Published online Nov 28, 2015. doi: 10.3748/wjg.v21.i44.12620
Peer-review started: April 7, 2015
First decision: June 19, 2015
Revised: July 7, 2015
Accepted: September 2, 2015
Article in press: September 2, 2015
Published online: November 28, 2015
Processing time: 236 Days and 12.9 Hours
AIM: To study the manifestations of perihepatic lymph nodes during the episode of acute hepatitis flare by point-of-care ultrasonography.
METHODS: One hundred and seventy-six patients with an episode of acute hepatitis flare (ALT value > 5 × upper normal limit) were enrolled retrospectively. Diagnosis of etiology of the acute hepatitis flare was based on chart records and serological and virological assays. The patients were categorized into two groups (viral origin and non-viral origin) and further defined into ten subgroups according to the etiologies. An ultrasonograpy was performed within 2 h to 72 h (median, 8 h). The maximum size of each noticeable lymph node was measured. Correlation between clinical parameters and nodal manifestations was analyzed
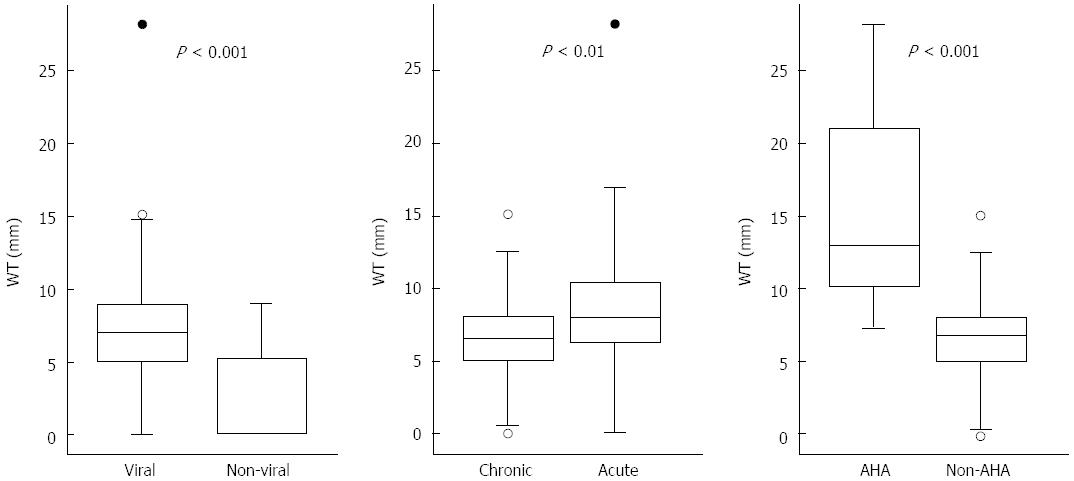
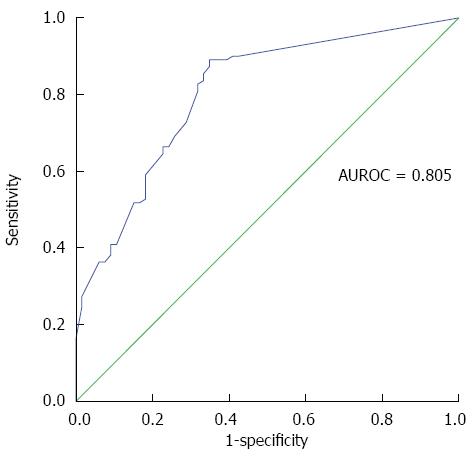
RESULTS: Enlarged lymph nodes (width ≥ 5mm) were noticeable in 110 (62.5%) patients, mostly in acute on chronic hepatitis B (54.5%). The viral group had a higher prevalence rate (89/110 = 80.9%) and larger nodal size (median, 7 mm) than those of the non-viral group (21/66 = 31.8%; median, 0 mm) (P < 0.001 for both). Meanwhile, there were significant differences in the nodal size between acute and chronic viral groups (P < 0.01), and between acute hepatitis A and non-hepatitis A viral groups (P < 0.001). In logistical regression analysis, the nodal width still showed strong significance in multivariate analysis (P < 0.0001) to stratify the two groups. The area under the curve of ROC was 0.805, with a sensitivity of 80.9%, a specificity of 68.2%, positive predictive value of 80.92%, negative predictive value of 68.18%, and an accuracy of 76.14%.
CONCLUSION: Point-of-care ultrasonography to detect perihepatic nodal change is valuable for clarifying the etiologies in an episode of acute hepatitis flare.
Core tip: The nodal manifestation in acute hepatitis flare has never been well studied especially in an endemic area of chronic hepatitis B. Our findings encouraged point-of-care ultrasonography to be performed as early as possible for clarifying the etiologies as well as early treatments. Those subjects with viral origin have a higher prevalence of nodal enlargement and larger nodal size as compared with those of non-viral origin. A 5 mm cut-off value for nodal shortest diameter (width) is convenient for defining nodal enlargement.
- Citation: Feng IC, Wang SJ, Sheu MJ, Koay LB, Lin CY, Ho CH, Sun CS, Kuo HT. Perihepatic nodes detected by point-of-care ultrasound in acute hepatitis and acute-on-chronic liver disease. World J Gastroenterol 2015; 21(44): 12620-12627
- URL: https://www.wjgnet.com/1007-9327/full/v21/i44/12620.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i44.12620
Acute hepatitis is often caused by one of several hepatotropic viruses, by systemic viral infections of the liver, or by drugs, toxic agents or hypoxia. Abruptly elevated alanine aminotransferase (ALT) level cannot be specific to any of the various causes. Hence, a definite diagnosis always depends on detailed history-taking, observations of clinical presentations and survey of serum markers, which is always time consuming and may delay targeting therapy toward the offending etiologies. Acute flare of chronic hepatitis has been observed in some viral hepatitis cases such as hepatitis B virus (HBV) and hepatitis C virus (HCV) infections as well as autoimmune hepatitis and alcoholic hepatitis. The manifestations of acute flare in chronic hepatitis are usually similar to those of acute hepatitis, which makes a differentiation between these two scenarios sometimes difficult. Liver biopsy is therefore clinically important.
The enlarged lymph nodes around the hepatoduodenal ligament (perihepatic nodes) are prevalent in chronic infections with HBV or HCV. The size of the noticeable lymph nodes seemed to be histologically and serologically correlated with the severity of hepatic inflammation[1-4]. Our comprehensive studies demonstrated that the prevalence of nodal enlargements (nodal width ≥ 5 mm) in chronic HBV infection and chronic HCV infection groups was significantly increased (56.9% and 69.4%, respectively, P < 0.001 for both) when compared with that of non-viral type; take note that this difference was independent of serum aminotransferase levels. Meanwhile, the nodal width was the only significant parameter when viral and non-viral groups were compared (P = 0.031)[5]. During episodes of hepatitis flare, enlarged perihepatic lymph nodes are always depicted by ultrasonography, however, its clinical significance is not clear[6-9].
The growth of point-of-care ultrasonography has progressed since the 1990s, Point-of-care ultrasonography refers to ultrasonography performed and interpreted by the clinician at the bedside for a real-time patient analysis, allowing immediate notations that could be directly correlated with the patient’s presenting signs and symptoms[10]. In this study, we tried to investigate whether the ultrasonographic assessment of perihepatic lymphadenopathy could facilitate the differential diagnosis in a cohort of patients with hepatitis flare of various etiologies.
From June 2003 to January 2013, patients with acute hepatitis flare presented to our institution were enrolled into this study. Inclusive criterion was abrupt elevation of alanine aminotransferase (ALT) > 250 U/L (> 5 × upper normal limit) with or without a known history of liver disease. All of the patients had undergone a pathophysical survey for the etiology, including routine abdominal sonographic examination and simultaneous measurement of the size of noticeable perihepatic lymph nodes. This study was designed as a retrospective study and the data were collected from chart review which was approved by the local ethics committee. The patients were categorized into two groups (viral origin and non-viral origin) and further defined into 10 subgroups according to the clinically diagnosed etiology. Diagnosis of acute hepatitis flare was based on chart records and serological and virological assays, which were reviewed by two of the authors (Kuo and Wang). Acute hepatitis A (AHA) was diagnosed by the presence of anti-HAV IgM. Acute hepatitis B (AHB) was diagnosed by the presence of a high titer of anti-HBc IgM. Acute hepatitis C (AHC) was diagnosed by positive seroconversion of anti-HCV antibody. All diagnoses included recent possible infection bouts but no known history of liver disease. Acute flare in chronic hepatitis B (CHB) or C (CHC) was diagnosed by high HBV DNA or HCV RNA viral load with a known history of chronic HBV or HCV infection, and positive HBsAg or anti HCV for more than 6 mo, without superinfection with other virus. Acute flare in chronic autoimmune hepatitis (AIH) was diagnosed by positive antinuclear antibody (ANA) titer > 80 ×, and high serum IgG level (> 1.1 × upper normal limit) without a known history of viral infections. Alcoholic hepatitis (AH) was diagnosed by a known history of alcohol consumption, high serum gamma-glutamyl transpeptidase (rGT) level without biliary obstructive signs, and no known viral infections or other etiologies. Drug induced liver disease (DILI) was diagnosed by a known intake of hepatotoxic drug, without known viral infections or other etiologies, and complete recovery after cessation of the target drugs. Bacterial infective hepatitis was diagnosed by toxic infection with evidence of infective foci including acute biliary tract infection or sepsis. Ischemic hepatitis was diagnosed by an episode of hemodynamic collapse without known viral infections or other etiologies, and rapid recovery after restoration of hemodynamic status. Patients with superinfection on chronic viral hepatitis, acute non-viral flare in chronic viral hepatitis, known malignancy, poor visualization of sonography, a history of abdominal surgery, or acute liver failure with mortality were excluded.
White blood cell (WBC) count, platelet (PLT) count, ALT level, rGT level, total bilirubin level, alfa-fetoprotein (AFP) level and ANA titer were measured during acute flare stage using routine automated techniques at our clinical pathology laboratories. Serum hepatitis markers, including HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HAV IgM, anti-HBc IgM, anti-HDV, and anti-HCV, were tested using the EIA kit (Abbott Diagnostics, North Chicago, IL, United States). Serum HBV DNA was tested using Roche Cobas Amplicor HBV Monitor test (Roche COBAS TaqMan HBV Test, lower limit of detection: 69 or 1.84 log 10 copies/mL; 12 or 1.08 log 10 IU/mL, Roche Diagnostics, Pleasanton, CA, United States). Serum HCV-RNA levels were determined using the COBAS AMPLICOR HCV MONITOR test, version 2.0 (Roche Diagnostics, Branchburg, NJ, United States), with a detection threshold between 600 and 850000 IU/mL.
After diagnosis of acute hepatitis flare, an ultrasonograpy was performed within 2 h to 72 h (median, 8 h). Two ultrasound scanners (SSD-5000 Aloka, Tokyo, Japan and Xario SSA-660A, Toshiba Medical Systems, Tokyo, Japan) equipped with a 3.75-5 MHz convex probe and color Doppler imaging were used. After the completion of the standard scanning of the whole abdomen, any other solid structures beside the common hepatic artery and/or the main portal vein were specifically studied. One or more ovoid masses that were less echogenic than the liver parenchyma were detected as solid masses on the Doppler sonography; these masses were clearly separated from adjacent organs and vessels on transverse, sagittal, and oblique scans. The maximum size of each noticeable lymph node was measured on the longest axis (length) and the corresponding perpendicular short axis (width). In patients with multiple lymph nodes, the measured and calculated values were added for a total value, and the maximal values were used for analysis. An enlarged lymph node was defined as the nodal width ≥ 5 mm[5]. The concurrent liver parenchymal manifestation by sonography was recorded as early change (normal/fatty liver) or late change (chronic liver parenchymal disease/liver cirrhosis). The wall thickness of the gallbladder, which has been a sonographic marker of acute hepatitis, was also recorded as normal or thickened when the typical three-layer appearance was depicted. The prevalence and size of enlarged lymph nodes were compared between groups and subgroups. Correlation between clinical parameters and nodal manifestations was also evaluated for surveillance accuracy in this hepatitis flare cohort.
Clinical and biochemical characteristic patterns of patients are expressed as mean ± SD or median with interquartile range (IQR). Data between groups/subgroups were tested by U test (Mann-Whitney) and Wilcoxon test, and P < 0.05 was judged to be statistically significant. Sensitivities and specificities were given with 95% confidence intervals. Comparisons between groups/subgroups were made by Pearson’s χ2 test or Fisher’s exact test for categorical variables. To find the independent factor, univariate logistic regression analysis was used to select possible variables as P-value < 0.1. Then, multiple logistic regression analysis was used to identify the independent factors. A P-value < 0.05 was considered significant in multiple logistic regression analysis. All analyses were performed using SPSS 20.0 statistical software.
There were a total of 203 patients who fulfilled the inclusive criteria and 27 patients were excluded due to variable reasons (9 for incomplete data, 8 for poor visualization of sonography, 4 for superinfection, 4 for unknown causes and 2 for mortality). One hundred and seventy-six patients were enrolled for this study, including cases of CHB with acute exacerbation (n = 77), CHC with acute exacerbation (n = 11), AHA (n = 5), AHB (n = 11), AHC (n = 6), AIH (n = 10), AH (n = 9), DILI (n = 24), infective hepatitis (n = 13), and ischemic hepatitis (n = 10) (Table 1). They were further divided into a viral origin group (n = 110) and a non-viral origin group (n = 66) (Table 2).
| Diagnosis according to etiology | n | Gender (%) male/female | WT≥5 mm (Yes/No) (%) | WT, median (Q1, Q3) | WT, mean ± SD |
| Viral | |||||
| CHBwAE | 77 | 64.9/35.1 | 77.9/22.1 | 6.5 (5, 8) | 6.32 ± 3.245 |
| CHCwAE | 11 | 63.6/36.4 | 100/0 | 6.1 (5, 7) | 6.79 ± 2.23 |
| Acute A | 5 | 60/40 | 100/0 | 13 (13, 14) | 15.12 ± 7.76 |
| Acute B | 11 | 54.5/45.5 | 63.6/36.4 | 6.8 (4, 8.1) | 6.35 ± 3.27 |
| Acute C | 6 | 50/50 | 100/0 | 8.15 (8, 9) | 7.92 ± 1.41 |
| Non-viral | |||||
| AIH | 10 | 20/80 | 50/50 | 4.65 (0, 7) | 3.83 ± 3.51 |
| AH | 9 | 88.9/11.1 | 44.4/55.6 | 3.3 (0, 7.6) | 3.77 ± 3.84 |
| DILI | 24 | 37.5/62.5 | 29.2/70.8 | 0 (0, 5.1) | 2.08 ± 2.90 |
| Infective | 13 | 30.8/69.2 | 30.8/69.2 | 0 (0, 5.2) | 2.57 ± 3.16 |
| Ischemic | 10 | 70/30 | 10/90 | 0 (0, 5.2) | 0.90 ± 1.91 |
| Viral group (n = 110) | Non-viral group (n = 66) | P value | |
| Gender (%) male/female | 62.7/37.3 | 45.5/54.5 | 0.025 |
| Age (years), mean ± SD | 40 ± 14.63 | 52.47 ± 17.82 | < 0.001 |
| ALT (IU/L), median (Q1-Q3) | 1055 (516.8-1869) | 821 (316-1520) | 0.022 |
| Total bilirubin (mg/dL), median (Q1-Q3) | 1.57 (0.72-5.15) | 2.49 (0.98-5.92) | 0.335 |
| AFP (ng/mL), median (Q1-Q3) | 8.22 (4.60-18.83) | 4.7 (0-7.6) | < 0.001 |
| WBC (/μL), median (Q1-Q3) | 5900 (5000-7500) | 7200 (5700-9500) | 0.003 |
| PLT (103/μL), median (Q1-Q3) | 186 (151-251) | 206.5 (142-263) | 0.543 |
| WT, median (Q1-Q3) | 7 (5-8.9) | 0 (0-5.4) | < 0.001 |
| Gallbladder wall thickening (%) (Yes/No) | 32.7/67.3 | 30.3/69.7 | 0.738 |
| Echogenecity (%) (1/0) | 30.6/69.4 | 20.3/79.7 | 0.142 |
| LN5 (%) (Yes/No) | 80.9/19.1 | 31.8/68.2 | < 0.001 |
Enlarged lymph nodes (width ≥ 5 mm) were noticeable in 110 patients with a total prevalence rate of 62.5%, most of which were diagnosed in CHB patients with acute flare (66/110 = 54.5%). The prevalence rates in all subgroups were as follows: CHB with acute flare (60/77 = 77.9%), CHC with acute flare (11/11 = 100%), AHA (5/5 = 100%), AHB (7/11 = 63.6%), AHC (6/6 = 100%), AIH(5/10 = 50%), AH (4/9 = 44.4%), DILI (7/24 = 29.2%), infective hepatitis (5/13 = 30.8%), and ischemic hepatitis (1/10 = 10%) (Table 1). The viral origin group had a higher prevalence rate (89/110 = 80.9%) than the non-viral origin group (21/66 = 31.8%) (Table 2) (P < 0.001). The median nodal width was 7 mm in the viral origin group and 0 mm in the non-viral origin group. In subgroups, the median nodal width was as follows: CHB with acute flare, 6.5 mm; CHC with acute flare, 6.1 mm; AHA, 13 mm; AHB, 6.8 mm; AHC, 8.15 mm; AIH, 4.65 mm; AH, 3.3 mm; DILI, 0 mm; infective hepatitis, 0 mm; and ischemic hepatitis, 0 mm. There were significant differences in nodal size between the viral and non-viral groups (P < 0.001), between the acute and chronic viral groups (P < 0.01), and between the AHA and non-AHA viral groups (P < 0.001) (Figure 1).
In logistical regression analysis, gender, age, WBC level and nodal width differed significantly between the viral and non-viral groups in univariate analysis (P = 0.026, < 0.0001, 0.003 and < 0.0001, respectively). The nodal width still had strong significance in multivariate analysis (P < 0.0001) (Table 3).
| Variables | Univariate analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Gender (M/F) | 2.020 | 1.087-3.753 | 0.026 | 0.280 | 0.108-0.725 | 0.009 |
| Age (yr) | 1.048 | 1.027-1.069 | < 0.0001 | 1.033 | 1.001-1.065 | 0.04 |
| ALT (IU/100 L) | 0.982 | 0.956-1.008 | 0.982 | 0.973 | 0.929-1.019 | 0.24 |
| Total bilirubin | 1.011 | 0.961-1.064 | 0.665 | 1.239 | 1.096-1.401 | 0.001 |
| AFP (ng/mL) | 0.992 | 0.982-1.003 | 0.141 | 0.971 | 0.955-0.987 | < 0.0001 |
| WBC (/100 μL) | 1.018 | 1.006-1.030 | 0.003 | 1.026 | 1.008-1.045 | 0.006 |
| PLT (103/μL) | 1.002 | 0.998-1.005 | 0.391 | 1.003 | 0.997-1.009 | 0.352 |
| Gallbladder wall thickening | 0.894 | 0.462-1.727 | 0.738 | 0.579 | 0.198-1.696 | 0.319 |
| Echogenecity | 0.579 | 0.278-1.207 | 0.579 | 0.803 | 0.272-2.370 | 0.691 |
| WT | 0.687 | 0.612-0.771 | < 0.0001 | 0.627 | 0.530-0.741 | < 0.0001 |
When using the value of nodal width to differentiate between the viral and non-viral groups, the area under the curve of ROC was 0.805 (Figure 2). If the cut-off value was 5 mm, it showed a sensitivity of 80.9%, a specificity of 68.2%, positive predictive value of 80.92%, negative predictive value of 68.18%, and an accuracy of 76.14%. A cut-off value of 3.7 mm resulted in a sensitivity of 89.1%, a specificity of 65.2% and the best accuracy rate of 80.14%.
In this study we found that the nodal enlargement was prevalence in acute hepatitis or acute flare on chronic liver disease especially in those caused by viral etiologies.
Among the various common etiologies, AHA manifested the most prominent nodal enlargement and ischemic hepatitis was of the least. The manifestations of perihepatic lymph nodes had been studied in liver disease of variable etiologies, mainly on chronic liver disease including AIH, primary biliary cirrhosis (PBC), CHB and CHC by ultrasound, CT and MRI and correlated to histopathology of the liver both by tissue biopsy and by graft from transplantation[2,3,11-19]. The accuracy of ultrasound detection of enlarged lymph nodes had been well validated by CT and MRI[19]. Correct sonographic detection of perihepatic lymph nodes has been confirmed by autopsy and verified by excision and histological examination during elective liver surgery with a high correlation coefficient (r = 0.94)[20].
However, there was less information about nodal change during acute hepatitis or acute flare on chronic liver disease. A small cohort study of acute hepatitis had been reported from Germany, where chronic viral hepatitis is not endemic[9]. Acute viral hepatitis had both a higher prevalence rate of noticeable nodes and larger nodal size than acute toxic liver disease.
Our study, within the endemic area of HBV and HCV infections, revealed that perihepatic lymphadenopathy (width ≥ 5 mm) in patients with acute hepatitis flare may predict a viral etiology, mostly CHB with acute flare. This is eminent in cases when urgent antiviral therapy is indicated, especially in those CHB patients with signs of hepatic decompensation. Ultrasound examination is naturally convenient, productive, affordable and safe. It can provide strong evidence before any results of laboratory examination are shown. Point-of-care ultrasonography should be widely promoted for its accurate depiction of the perihepatic nodal change during episode of acute hepatitis flare[10]. HBV and HCV are non-cytopathic viruses and thus immunologically mediated events are critical factors in the pathogenesis and outcome of these infections. The adaptive immune response mediates virtually all of the liver disease associated with viral hepatitis[21].
In this study, we found that AHA has more prominent nodal size than other acute viral hepatitis or chronic viral hepatitis with acute flare. AHA caused by primary infection with hepatitis A virus (HAV) is a common form of acute viral hepatitis. HAV is also a non-cytopathic virus, and hepatocyte injury is mediated by activated T cells in AHA. The frequency of circulating Tregs was reduced during AHA. A decrease in Treg numbers led to reduced suppressive activity of the Treg pool and consequently resulted in severe liver injury during AHA which was not observed in other acute liver diseases such as acute hepatitis B, acute hepatitis C and toxic/drug-induced hepatitis[22]. Thus, nodal reaction in acute hepatitis A could be a special sonographic detection for the diagnosis.
Immune mechanisms play a critical pathogenetic role in the majority of liver diseases, and are of crucial importance in particular infections and autoimmune disease affecting the liver[23]. Our study revealed variable prevalence and measurable size between variable non-viral etiologies of acute hepatitis flare, which may reflect the variable magnitudes of intrahepatic immune responses. Earlier studies of perihepatic lymph nodes revealed that autoimmune hepatobiliary diseases including PBC and AIH were associated with nodal enlargement. The nodal size and prevalence both correlated with the inflammatory severity[1,13]. We found that most of the non-viral hepatitis patients, however, presented with less prominent and less prevalent nodal enlargement than the viral group. Interestingly, four of nine alcoholic hepatitis patients also manifested with prominent nodal reaction, which, to our knowledge, has never been reported before. Immune response has been explored to be the mechanism of alcoholic liver disease. Immune responses were increased in the group of patients who are actively drinking or abstinent < 6 mo and although weaker, persisted in the abstinent patients[24]. The meaning of nodal enlargement in some alcoholic hepatitis cases requires further studies to evaluate its contribution to pathophysiology and management. The mechanism of acute ischemic hepatitis is more hemodynamic than immunologic and presents less nodal reaction (10% in the present study). In a patient with acute hepatitis flare with hemodynamic collapse, an enlarged perihepatic lymph node detected by ultrasound examination indicates etiologies other than ischemic hepatitis alone.
The mechanism of perihepatic lymph nodal reaction in liver disease, an important immune response, had not been well studied before. There was some evidence from previous studies of the celiac node, which is one of the main perihepatic lymph nodes. Matsuno et al[25] had identified the celiac node as a drainage pathway of the lymphatic macrophages from the hepatic sinusoids. In an animal study, orally administered antigen could induce antigen-specific regulatory T cells in the liver-draining celiac lymph node[26]. Recently, by using Evans Blue and purified dendritic cells, the celiac node was proved as lymphatic drainage of liver tissue in mice, which could reflect the intra-hepatic immune response appropriately[27]. Furthermore, a recent study about hepatitis B provided the evidence that the liver-draining lymph nodes induce an anti-HBV-specific immune response responsible for HBV clearance after hydrodynamic injection of HBV plasmid[28].
All of the above evidence suggested an immune responsive correlation between the perihepatic lymph nodes and the liver parenchyma. The nodal manifestations in variable liver diseases with different inflammatory stages merit further evaluations.
The issues for measurements of the nodal size and detectable number are critical when the magnitude of immune response is concerned. With inconsistent correlations, however, associations of the nodal size and number with serum parameters of cytolysis, severity of histologic damage, viremia, and a high CD8 lymphocyte level have been reported in many CHC studies[4,15,20]. Previously, the nodal size was measured by the nodal maximal diameter (long axis or short axis) or area (long axis multiplies short axis) with a maximal cut in transverse view or sagittal view[13,14]. A more convincing measurement was to calculate the nodal volume assumed with an ellipsoid shape[20]. However, an enlarged node around the common hepatic artery does not necessary form as an ellipsoid shape[29,30]. The detectable number is also influenced by various factors including the operator’s experience, definition of nodal enlargement and the resolution power of the machine. In our previous large series study, a nodal width (the short diameter) greater than 5 mm has the same predictable and more convenient value than the nodal volume. Recently, Shu et al[30] also noted that enlarged lymph nodes (more than 5 mm in the short diameter) could be found in about 90% of CHB patients by using MRI, which also proved that nodal width is related to the degree of liver inflammation (grade), when compared with liver biopsy pathology report. In this cohort of patients with acute hepatitis flare, nodal width also provided a better discrimination between viral and non-viral subgroups with the AUROC of 0.805. Actually, in this study cohort, a cut-point of 3.7 mm had a sensitivity of 89.1%, a specificity of 65.2% and a better accuracy rate of 80.14% than that of 5 mm. However, 5 mm may be clinically more practical for operation and no analytical difference was noted when either 5 mm or 3.7 mm was applied.
It is difficult to make a correct differential diagnosis only by history-taking and laboratory data without pathology between AHB and CHB or between AHC and CHC. None of our patients has undergone a liver biopsy for diagnosis and some patients in the acute subgroup may belong to the chronic hepatitis with acute flare subgroup if a non-detectable chronic infection was present. However, the liver biopsy during acute flare hepatitis might be contraindicated due to impaired coagulative functions.
Based on our observations, point-of-care ultrasonography to detect perihepatic nodal change when performed in acute flare hepatitis is valuable to differentiate the etiologies of viral origins from non-viral origins. In an endemic area of HBV infection, CHB with acute flare presents the most prevalent nodal enlargement. In a hemodynamic critical patient with acute hepatic flare, an enlarged perihepatic lymph node detected by ultrasonography indicates etiologies other than ischemic hepatitis alone.
