. pylori infection, especially in areas with moderate or high clarithromycin resistance.
P- Reviewer: Tovey FI S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011;8:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ; European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1588] [Article Influence: 122.2] [Reference Citation Analysis (5)] |
| 3. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 4. | Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [PubMed] |
| 5. | Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 281] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Gumurdulu Y, Serin E, Ozer B, Kayaselcuk F, Ozsahin K, Cosar AM, Gursoy M, Gur G, Yilmaz U, Boyacioglu S. Low eradication rate of Helicobacter pylori with triple 7-14 days and quadriple therapy in Turkey. World J Gastroenterol. 2004;10:668-671. [PubMed] |
| 7. | De Francesco V, Margiotta M, Zullo A, Hassan C, Giorgio F, Burattini O, Stoppino G, Cea U, Pace A, Zotti M. Prevalence of primary clarithromycin resistance in Helicobacter pylori strains over a 15 year period in Italy. J Antimicrob Chemother. 2007;59:783-785. [PubMed] |
| 8. | Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353-1357. [PubMed] |
| 9. | Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:109-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Hsu PI, Kao SS. Therapy of Helicobacter pylori Infection: Many Drugs for Which Association? Helicobacter pylori. New York: Nova Science Publishers, Inc 2013; 347-362. |
| 11. | Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter. 2013;18:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Zullo A, Scaccianoce G, De Francesco V, Ruggiero V, D’Ambrosio P, Castorani L, Bonfrate L, Vannella L, Hassan C, Portincasa P. Concomitant, sequential, and hybrid therapy for H. pylori eradication: a pilot study. Clin Res Hepatol Gastroenterol. 2013;37:647-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | De Francesco V, Hassan C, Ridola L, Giorgio F, Ierardi E, Zullo A. Sequential, concomitant and hybrid first-line therapies for Helicobacter pylori eradication: a prospective randomized study. J Med Microbiol. 2014;63:748-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Oh DH, Lee DH, Kang KK, Park YS, Shin CM, Kim N, Yoon H, Hwang JH, Jeoung SH, Kim JW. Efficacy of hybrid therapy as first-line regimen for Helicobacter pylori infection compared with sequential therapy. J Gastroenterol Hepatol. 2014;29:1171-1176. [PubMed] |
| 16. | Molina-Infante J, Romano M, Fernandez-Bermejo M, Federico A, Gravina AG, Pozzati L, Garcia-Abadia E, Vinagre-Rodriguez G, Martinez-Alcala C, Hernandez-Alonso M. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013;145:121-128.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Hsu PI, Wu DC, Tsay , FW . Taiwan Acid Related Disease (TARD) Study Group. Reverse hybrid therapy versus standard triple therapy for Helicobacter pylori infection - A multi-center, randomized, controlled trial. Medicine. 2015;in press. |
| 18. | Chuah SK, Tsay FW, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol. 2011;17:3971-3975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Georgopoulos SD, Papastergiou V, Karatapanis S. Helicobacter pylori Eradication Therapies in the Era of Increasing Antibiotic Resistance: A Paradigm Shift to Improved Efficacy. Gastroenterol Res Pract. 2012;2012:757926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [PubMed] |
| 21. | Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719-748. [PubMed] |
| 22. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [PubMed] |
| 23. | Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, Hassan C, Bernabucci V, Tampieri A, Morini S. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146:556-563. [PubMed] |
| 24. | Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177-186.e3; Discussion e12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 254] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 25. | Hsu PI, Wu DC, Chen WC, Tseng HH, Yu HC, Wang HM, Kao SS, Lai KH, Chen A, Tsay FW. Randomized controlled trial comparing 7-day triple, 10-day sequential, and 7-day concomitant therapies for Helicobacter pylori infection. Antimicrob Agents Chemother. 2014;58:5936-5942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, Lee JY, Hsu SJ, Luo JC, Chang WH. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013;381:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 199] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 27. | Wu JY, Hsu PI, Wu DC, Graham DY, Wang WM. Feasibility of shortening 14-day hybrid therapy while maintaining an excellent Helicobacter pylori eradication rate. Helicobacter. 2014;19:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374-1384. [PubMed] |
| 29. | Houben MH, van de Beek D, Hensen EF, de Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy--the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999;13:1047-1055. [PubMed] |
| 30. | Gisbert JP, Calvet X, O’Connor A, Mégraud F, O’Morain CA. Sequential therapy for Helicobacter pylori eradication: a critical review. J Clin Gastroenterol. 2010;44:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 31. | Murakami K, Fujioka T, Okimoto T, Sato R, Kodama M, Nasu M. Drug combinations with amoxycillin reduce selection of clarithromycin resistance during Helicobacter pylori eradication therapy. Int J Antimicrob Agents. 2002;19:67-70. [PubMed] |
| 32. | Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (95)] |
| 33. | Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, Wang SS, Chen A, Hung WC, Graham DY. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010;8:36-41.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 34. | Hsu PI, Wu DC, Wu JY, Graham DY. Is there a benefit to extending the duration of Helicobacter pylori sequential therapy to 14 days? Helicobacter. 2011;16:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Gisbert JP, Calvet X. Review article: non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment Pharmacol Ther. 2011;34:604-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |