Published online Dec 7, 2015. doi: 10.3748/wjg.v21.i45.12814
Peer-review started: March 29, 2015
First decision: April 24, 2015
Revised: July 4, 2015
Accepted: September 15, 2015
Article in press: September 15, 2015
Published online: December 7, 2015
Processing time: 252 Days and 21.3 Hours
AIM: To investigate small interfering RNA (siRNA)-mediated inhibition of nuclear factor-kappa B (NF-κB) activation and multidrug-resistant (MDR) phenotype formation in human HepG2 cells.
METHODS: Total RNA was extracted from human HepG2 or LO2 cells. NF-κB/p65 mRNA was amplified by nested reverse transcription polymerase chain reaction and confirmed by sequencing. NF-κB/p65 was analyzed by immunohistochemistry. Specific-siRNA was transfected to HepG2 cells to knock down NF-κB/p65 expression. The effects on cell proliferation, survival, and apoptosis were assessed, and the level of NF-κB/p65 or P-glycoprotein (P-gp) was quantitatively analyzed by enzyme-linked immunosorbent assay.
RESULTS: HepG2 cells express NF-κB/p65 and express relatively less phosphorylated p65 (P-p65) and little P-gp. After treatment of HepG2 cells with different doses of doxorubicin, the expression of NF-κB/p65, P-p65, and especially P-gp were dose-dependently upregulated. After HepG2 cells were transfected with NF-κB/p65 siRNA (100 nmol/L), the expression of NF-κB/p65, P-p65, and P-gp were downregulated significantly and dose-dependently. The viability of HepG2 cells was decreased to 23% in the combination NF-κB/p65 siRNA (100 nmol/L) and doxorubicin (0.5 μmol/L) group and 47% in the doxorubicin (0.5 μmol/L) group (t = 7.043, P < 0.001).
CONCLUSION: Knockdown of NF-κB/p65 with siRNA is an effective strategy for inhibiting HepG2 cell growth by downregulating P-gp expression associated chemosensitization and apoptosis induction.
Core tip: Hepatic nuclear factor-kappa B (NF-κB) signaling pathway could be a potential target for designing highly effective therapeutic agents for the chemoprevention of hepatocellular carcinoma (HCC). Specific siRNAs used in combination with doxorubicin could enhance doxorubicin cytotoxicity in human HepG2 cells by downregulating NF-κB and P-glycoprotein expression. Stable NF-κB inhibition and chemosensitization could significantly inhibit tumor cell proliferation. Thus, the modulation of NF-κB might represent an advance in HCC therapy efficacy and is worthy of further research and investigation.
- Citation: Shi Y, Wang SY, Yao M, Sai WL, Wu W, Yang JL, Cai Y, Zheng WJ, Yao DF. Chemosensitization of HepG2 cells by suppression of NF-κB/p65 gene transcription with specific-siRNA. World J Gastroenterol 2015; 21(45): 12814-12821
- URL: https://www.wjgnet.com/1007-9327/full/v21/i45/12814.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i45.12814
Hepatocellular carcinoma (HCC) is one of the most common cancers and causes of mortality in China[1,2]. A great deal of progress in understanding the mechanisms of hepatocarcinogenesis has been achieved in recent years[3-5]. Many genes, such as protooncogenes, tumor suppressor genes, and growth factor genes, have been suggested to play an important role in this process[6]. Infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) is involved in HCC development and progression. Tumorigenic protein (HBx or core protein of HCV) activates a variety of signaling pathways, including nuclear- transcription factor kappa B (NF-κB) and tumor necrosis factor (TNF)-α[7,8]. Abnormal activation of NF-κB has been shown to modulate the transcription and expression of many genes in hepatocarcinogenesis[9,10]. NF-κB is formed by five subunits, p65, RelB, C-Rel, p50, and p52. The p50/p65 dimers play the common biological role and can be activated by chemotherapy drugs, cytokines, and viruses induced into nucleus[11-13].
HCC is one of the most resistant tumors to systemic chemotherapy, and doxorubicin is one of the chemotherapy drugs used in HCC[14,15]. Both NF-κB and P-glycoprotein (P-gp) have been described as important mediators of chemotherapy-induced cell death[16-18]. Because the first exon of multi-drug resistance (MDR) gene promoter contains the NF-κB binding sequence, it may be one of the downstream target genes of NF-κB[19,20]. A variety of chemotherapeutic drugs can induce formation of the MDR phenotype in malignant cells, thereby restricting the efficacy of these drugs. P-gp is encoded by the MDR gene and is a membrane bound ATP-dependent flow pump. It can pump drugs out of the cell with the energy provided by ATP, and this is the main mechanism underlying the MDR phenomenon[21,22]. However, the role of NF-κB signaling pathway in chemotherapy-induced MDR remains to be elucidated. In this study, we used small interference RNA (siRNA)-mediated inhibition of NF-κB expression in combination with chemotherapy drugs to explore the role of NF-κB in human HepG2 cell growth.
Human hepatoma cell (HepG2) and normal liver cell (LO2) lines were purchased from the Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China) and cultured at 37 °C with 5% CO2 in Roswell Park Memorial Institute (RPMI)-1640 supplemented with 2 mmol/L L-glutamine and 10% fetal calf serum (FCS). The cells were digested by parenzyme. Recombinant human TNF-α was purchased from the Prepro Tech, Inc. (Rocky Hill, NJ, United States). Doxorubicin and all chemicals not otherwise specified below were purchased from Sigma (St Louis, MI, United States).
To the cells (5 × 105), 1.0 ml of Trizol reagent (Promega, Madison, WI, United States) was added. Total RNA was isolated according to standard procedures and the protocols outlined by the manufacturer. RNA purity was estimated from the ratio of absorbance (A) readings at 260 and 280 nm, with an A260/280 ratio between 1.8 and 2.0 indicating sufficient purity. The RNA samples were kept frozen at -85 °C until required. For synthesis of NF-κB complementary DNA (cDNA), 2 μg of total RNA was denatured in the presence of random hexamers (100 pMol/L, Promega) and reverse-transcriptase (Gibco, Carlsbad, CA, United States) at 23 °C for 10 min, 42 °C for 60 min, 95 °C for 10 min, on ice for 5 min, and then stored at -20 °C for polymerase chain reaction (PCR) amplification.
A set of primers, NF-κB-P1 (sense), 5’-AGCACAGATACCACCAAGAC-3’ (nt398-417) and NF-κB-P2 (antisense), 5’-TGGTCCCGTGAAATACACCT-3’ (nt523-542) were designed according to NF-κB/p65 sequences obtained from Genebank (NM-021975) and synthesized in the Shanghai Institute of Cell Biology, Chinese Academy Sciences, China, and the size of amplified fragment was 145 bp. The PCR amplification consisted of initial denaturation at 94 °C for 5 min, followed by 94 °C for 25 s, 55 °C for 30 s, and 72 °C for 90 s for 30 cycles. The glyceraldehyde-3- phosphate dehydrogenase (GAPDH) genome was used as a control. A set of primer sequences was GAPDH-1 (sense), 5’-AGAAGGCTGGGGCTCATTTG-3’ and GAPDH-2 (antisense), 5’-AGGGGCCATCCACAGTCTTC-3’, and the size of designed fragment was 258 bp. The amplified products were separated by electrophoresis on 2% agarose gels with ethidium bromide staining. The fragment sizes were evaluated using DNA markers (Promega) as molecular weight standards, and Molecular Imager Gel DocTM System (Biorad, Hercules, CA, United States).
The human NF-κB/p65 siRNA was purchased from the Biomics Biotechnologies Co. (Nantong, China). The sequences of siRNA were 5’-GA UGAGAUCUUCCUACUGUdTdT-3’ for p65 and 5’-UUCUCCGAACGUGU CACGUTTdTdT-3’ for negative control. Cells at 50% confluence were transfected with 100 nmol/L NF-κB/p65 siRNA using LipofectamineTM 2000 Transfection Reagent (Invitrogen), according to the manufacturer’s specifications. The cells were seeded the day before transfection, using RPMI-1640 with 10% FCS without antibiotics. After 2 d, the cells were subjected to Western blotting analysis or 3-(4,5-dimethylthiazol-2-Yl)-2, 5-diphenyltetrazoliumbromide (MTT) assay in the presence or absence of doxorubicin.
The effect of doxorubicin on the viability of HepG2 cells was studied using the MTT Cell Proliferation and Cytotoxicity Assay Kit purchased from the Nanjing KeyGen Biotech CO., China. MTT assay was performed in 96-well flat-bottomed plates (Nunc, Rochester, NY, United States). Approximately 5 × 104 cells were seeded in 100 μL of drug free media and incubated for 24 h before drug treatment or siRNA transfection. Different concentrations of doxorubicin were added for 24 h. Then, 10 μL of MTT solution was added and incubated for 4 h, and, subsequently, 100 μL formanzan solution was added and incubated for 4 h. The amount of soluble formanzan produced, by cellular reduction of MTT, was measured at 570 nm. Approximate IC50 values were determined from a dose response curve. Data were derived from at least three independent experiments (n = 3). The effects of doxorubicin with or without NF-κB/p65 siRNA on the viability of HepG2 cells were studied using MTT assay. Twenty four hours after seeding, the cells were transfected with NF-κB/p65 siRNA or negative control siRNA. After 24 h, doxorubicin was added for an additional 24 h. The MTT assays were then performed, as described above.
For Western blotting, the cytoplasmic proteins were purified from cells cultured in 6-wells plates and lysed with a hypotonic buffer (20 mmol/L Tris-buffer, pH 8.0, 150 mmol/L NaCl, 100 mMol/L NaF, 10% of glycerol, 1% of Nonidet P-40, 1 mmol/L PMSF, 40 μg/mL leupeptin, and 20 μg/mL arotinin) for 30 min at 4 °C. After centrifuged, equal amounts of protein (25 μg/lane) were resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane. Membranes were blocked in Tris-buffered saline (TBS), containing 2% glycine and 3% non-fat dried milk overnight at 4 °C, and then incubated with specific primary antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX, United States) to NF-κB/p65, phosphorylated p65, P-gp, and β-actin for 2 h at 37 °C. Membranes were them incubated with horseradish peroxidase-labeled secondary antibody for 1.5 h at 37 °C. The reaction was developed using a chemiluminescence detection system.
The cells were fixed with 10% formaldehyde, and then the streptavidin-peroxidase (S-P) method with empirical procedure directions was performed. Phosphate buffered saline (PBS) was used to substitute for the primary antibody and served as a negative control. The positive material of NF-κB/p65 was a brown-yellow fine particle layer localized in the nucleus or cytoplasm. NF-κB/p65 staining was evaluated semi-quantitatively according to the percentage of positive cells.
The nuclear protein was extracted after cell transfection, according to the instructions for the nuclear and cytoplasmic protein extraction kit, and quantified spectro- photometrically using the BCA assay kit (Beyotime, Haimen, China). The level of NF-κB/p65 was detected according to the human NF-κB/p65 enzyme-linked immunosorbent assay kit (Cusabio Biotech, Wuhan, China), with 30 μL of complete combining buffer, 10 μL of nuclear protein extraction agent, and 20 μL of complete lysis buffer (CLB). The positive control consisted of 2.5 μg of provided nuclear extract diluted in 20 μL of CLB per well; the blank well contained only 20 μL of CLB. Twenty microliters of the appropriate standard diluted in the CLB was added to each well. Solutions were incubated with mild agitation for 1 h at room temperature. Each well was washed three times with 200 μL of washing buffer, and then 100 μL of diluted NF-κB antibody was added. The plate was covered and incubated for 1 h with mild agitation, washed four times, and 100 μL of Developing Solution was added. After 10 min incubation in the dark, 100 μL of stop solution was added, and within 5 min, the A450 was measured with a spectrophotometer and reference wavelength at 655 nm. NF-κB level was calculated according to a standard curve.
Data was expressed as the mean ± standard deviation (SD). Statistical analyses were done using the SPSS 10.0 software package (Chicago, IL, United States). Differences between groups were assessed using Fisher’s exact test or the χ2 test. P≤ 0.05 was regarded as statistically significant.
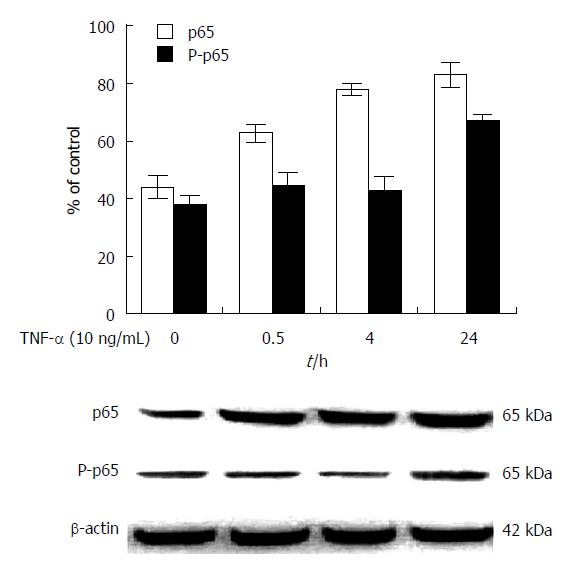
The comparative analysis of NF-κB/p65 and P-p65 expression in human HepG2 cells induced with TNF-α are shown in Figure 1. The ratio of NF-κB/p65 and the relative expression of P-p65 to β-actin were increased in HepG2 cells treated with a time course of 10 ng/mL of TNF-α (Figure 1A), as shown with Western blotting (Figure 1B). The expression of NF-κB/p65 or P-p65 was slight in HepG2 cells. Incubation of HepG2 cells with TNF-α (10 ng/mL) significantly increased the expression of NF-κB/p65 at 0.5, 4, and 24 h relative to that at 0 h (P < 0.01). The expression of P-p65 was significantly higher (P < 0.01) at 24 h than at 0, 0.5, and 4 h[23].
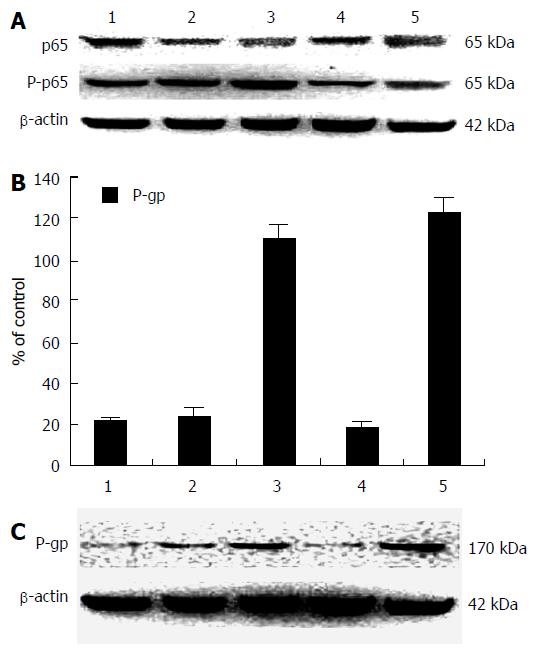
The expression of NF-κB/p65, P-p65, and P-gp induced by different doses of doxorubicin in HepG2 cells are shown in Figure 2. The expression of NF-κB/p65 and P-p65 with different doses of doxorubicin was analyzed using Western blotting (Figure 2A). There were no significant changes between NF-κB/p65 or P-p65 with various doses or incubation times of doxorubicin. The ratio of P-gp expression to β-actin in human hepG2 cells with different doses of doxorubicin at different times (Figure 2B) was analyzed by Western blotting (Figure 2C). P-gp expression in cells without doxorubicin was minimal, with no significant change found in the cells with doxorubicin at 4 h. P-p65 expression at 24 h was 6- or 7-fold higher than that at 4 h.
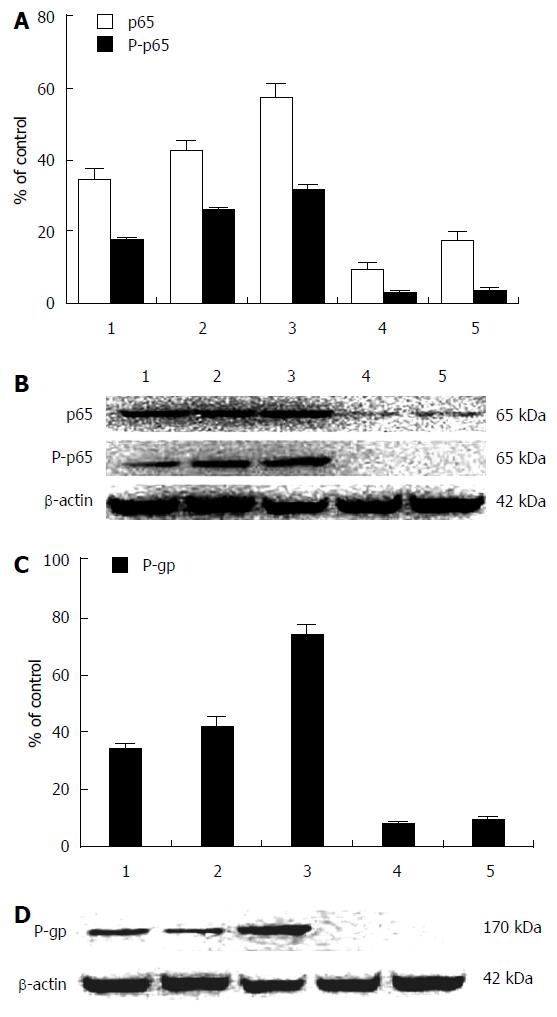
The downregulation of NF-κB/p65, P-p65, and P-gp expression in HepG2 cells after siRNA transfection is shown in Figure 3. In HepG2 cells transfected with siRNA, the ratios of NF-κB/p65, P-p65, or P-gp expression to β-actin (Figure 3A and C) were decreased significantly, and this was confirmed by Western blotting (Figure 3B and D). The expression of NF-κB/p65, P-p65, and P-gp was inhibited significantly (P < 0.001) in compared with the control or doxorubicin group.
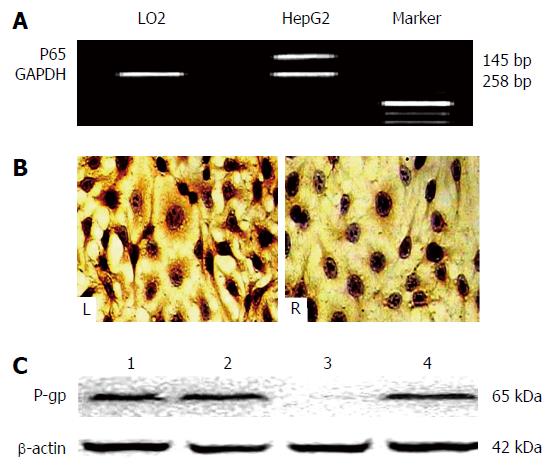
The expression of NF-κB/p65 and NF-κB/p65 mRNA in HepG2 cells before and after siRNA transfection is shown in Figure 4. The expression of NF-κB/p65 mRNA was significantly higher in HepG2 cells than LO2 cells (P < 0.001, Figure 4A) before siRNA transfection. The relative ratio of NF-κB/p65 mRNA to GAPDH was 1.13 ± 0.03 in HepG2 cells and 0.29 ± 0.07 in LO2 cells. NF-κB/p65 positive material was a brown-yellow fine particle layer and localized in cytoplasm and nucleus of HepG2 cells; and this expression pattern was different in the cytoplasm and nucleus of HepG2 cells transfected with siRNA [P < 0.001, Figure 4B(L) vs Figure 4B(R)]. The incidence of NF-κB/p65 expression decreased significantly in the cytoplasm of HepG2 cells with siRNA transfection but not in nucleus of HepG2 cells (Figure 4C), as confirmed by Western blotting.