The enlarged lymph nodes around the hepatoduodenal ligament are prevalent in chronic liver diseases including chronic viral hepatitis and immune associated liver disease. The size of the noticeable lymph nodes seemed to be histologically and serologically correlated with the severity of hepatic inflammation, thus, they had been used to predict the effect of treatments and disease prognosis.
Acute hepatitis flare is a life threatening disease and has variable etiologies that need different treatments. The perihepatic nodal appearances have not well been understood. Point-of-care ultrasonography to evaluate the perihepatic nodes may provide valuable evidence for episodes of acute hepatitis flare.
Enlarged lymph nodes were noticeable in 62.5% of acute hepatitis flare episodes and mostly acute on chronic hepatitis B (54.5%) in an endemic area of chronic hepatitis B. The viral group has a higher prevalence rate (80.9%) and larger nodal size (median width, 7 mm) than the non-viral group (31.8%; median width, 0 mm). Meanwhile, there were significant differences in the nodal size between acute and chronic viral groups (P < 0.01), and between acute hepatitis A and non-hepatitis A viral groups (P < 0.001). In logistical regression analysis, the nodal width still showed strong significance in multivariate analysis (P < 0.0001) to stratify the viral and non-viral groups. When a 5 mm cut-off value for nodal width was used, the diagnostic performance for differentiating the viral and non-viral groups was as follows: AUROC, 0.805; sensitivity, 80.9%; specificity, 68.2%; positive predictive value, 80.92%; negative predictive value, 68.18%; and accuracy, 76.14%.
In a victim of acute hepatitis flare, point-of-care ultrasonography to detect perihepatic nodes contributes an initial differential diagnosis of viral and non-viral origins. In an endemic area of chronic hepatitis B, an enlarged perihatic node usually directs acute-on-chronic hepatitis B. The appearance of multiple enlarged perihepatic nodes should lead one to diagnose acute hepatitis A.
The perihepatic lymph nodes consist of hepatic portal lymph nodes, celiac lymph nodes, posterior mediastinal lymph nodes, supradiaphragmatic lymph nodes, and parasternal lymph nodes. Inflammatory process in the liver frequently leads to hyperplasia of regional lymph nodes. Point-of-care ultrasonography refers to ultrasonography performed and interpreted by the clinician at the bedside for a real-time patient analysis, allowing immediate notations that could be directly correlated with the patient’s presenting signs and symptoms.
Overall this is a good paper.
P- Reviewer: Hall TR S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Cassani F, Zoli M, Baffoni L, Cordiani MR, Brunori A, Bianchi FB, Pisi E. Prevalence and significance of abdominal lymphadenopathy in patients with chronic liver disease: an ultrasound study. J Clin Gastroenterol. 1990;12:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Soresi M, Carroccio A, Bonfissuto G, Agate V, Magliarisi C, Aragona F, Levrero M, Notarbartolo A, Montalto G. Ultrasound detection of abdominal lymphadenomegaly in subjects with hepatitis C virus infection and persistently normal transaminases: a predictive index of liver histology severity. J Hepatol. 1998;28:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Soresi M, Carroccio A, Agate V, Bonfissuto GD, Magliarisi C, Fulco M, Aragona F, Montalto G. Evaluation by ultrasound of abdominal lymphadenopathy in chronic hepatitis C. Am J Gastroenterol. 1999;94:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Muller P, Renou C, Harafa A, Jouve E, Kaplanski G, Ville E, Bertrand JJ, Masson C, Benderitter T, Halfon P. Lymph node enlargement within the hepatoduodenal ligament in patients with chronic hepatitis C reflects the immunological cellular response of the host. J Hepatol. 2003;39:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Kuo HT, Lin CY, Chen JJ, Tsai SL. Enlarged lymph nodes in porta hepatis: sonographic sign of chronic hepatitis B and C infections. J Clin Ultrasound. 2006;34:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Jimenez-Saenz M, Maldonado-Perez B, Romero-Vázquez J, Herrerias-Gutierrez JM. Intra-abdominal lymphadenopathy and acute hepatitis A in an adult patient: a anecdotal radiological finding. J Clin Gastroenterol. 2007;41:119-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Ozaras R, Ipekci S, Kumbasar H, Aybar Y, Tahan V, Mert A, Ozturk R, Tabak F. Does the presence of peripheral and intra-abdominal lymphadenopathy predict the etiology of acute hepatitis? J Clin Gastroenterol. 2009;43:196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Nardi P, Biagi P, Bocchini S. [Enlargement of the lymph nodes of the hilus hepatis: a further ultrasonographic sign of acute viral hepatitis]. Radiol Med. 1990;79:212-214. [PubMed] |