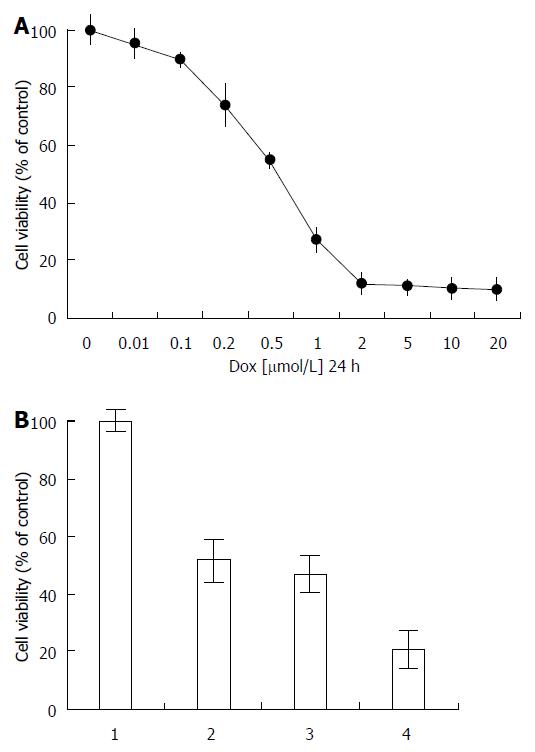
The effects of human HepG2 cell viability with doxorubicin are shown in Figure 5A. The viability of HepG2 cells was analyzed after the cells were incubated with different doses of doxorubicin for 24 h. Drug cytotoxicity in HepG2 cells was dose-dependent, and the IC50 was between 0.5-1.0 μmol/L during 24 h. The viability of HepG2 cells with combination siRNA transfection and doxorubicin is shown in Figure 5B. Viability in cells treated with doxorubicin (0.5 μmol/L) for 24 h was reduced to 51% compared with the control group. In cells transfected with negative siRNA for 1 d and then treated with doxorubicin for 1 d, the cell viability was reduced to 47% compared with control. There was no significant difference between the doxorubicin only group and the combination negative-siRNA and doxorubicin group. In cells transfected with siRNA for 1 d and then treated with doxorubicin for 1 d, the cell viability was reduced to 23%. The cell viability with siRNA and doxorubicin was significantly lower than that in the doxorubicin only group (P < 0.001). In HepG2 cells transfected with siRNA and treated with doxorubicin, the expression of NF-κB/p65, P-p65, and P-gp was decreased relative to doxorubicin treatment alone.
HCC is one of the most common cancers and one of the most resistant tumors to systemic chemotherapy[24,25]. Chronic infections with HBV and HCV are etiologically linked to hepatitis, liver cirrhosis, and HCC. Both viruses may induce activation of NF-κB in hepatocytes, which plays a crucial role in the regulation of cell growth and apoptosis[26-28]. NF-κB signaling complexes in both viruses in HCC tumor and non-tumor tissues may disclose possible common mechanisms in hepatocarcinogenesis[29,30]. NF-κB and P-gp are important mediators of chemotherapy-induced cell death. In the present study, we evaluated siRNA-mediated inhibition of NF-κB expression and application of chemotherapy to explore the anti-cancer effect on HepG2 cells.
Doxorubicin is a topoisomerase II inhibitor and can destruct the synthesis of DNA, induce NF-κB activation, and inhibit cell apoptosis[31,32]. The viability of HepG2 cells was analyzed in cells incubated with different doses of doxorubicin for 1 d. However, extracellular stimulation, including antineoplastic agents, mitogens, hormones, cytokines, and growth factors, can upregulate the expression of the MDR gene. We showed TNF-α can induce NF-κB expression, as shown by the time-dependent increase of NF-κB/p65 in HepG2 cells incubated with TNF-α (10 ng/mL) (Figure 1). In addition, P-p65, the active form of NF-κB/p65 that is phosphorylated at Ser536 of the C-terminal transactivation domain during the phosphorylation and degradation of IκB, was increased with 24 h of TNF-α treatment[13]. Our results indicated that HCC is a tumor with high NF-κB expression.
In the process of liver formation or liver regeneration, activity of MDR1 is abnormal. The human MDR gene family includes two members, MDR1 and MDR2, and abnormally regulated MDR1 is closely linked to the MDR phenotype[15,17]. NF-κB was moderately expressed in resting HepG2 cells. In cells treated with doxorubicin, NF-κB/p65 and P-p65 expression was significantly and dose-dependently increased (Figure 2A). However, the P-gp expression in HepG2 cells was related to drug dose and treatment duration. Interestingly, the expression of P-p65 at 24 h was 6-fold to 7-fold higher than that at 4 h (Figure 2B and C). Doxorubicin induced P-gp expression in HepG2 cells, whereas the control HepG2 cells expressed very low P-gp. The first exon of MDR gene promoter contains the NF-κB binding sequence, and a variety of chemotherapeutic drugs can induce formation of MDR phenotype in malignant cells that restrict the efficacy of drugs. These data suggest that activated P-gp is involved in the formation of the MDR phenotype.
NF-κB activation in tumor cells was resistant to doxorubicin-based chemotherapy[16]. The NF-κB/p65 pathway acts as a bridge in the process of doxorubicin-mediated upregulation of P-gp expression. The expression of NF-κB/p65, P-p65, and P-gp was significantly downregulated in HepG2 cells transfected with siRNA (Figure 3) compared with cells in the control or doxorubicin alone groups. The mRNA expression of NF-κB/p65 and NF-κB/p65 in HepG2 cells before and after siRNA transfection was also significantly different (Figure 4). The NF-κB/p65 mRNA expression or the ratio of NF-κB/p65 mRNA to GAPDH was significantly higher in HepG2 cells than that in LO2 cells before siRNA transfection. After HepG2 cells were transfected with siRNA, positive NF-κB/p65 expression decreased significantly in cytoplasm but not in nucleus, suggesting that inhibiting NF-κB activity can enhance the cytotoxicity of drugs in liver cancer cells.
It is speculated that doxorubicin-induced upregulation of MDR expression is mediated by activation of the NF-κB signaling pathway[33,34]. The drug cytotoxicity on HepG2 cells was dose-dependent, with an IC50 between 0.5-1.0 μmol/L (Figure 5A). In order to confirm this conjecture, p65-specific siRNA was designed and transfected in HepG2 cells. The cell viability of HepG2 cells with negative-siRNA treated with doxorubicin was reduced to 47% (Figure 5B), whereas viability of cells with NF-κB/p65-siRNA treated with doxorubicin was reduced to 23%, suggesting that siRNA-mediated inhibition of NF-κB/p65 downregulated P-gp expression, decreased formation of MDR phenotype, and sensitized tumor cells to drug[3].
NF-κB signaling pathway could be a potential target for the design of highly effective therapeutic agents for the chemoprevention of HCC[35,36]. Specific siRNA combined with doxorubicin can enhance doxorubicin cytotoxicity to HepG2 cells by downregulating NF-κB and P-gp expression. Furthermore, stable NF-κB inhibition and chemosensitization can significantly inhibit tumor cell proliferation. Thus, the modulation of NF-κB may represent an advance in HCC therapy efficacy and is worthy of further research and investigation.
The authors thank Dr. T. FitzGibbon for comments on earlier drafts of the manuscript.
Hepatocellular carcinoma (HCC) is one of the most resistant tumors to systemic chemotherapy, and doxorubicin is one of the chemotherapy drugs used in the treatment of HCC. Nuclear factor-kappa B (NF-κB) has been described as an important mediator of chemotherapy-induced cell death. Because the first exon of the multi-drug resistance (MDR) gene promoter contains the NF-κB binding sequence, it may be one of the downstream target genes of NF-κB. A variety of chemotherapeutic drugs can induce formation of MDR phenotype in malignant cells, thereby restricting the efficacy of drugs. However, the role of NF-κB signaling pathway in chemotherapy-induced MDR remains to be elucidated.
Recently, Gu et al investigated the inhibitory effects of intervention of the tumor necrosis factor (TNF)-α/NF-κB signaling pathway on HCC cell proliferation. HepG2 cells were cultured in vitro and treated with anti-TNFa mAb to down-regulate its expression or transfected with NF-κBp65 siRNA to inhibit its activation, and suggesting that the proliferation of hepatoma cells might be significantly inhibited by intervening in NF-κB signaling pathway activation, which promotes cell apoptosis and blocks cell cycling.
Hepatic NF-κB signaling pathway could be a potential target for designing highly effective therapeutic agents and chemoprevention of HCC. Specific siRNA combined with doxorubicin could enhance the doxorubicin cytotoxicity to human HepG2 cells by downregulating NF-κB and P-glycoprotein (P-gp) expression. Stable NF-κB inhibition and chemo- sensitization could significantly inhibit tumor cell proliferation. Thus, the modulation of NF-κB might represent an improvement in HCC therapy efficacy, and it is worthy of further research and investigation.
Tumorigenic protein (HBx or core protein of HCV) activates a variety of signaling pathways, including NF-κB activation and TNF-α. Abnormal activation of NF-κB modulates the transcription and expression of many genes in hepatocarcinogenesis. Understanding the role of NF-κB, a master regulator of inflammation and cell death, in the development of hepatocellular injury, liver fibrosis, and HCC, with a particular focus on the role of NF-κB in different cellular compartments of the liver is important. The application of NF-κB/p65 siRNA is an effective strategy for inhibiting HepG2 cell growth by down-regulating P-gp expression towards chemosensitization and apoptosis induction.
NF-κB is formed by five subunits, p65, Rel B, C-Rel, p50, and p52. The p50/p65 dimers play the common biological role, and it can be activated by chemotherapy drugs, cytokines, and viruses induced into nucleus. NF-κB acts as a central link between hepatic injury, fibrosis, and HCC, and it may represent a target for the prevention or treatment of liver fibrosis and HCC. NF-κB acts as a two-edged sword, and its inhibition may not only exert beneficial effects but also negatively impact hepatocyte viability. Finding appropriate targets or identifying drugs that either exert only a moderate effect on its activity or that can be specifically delivered to nonparenchymal cells will be essential to avoid the potential increase in liver injury associated with NF-κB blockade in hepatocytes.
Authors have done excellent work in this study. They have investigated small interference RNA (siRNA)-mediated inhibition of NF-κB activation in human HepG2 cells exposed to an anti-cancer drug. The application of NF-κB/p65 siRNA is an effective strategy for inhibiting HepG2 cell growth by down-regulating P-gp expression towards chemosensitization and apoptosis induction
P- Reviewer: Guo ZJ, Hu YH S- Editor: Yu J L- Editor: Filipodia E- Editor: Wang CH
| 1. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [PubMed] |
| 2. | Wu L, Tang ZY, Li Y. Experimental models of hepatocellular carcinoma: developments and evolution. J Cancer Res Clin Oncol. 2009;135:969-981. [PubMed] |
| 3. | Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312-1327. [PubMed] |
| 4. | Yao DF, Dong ZZ, Yao M. Specific molecular markers in hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2007;6:241-247. [PubMed] |
| 5. | Frau M, Biasi F, Feo F, Pascale RM. Prognostic markers and putative therapeutic targets for hepatocellular carcinoma. Mol Aspects Med. 2010;31:179-193. [PubMed] |
| 6. | Feo F, Frau M, Tomasi ML, Brozzetti S, Pascale RM. Genetic and epigenetic control of molecular alterations in hepatocellular carcinoma. Exp Biol Med (Maywood). 2009;234:726-736. [PubMed] |
| 7. | Liu LP, Liang HF, Chen XP, Zhang WG, Yang SL, Xu T, Ren L. The role of NF-kappaB in Hepatitis b virus X protein-mediated upregulation of VEGF and MMPs. Cancer Invest. 2010;28:443-451. [PubMed] |
| 8. | Kim HR, Lee SH, Jung G. The hepatitis B viral X protein activates NF-kappaB signaling pathway through the up-regulation of TBK1. FEBS Lett. 2010;584:525-530. [PubMed] |
| 9. | Dong ZZ, Yao DF, Wu W, Yao M, Yu HB, Shen JJ, Qiu LW, Yao NH, Sai WL, Yang JL. Delayed hepatocarcinogenesis through antiangiogenic intervention in the nuclear factor-kappa B activation pathway in rats. Hepatobiliary Pancreat Dis Int. 2010;9:169-174. [PubMed] |
| 10. | Wu W, Yao DF, Qiu LW, Sai WL, Shen JJ, Yu HB, Wu XH, Li YM, Wang YL, Gu WJ. Characteristics of hepatic nuclear-transcription factor-kappa B expression and quantitative analysis in rat hepatocarcinogenesis. Hepatobiliary Pancreat Dis Int. 2009;8:504-509. [PubMed] |
| 11. | Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431-436. [PubMed] |
| 12. | Hayden MS, West AP, Ghosh S. SnapShot: NF-kappaB signaling pathways. Cell. 2006;127:1286-1287. [PubMed] |
| 13. | O’Neil BH, Bůzková P, Farrah H, Kashatus D, Sanoff H, Goldberg RM, Baldwin AS, Funkhouser WK. Expression of nuclear factor-kappaB family proteins in hepatocellular carcinomas. Oncology. 2007;72:97-104. [PubMed] |
| 14. | Yan F, Wang XM, Liu ZC, Pan C, Yuan SB, Ma QM. JNK1, JNK2, and JNK3 are involved in P-glycoprotein-mediated multidrug resistance of hepatocellular carcinoma cells. Hepatobiliary Pancreat Dis Int. 2010;9:287-295. [PubMed] |
| 15. | Sun Z, Zhao Z, Li G, Dong S, Huang Z, Ye L, Liang H, Qu J, Ai X, Zhang W. Relevance of two genes in the multidrug resistance of hepatocellular carcinoma: in vivo and clinical studies. Tumori. 2010;96:90-96. [PubMed] |
| 16. | Li G, Dong S, Qu J, Sun Z, Huang Z, Ye L, Liang H, Ai X, Zhang W, Chen X. Synergism of hydroxyapatite nanoparticles and recombinant mutant human tumour necrosis factor-alpha in chemotherapy of multidrug-resistant hepatocellular carcinoma. Liver Int. 2010;30:585-592. [PubMed] |
| 17. | Yan F, Wang XM, Pan C, Ma QM. Down-regulation of extracellular signal-regulated kinase 1/2 activity in P-glycoprotein-mediated multidrug resistant hepatocellular carcinoma cells. World J Gastroenterol. 2009;15:1443-1451. [PubMed] |
| 18. | Vainer GW, Pikarsky E, Ben-Neriah Y. Contradictory functions of NF-kappaB in liver physiology and cancer. Cancer Lett. 2008;267:182-188. [PubMed] |
| 19. | McCarty MF, Block KI. Preadministration of high-dose salicylates, suppressors of NF-kappaB activation, may increase the chemosensitivity of many cancers: an example of proapoptotic signal modulation therapy. Integr Cancer Ther. 2006;5:252-268. [PubMed] |
| 20. | Ho WC, Dickson KM, Barker PA. Nuclear factor-kappaB induced by doxorubicin is deficient in phosphorylation and acetylation and represses nuclear factor-kappaB-dependent transcription in cancer cells. Cancer Res. 2005;65:4273-4281. [PubMed] |
| 21. | Shareef MM, Brown B, Shajahan S, Sathishkumar S, Arnold SM, Mohiuddin M, Ahmed MM, Spring PM. Lack of P-glycoprotein expression by low-dose fractionated radiation results from loss of nuclear factor-kappaB and NF-Y activation in oral carcinoma cells. Mol Cancer Res. 2008;6:89-98. [PubMed] |
| 22. | Vander Borght S, Komuta M, Libbrecht L, Katoonizadeh A, Aerts R, Dymarkowski S, Verslype C, Nevens F, Roskams T. Expression of multidrug resistance-associated protein 1 in hepatocellular carcinoma is associated with a more aggressive tumour phenotype and may reflect a progenitor cell origin. Liver Int. 2008;28:1370-1380. [PubMed] |
| 23. | Wang J, Tokoro T, Higa S, Kitajima I. Anti-inflammatory effect of pitavastatin on NF-kappaB activated by TNF-alpha in hepatocellular carcinoma cells. Biol Pharm Bull. 2006;29:634-639. [PubMed] |
| 24. | Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047-2063. [PubMed] |
| 25. | Butterfield LH. Recent advances in immunotherapy for hepatocellular cancer. Swiss Med Wkly. 2007;137:83-90. [PubMed] |
| 26. | Chang CS, Huang SM, Lin HH, Wu CC, Wang CJ. Different expression of apoptotic proteins between HBV-infected and non-HBV-infected hepatocellular carcinoma. Hepatogastroenterology. 2007;54:2061-2068. [PubMed] |
| 27. | Sato Y, Kato J, Takimoto R, Takada K, Kawano Y, Miyanishi K, Kobune M, Sato Y, Takayama T, Matunaga T. Hepatitis C virus core protein promotes proliferation of human hepatoma cells through enhancement of transforming growth factor alpha expression via activation of nuclear factor-kappaB. Gut. 2006;55:1801-1808. [PubMed] |
| 28. | Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749-759. [PubMed] |
| 29. | Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008;99:836-842. [PubMed] |
| 30. | Guo L, Guo Y, Xiao S. Expression of tyrosine kinase Etk/Bmx and its relationship with AP-1- and NF-kappaB-associated proteins in hepatocellular carcinoma. Oncology. 2007;72:410-416. [PubMed] |
| 31. | Assef Y, Rubio F, Coló G, del Mónaco S, Costas MA, Kotsias BA. Imatinib resistance in multidrug-resistant K562 human leukemic cells. Leuk Res. 2009;33:710-716. [PubMed] |
| 32. | Barraud L, Merle P, Soma E, Lefrançois L, Guerret S, Chevallier M, Dubernet C, Couvreur P, Trépo C, Vitvitski L. Increase of doxorubicin sensitivity by doxorubicin-loading into nanoparticles for hepatocellular carcinoma cells in vitro and in vivo. J Hepatol. 2005;42:736-743. [PubMed] |
| 33. | Xie Z, Choong PF, Poon LF, Zhou J, Khng J, Jasinghe VJ, Palaniyandi S, Chen CS. Inhibition of CD44 expression in hepatocellular carcinoma cells enhances apoptosis, chemosensitivity, and reduces tumorigenesis and invasion. Cancer Chemother Pharmacol. 2008;62:949-957. [PubMed] |
| 34. | Calvisi DF, Pascale RM, Feo F. Dissection of signal transduction pathways as a tool for the development of targeted therapies of hepatocellular carcinoma. Rev Recent Clin Trials. 2007;2:217-236. [PubMed] |
| 35. | Van Waes C. Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Cancer Res. 2007;13:1076-1082. [PubMed] |
| 36. | Cho HA, Park IS, Kim TW, Oh YK, Yang KS, Kim JS. Suppression of hepatitis B virus-derived human hepatocellular carcinoma by NF-kappaB-inducing kinase-specific siRNA using liver-targeting liposomes. Arch Pharm Res. 2009;32:1077-1086. [PubMed] |