| 9. | Braden B, Faust D, Ignee A, Schreiber D, Hirche T, Dietrich CF. Clinical relevance of perihepatic lymphadenopathy in acute and chronic liver disease. J Clin Gastroenterol. 2008;42:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1110] [Cited by in RCA: 1145] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 11. | Forsberg L, Florén CH, Hederström E, Prytz H. Ultrasound examination in diffuse liver disease. Clinical significance of enlarged lymph nodes in the hepato-duodenal ligament. Acta Radiol. 1987;28:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Deutch SJ, Sandler MA, Alpern MB. Abdominal lymphadenopathy in benign diseases: CT detection. Radiology. 1987;163:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Lyttkens K, Prytz H, Forsberg L, Hederström E, Hägerstrand I. Ultrasound, hepatic lymph nodes and chronic active hepatitis. J Hepatol. 1994;21:578-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Cassani F, Valentini P, Cataleta M, Manotti P, Francesconi R, Giostra F, Ballardini G, Lenzi M, Zauli D, Bianchi FB. Ultrasound-detected abdominal lymphadenopathy in chronic hepatitis C: high frequency and relationship with viremia. J Hepatol. 1997;26:479-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Dietrich CF, Stryjek-Kaminska D, Teuber G, Lee JH, Caspary WF, Zeuzem S. Perihepatic lymph nodes as a marker of antiviral response in patients with chronic hepatitis C infection. AJR Am J Roentgenol. 2000;174:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Zhang XM, Mitchell DG, Shi H, Holland GA, Parker L, Herrine SK, Pasqualin D, Rubin R. Chronic hepatitis C activity: correlation with lymphadenopathy on MR imaging. AJR Am J Roentgenol. 2002;179:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Dodd GD, Baron RL, Oliver JH, Federle MP, Baumgartel PB. Enlarged abdominal lymph nodes in end-stage cirrhosis: CT-histopathologic correlation in 507 patients. Radiology. 1997;203:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Ko YL, Sun CS, Chung KM, Lin YM, Feng IC, Sheu MJ, Koay LB, Lin CY, Ho CH, Kuo HT. Manifestations of perihepatic lymph nodes in acute flare of chronic hepatitis B: association with HBeAg status and with HBeAg seroconversion. PLoS One. 2015;10:e0117590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Okada Y, Yao YK, Yunoki M, Sugita T. Lymph nodes in the hepatoduodenal ligament: US appearances with CT and MR correlation. Clin Radiol. 1996;51:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Dietrich CF, Lee JH, Herrmann G, Teuber G, Roth WK, Caspary WF, Zeuzem S. Enlargement of perihepatic lymph nodes in relation to liver histology and viremia in patients with chronic hepatitis C. Hepatology. 1997;26:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 595] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 22. | Choi YS, Lee J, Lee HW, Chang DY, Sung PS, Jung MK, Park JY, Kim JK, Lee JI, Park H. Liver injury in acute hepatitis A is associated with decreased frequency of regulatory T cells caused by Fas-mediated apoptosis. Gut. 2015;64:1303-1313. [PubMed] |
| 23. | Lohse AW, Weiler-Normann C, Tiegs G. Immune-mediated liver injury. J Hepatol. 2010;52:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Lin F, Taylor NJ, Su H, Huang X, Hussain MJ, Abeles RD, Blackmore L, Zhou Y, Ikbal MM, Heaton N. Alcohol dehydrogenase-specific T-cell responses are associated with alcohol consumption in patients with alcohol-related cirrhosis. Hepatology. 2013;58:314-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Matsuno K, Miyakawa K, Ezaki T, Kotani M. The liver lymphatics as a migratory pathway of macrophages from the sinusoids to the celiac lymph nodes in the rat. Arch Histol Cytol. 1990;53 Suppl:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Hultkrantz S, Ostman S, Telemo E. Induction of antigen-specific regulatory T cells in the liver-draining celiac lymph node following oral antigen administration. Immunology. 2005;116:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Barbier L, Tay SS, McGuffog C, Triccas JA, McCaughan GW, Bowen DG, Bertolino P. Two lymph nodes draining the mouse liver are the preferential site of DC migration and T cell activation. J Hepatol. 2012;57:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Zheng M, Yu J, Tian Z. Characterization of the liver-draining lymph nodes in mice and their role in mounting regional immunity to HBV. Cell Mol Immunol. 2013;10:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Metreweli C, Ward SC. Ultrasound demonstration of lymph nodes in the hepatoduodenal ligament (‘Daisy Chain nodes’) in normal subjects. Clin Radiol. 1995;50:99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Shu J, Zhao JN, Han FG, Tang GC, Luo YD, Luo L, Chen X. Chronic hepatitis B: Enlarged perihepatic lymph nodes correlated with hepatic histopathology. World J Radiol. 2013;5:208-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |