Published online Nov 14, 2015. doi: 10.3748/wjg.v21.i42.11904
Peer-review started: April 15, 2015
First decision: May 18, 2015
Revised: June 3, 2015
Accepted: September 30, 2015
Article in press: September 30, 2015
Published online: November 14, 2015
Processing time: 211 Days and 16.5 Hours
Acute alcoholic hepatitis (AAH) is a serious complication of alcohol misuse and has high short term mortality. It is a clinical syndrome characterised by jaundice and coagulopathy in a patient with a history of recent heavy alcohol use and is associated with profound immune dysfunction with a primed but ineffective immune response against pathogens. Here, we review the current knowledge of the pathogenesis and immune defects of AAH and identify areas requiring further study. Alcohol activates the immune system primarily through the disruption of gut tight junction integrity allowing the escape of pathogen-associated molecular particles (PAMPs) into the portal venous system. PAMPs stimulate cells expressing toll-like receptors (mainly myeloid derived cells) and initiate a network of intercellular signalling by secretion of many soluble mediators including cytokines and chemokines. The latter coordinates the infiltration of neutrophils, monocytes and T cells and results in hepatic stellate cell activation, cellular damage and hepatocyte death by necrosis or apoptosis. On the converse of this immune activation is the growing evidence of impaired microbial defence. Neutrophils have reduced phagocytic capacity and oxidative burst and there is recent evidence that T cell exhaustion plays a role in this.
Core tip: Acute alcoholic hepatitis (AAH) has high short-term mortality and is challenging to treat with only glucocorticoids demonstrating proven survival benefit. Development of other effective treatment requires a clear understanding of the mechanisms of immune dysfunction in AAH. Here, we review recent progress in the field and identify areas in need of further research; particularly the role of gut dysbiosis in allowing presentation of pathogen associated molecular patterns to innate receptors on myeloid cells and the subsequent recruitment of immune cell subsets. Recent data demonstrating that T cells have an exhausted phenotype and result in impaired antimicrobial defence is also discussed.
- Citation: Dhanda AD, Collins PL. Immune dysfunction in acute alcoholic hepatitis. World J Gastroenterol 2015; 21(42): 11904-11913
- URL: https://www.wjgnet.com/1007-9327/full/v21/i42/11904.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i42.11904
The United Kingdom has seen an increasing burden of liver related mortality over recent decades with rates increasing 4-fold since the 1970s and 5-fold in the under-65s[1]. This is closely mirrored by the relative affordability of alcohol over this time[2], which is 61% more affordable in 2013 than 1980[3] suggesting that alcohol is an important driving factor for liver disease in the United Kingdom. However, this is not a problem unique to the United Kingdom, similar changes in alcohol consumption and alcohol related mortality have been observed in Northern and Eastern Europe[1] as well as in sub-Saharan Africa, South America and Asia[4].
Given the increasing global consumption of alcohol, it is not surprising that the incidence of acute alcoholic hepatitis (AAH), a serious complication of harmful alcohol use, has also been rising over recent years[5]. AAH is a clinical syndrome characterised by jaundice and coagulopathy in a patient with a recent history of heavy alcohol consumption[6] and has a high short term mortality of up to 40%[7]. It should be clearly differentiated from alcoholic steatohepatitis, a histological diagnosis, which can occur outside the context of current alcohol misuse[8-10].
AAH is increasingly recognised as a systemic inflammatory condition, leading to progressive organ dysfunction and the presence of a systemic inflammatory response syndrome confers a poor prognosis[9]. As well as marked immune activation, there is severe impairment of immune protection against pathogens[11,12]. The discordance between the primed state of the immune system and its failure in microbial defence is yet to be fully explained and an understanding of this dysfunction would certainly help in identifying novel therapeutic targets. To date, therapy has focused on the suppression of an activated immune system and numerous clinical trials have been conducted to evaluate immuno-modulatory (for example, glucocorticoids) or anti-inflammatory therapies [for example, tumour necrosis factor (TNF) alpha antagonists] which target systemic immune activation. Initial promising results from an open label study of infliximab[13] were not confirmed in a randomised controlled trial which was stopped due to excess mortality and infection[14]. Similarly, etanercept treatment was associated with increased mortality[15]. Pentoxifylline, a non-selective phosphodiesterase inhibitor with anti-TNF properties, has also shown no benefit either in combination with glucocorticoids[16,17] or alone[18]. To date only glucocorticoids have a proven short term survival benefit[19,20]. The challenge is how to strike the correct balance between supressing an overactive immune system without further impairing its protective role since death through sepsis remains a significant issue with immunosuppressive treatments[13,21].
The rising incidence of AAH together with its high mortality and limited treatment options has resulted in the European Association for the Study of the Liver identifying AAH as a priority area for research with a specific aim to investigate molecular signals which may predict clinical outcome[22]. Here, we review the current knowledge of the immune mechanisms involved in the pathogenesis of AAH and focus on areas in need of future study. We have not discussed the direct toxic effects on the liver of alcohol and its metabolites including oxidative stress and acetaldehyde adducts which have been reviewed in detail elsewhere[23,24].
The mechanisms by which alcohol activates the immune system were first conclusively elucidated by Thurman et al[25] in 1999. The presence of alcohol allowed the presentation of pathogen associated molecular patterns (PAMPs) to hepatic macrophages (Kupffer cells) by modulating intestinal permeability[25]. Subsequent studies have highlighted the importance of the effects of alcohol on the gut microbiome itself with alterations in both the number and balance of organisms which contribute to the breakdown of the intestinal barrier[26]. In a murine model of alcohol related liver disease (ALD), intestinal bacterial overgrowth occurs with a corresponding reduction in probiotic species such as Lactobacillus[26]. In humans, bacterial overgrowth has been found in jejunal aspirates from chronic alcohol misusers[27] but the species of bacteria is also important. In a randomised controlled trial (RCT) of Lactobacillus and Bifidobacterium probiotic therapy in patients with alcoholic psychosis, baseline levels of intestinal probiotic species were lower than healthy controls and short term probiotic treatment significantly improved biochemical indices[28]. Improvement in clinical disease score has also been demonstrated in a small RCT by Escherichia coli Nissle treatment of patients with stable cirrhosis[29]. There is also preliminary data that treatment with probiotics can improve neutrophil phagocytic function in stable cirrhosis with normalisation of phagocytosis after 7 d of treatment with Lactobacillus casei Shirota[30].
To date, the most detailed human study of the effects of alcohol on gut dysbiosis compared patients with ALD cirrhosis, patients with alcohol dependence and healthy controls using next generation sequencing techniques to analyse the 16S ribosomal RNA (rRNA)[31]. Compared to the control group a subset of patients displayed gut dysbiosis with significantly lower levels of Bacteroides and higher levels of Proteobacteria, which was associated with increased systemic endotoxin. Next generation sequencing rRNA studies have yet to be performed in patients with AAH.
Gut dysbiosis alters the intestinal lumen integrity through mechanisms that are incompletely understood. It is clear that the microbiota are key in increasing gut permeability through murine experiments in which the sterilised gut protects against alcohol-induced intestinal barrier leakage[32]. Disruption of tight junctions is probably mediated by microbial metabolism of alcohol to acetaldehyde[33]. However, systemic TNFα and IL-1β, which are increased in patients with AAH, also reduce tight junction integrity so there may be a positive feedback loop in these patients[34]. Furthermore, the loss of probiotic bacterial species may reduce barrier protection since transfer of Lactobacillus ameliorates ALD in a mouse model[35]. In a more acute murine model of alcoholic steatohepatitis Lactobacillus treatment restored intestinal integrity, reduced oxidative stress and improved histological liver damage[36].
The leaky gut seen in patients with AAH results in presentation of PAMPs to hepatic innate immune cells, particularly Kupffer cells. Chronic alcohol misusers have higher levels of endotoxin [lipopolysaccharide (LPS)] systemically[37] as well as in the portal vein[38] suggesting that there is greater exposure of the liver to microbial components. Interestingly, this defect may be rapidly reversible: in a study of alcohol dependent patients, both intestinal permeability and LPS levels were elevated compared to normal controls but returned to normality after 3 wk of abstinence[39].
Most of our understanding of gut dysbiosis in AAH comes from animal models and patients with chronic ALD. Future study should be directed at analysing the gut microbiome of patients with AAH compared to healthy controls and patients with cirrhosis. A well powered RCT should then be conducted to test the efficacy of specific probiotic therapy designed to restore the microbiome. Work in this area is underway as demonstrated by a single trial registered at Clinicaltrials.gov which is investigating 7 d of treatment with several probiotic regimes but this is not powered to detect survival differences[40]. Prevention of the disruption of gut tight junctions is also an appealing therapeutic target but this is likely to be due to a complex interplay between gut bacteria and innate immunity and a more detailed understanding of the mechanisms of disruption is first required.
Toll-like receptors (TLRs) are innate pattern recognition receptors for a wide variety of PAMPs such as microbial components, endogenous molecules and danger signals[41]. The TLR family consists of 10 receptors principally expressed by granulocytes and cells of myeloid lineage. The net action of ligand binding is the activation of the nuclear factor kappa B (NF-κB), activating protein 1 (AP1) and interferon regulatory factor (IRF) families and rapid and robust transcription of pro-inflammatory mediators[42]. In animal models of ALD, increased expression of TLR1, 2, 4, 6, 7, 8, and 9 is reported with increased sensitivity to their respective ligands[43] while in humans, TLR2, 4 and 9 are upregulated in neutrophils from AAH patients[44] suggesting that the TLR signalling pathway is important in the pathogenesis of the disease.
LPS acting via TLR4 appears to be the most important interaction in the pathogenesis of AAH. Mice with non-functional mutant TLR4 are protected from alcoholic liver injury[45] as are those with inactivated Kupffer cells[46]. However, both Kupffer cells and non-bone marrow derived liver cells are involved in TLR4-mediated alcoholic liver injury shown by development of ALD in TLR4-/- mice transferred with wildtype bone marrow cells[47]. Furthermore, deficiency in IL-1 receptor associated kinase (IRAK)-M (the negative regulator of TLR4) conferred more severe ALD[48].
The action of LPS and other PAMPs via innate receptors on intrahepatic cells initiates a sequence of pro-inflammatory responses. Patients with AAH have elevated levels of pro-inflammatory cytokines mostly produced by myeloid cells including IL-1, IL-6, IL-8 and TNFα (the latter is also related to disease severity)[49-51]. This results in hepatocellular damage via TNF Receptor 1 and intrinsic death pathways[52]. In addition, alcohol sensitises Kupffer cells to the effects of LPS[53] and hepatic macrophages and Kupffer cells produce reactive oxygen species in response to chronic alcohol exposure or LPS[54], driving further liver damage.
The TLR system has the potential to be modulated to reduce pro-inflammatory signalling in AAH but still requires more thorough evaluation. TLR expression has been studied in detail in neutrophils from patients with AAH but blockade of the overexpressed TLRs did not result in restoration of normal neutrophil function[44]. The function of other immune subsets with high TLR expression (especially monocytes, macrophages and Kupffer cells) should also be examined.
Stimulation of both immune and non-immune intrahepatic cells results in the secretion of an array of soluble mediators including cytokines and chemokines which co-ordinate the subsequent immune response and determine the balance between liver damage and resolution of inflammation. Chemokines and their respective receptors control the influx of leucocyte subsets into the liver and have been shown to play important roles in shaping the immune response in a variety of liver diseases[55]. Chemokines are low molecular weight proteins which bind to transmembrane receptors triggering a signalling cascade which alters integrin expression allowing interaction with endothelial adhesion molecules. The gradient of chemokine expression increases near the site of inflammation, which ensures the leucocyte is attracted to the appropriate site before migrating through the vascular endothelium.
Interest in chemokines and their receptors as therapeutic targets has increased over recent years since they can control the ingress of specific pro-inflammatory leucocyte subsets into sites of inflammation or injury. Currently, a number of clinical trials evaluating several chemokine and chemokine receptor antagonists for the treatment of inflammatory diseases including asthma, inflammatory bowel diseases and primary biliary cirrhosis have been registered at clinicaltrials.gov. In the context of AAH, many different chemokines have been implicated and the challenge is to determine which pathway to block. Neutrophils may be the most appropriate target since liver tissue from patients with AAH demonstrates a significant neutrophilic infiltration, the degree of which correlates with disease severity[56].
Ischaemic models of acute liver injury demonstrate that neutrophils are activated by TNFα, IL-1β and IL-17 and recruited by CXC chemokines such as CXCL1 (GROα) and CXCL8 (IL-8)[57]. Elevated levels of these chemokines among others have been confirmed in transcriptome microarray and PCR analysis of homogenised liver biopsy material from patients with AAH[58]. Levels of CXCL1, 5, 6 and 8 were all elevated in AAH vs normal liver and correlated with neutrophil infiltration and degree of portal hypertension and were associated with a poor prognosis at 90 d[58]. However, the exact role of the neutrophil in the pathogenesis of AAH is unclear[11] and the control of their entry into the liver is not fully understood. Moreover, chemokines that are known to attract neutrophils will also attract other immune cell types. CXCL1, 5, 6, and 8 specifically attract both neutrophils and monocytes which both express the relevant CXCR1 and CXCR2 receptors for these chemokines. Therefore, a clearer understanding of the complex pathways of leucocyte trafficking in AAH is required and blockade of a single component of the pathway may not translate into a clinical benefit[59].
The same transcriptome study also identified CCL20 as being the third most upregulated gene expressed in AAH liver tissue compared to controls[58]. CCL20 binds to CCR6 which is expressed on the Th17 subset of T cells as well as on hepatic stellate cells (HSCs) and γδT cells and is likely to play an important role in the adaptive immune response.
The Th17 cell subset, defined by its production of IL-17 and expression of RORγt, is derived from naïve CD4+ T cells under the influence of cytokines IL-1β and IL-6[60]. As well as being important in the clearance of extracellular pathogens, it is also implicated in the pathogenesis of several autoimmune[61] and inflammatory diseases including ALD and AAH[62,63]. IL-17 enhances the inflammatory response by stimulating a wide variety of cells including monocytes, endothelial cells and fibroblasts, to secrete CXCL8, a neutrophil chemoattractant[64]. In a positive feedback loop, IL-17 also stimulates CCL20 secretion, itself a Th17 chemoattractant, with high levels of its receptor CCR6 expressed by Th17 cells[65,66].
Th17 cells have been implicated in the pathogenesis of AAH[63]. IL-17 protein in serum from patients with AAH was elevated as was peripheral CD4+ T cell capacity to produce IL-17 on stimulation compared to healthy controls. In AAH liver tissue, there was an enrichment of IL-17+ cells which were T cells and neutrophils and numbers correlated with degree of fibrosis. Furthermore, it was shown that HSCs have the IL-17 receptor and their secretion of important fibrotic mediators was dependent on IL-17[34].
The liver transcriptome study[58] suggests that in AAH, the high expression of CCL20 results in the infiltration of Th17 cells. Further work has shown that CCL20 levels correlate with clinical severity score, degree of portal hypertension and survival in patients with AAH and, using an animal model of acute on chronic ALD [mice treated with carbon tetrachloride (CCl4), ethanol and LPS], that macrophages and HSCs are the primary source of CCL20[67]. In addition, exposure of primary HSCs in vitro to CCL20 promotes fibrogenesis[67].
Therefore, it is likely that CCL20 mediates hepatic inflammation and fibrosis in AAH by direct effects on HSCs and via recruitment of Th17 cells. The Th17 cytokine IL-22 has also been shown to be upregulated in peripheral blood of patients with AH and increased levels subsequently predicted better patient outcome[68]. Plasma IL-17 (but not IL-21 or IL-23) was also elevated compared to healthy controls but not related to outcome. Data was not presented to determine whether IL-17 and IL-22 were co-expressed so it is possible that IL-22 may be from the novel Th22 cells rather than pathogenic Th17 cells and hence may have a hepatoprotective effect. The protective effects of IL-22 have been demonstrated in a chronic/binge ethanol feeding model where administration of exogenous IL-22 ameliorated liver injury and oxidative stress via a STAT3 mechanism[69]. A phase 2 clinical trial of recombinant human IL-22 is currently underway in patients with AAH.
Interestingly, a recent study extensively characterised pathogenic Th17 cells from healthy controls and then confirmed that they were enriched in the peripheral blood and inflamed gut of patients with Crohn’s disease[70]. These pathogenic Th17 cells were resistant to steroid-mediated T cell suppression in terms of pro-inflammatory cytokine production and proliferation[70]. An animal model of airways inflammation has also suggested that Th17 cells are steroid resistant[71]. This has been further assessed by gene profile analysis, cytokine expression and proliferation in Th17 cells derived from patients with autoimmune uveitis as well as murine Th17 cells in an experimental model of uveitis, which were shown to be resistant to steroid treatment[72]. The latter study also demonstrated that both human Th17 cells in vitro and murine Th17 cells in vivo were selectively inhibited by the calcineurin inhibitor, ciclosporin A (CsA)[72].
The observation that Th17 cells are enriched in AAH but may be resistant to steroid treatment may help to explain why some patients do not respond clinically to steroid treatment. Further research is needed to clarify whether these cells are indeed steroid resistant in the context of AAH and whether there are different characteristics in this T cell subset between steroid responders and non-responders. As suggested by the recent in vitro and in vivo data discussed above[72], rescue therapy with CsA in patients with steroid resistant AAH may be efficacious. It is already well-established therapy for the treatment of steroid resistant acute severe ulcerative colitis albeit with significant toxicities and side-effects[73] but the risk of sepsis with such a potent immunosuppressive agent would be too high to justify a clinical trial in AAH patients unless a method to specifically target Th17 cells could be found. Alternatively, if steroid non-responders could be accurately identified, for example by the bioassay recently reported by our group[74], CsA could be selectively offered to these patients with the highest risk of death from AAH. Another perhaps less toxic approach is to prevent Th17 cell ingress to the liver with anti-CCL20 antibody, which may be particularly efficacious in patients who do not respond to steroid treatment.
The phenomenon of a primed immune system but with failure of pathogen defence may be caused by a defect in effector cell negative regulatory signalling. Inhibitory pathways exist to maintain immune homeostasis to prevent over-activation and exhaustion of immune cells but allow appropriate clearance of pathogens and tumours. Several pathways exist; the best studied involves the cytotoxic T-lymphocyte associated protein 4 (CTLA-4) family. CTLA-4, a T cell surface receptor, by binding to the same molecules on antigen presenting cells, competitively antagonises CD28, the T cell co-stimulation receptor and prevents T cell activation[75]. Deletion of CTLA-4 in murine models leads to the development of fatal multiorgan autoimmune disease[76], probably as a result of unchecked CD28-mediated T cell stimulation, demonstrating its importance in immune control. A member of the CTLA-4 family, programmed death 1 (PD-1) serves a similar purpose to maintain balance of effector T cell function[77] while T-cell immunoglobulin and mucin domain 3 (TIM-3) also has potent inhibitory functions on both T cells and innate immune cells[78]. It has recently been proposed that inappropriate expression of PD-1 and TIM-3 plays a role in the immune paresis seen in AAH[79].
Impairment in both innate and adaptive immunity was seen in patients with AAH; poor neutrophil anti-microbial function and reduced T cell interferon-γ (IFNγ) production was demonstrated. In addition, PD-1, TIM-3 and their ligands were overexpressed on T cells from the peripheral blood of patients with AAH compared to patients with ALD or healthy controls but when these receptors were blocked the immune defect was overcome. It was shown that the overexpression of these inhibitory molecules was mediated by LPS binding to TLR4 on CD14+ monocytes[79].
These intriguing data inform new paradigms of how an active immune system exposed to many PAMPs can remain impaired at pathogen clearance and suggest that gut dysbiosis is central to the pathogenesis of the disease. However, the effect of other TLR ligands, cytokines and the direct effect of alcohol on negative regulatory molecule expression (PD-1, TIM-3 and others) have not been investigated. It is important to note that this study was conducted using peripheral blood derived immune cells which may not accurately reflect what occurs within the liver and therefore a similar effect needs to be demonstrated on intrahepatic immune cell subsets. Finally, careful consideration of how to translate these findings to a possible therapy is required. Restoration of immune homeostasis involves the rebalancing of pro- and anti-inflammatory pathways. Improving host defence by blockade of these regulatory pathways may result in the tipping of the balance too far in favour of immune activation which may drive further liver damage. An experimental model of AAH should be first employed to assess whether this strategy would have a beneficial effect.
The cytokine granulocyte colony-stimulating factor (G-CSF) stimulates bone marrow production of granulocytes and haematopoietic stem cells and is involved in the proliferation and differentiation of neutrophils but may also play a role in hepatic regeneration[80]. G-CSF treatment enhances the bactericidal and phagocytic capacity of human neutrophils from healthy subjects as well as impaired neutrophils from HIV-1 infected individuals[81]. It is therefore an appealing therapy for AAH which has the potential to both enhance neutrophil function and hepatocyte regeneration.
G-CSF was well tolerated in patients with cirrhosis and alcoholic steatohepatitis; 5 d of treatment was associated with an increase in circulating CD34+ cells (a surrogate for haematopoietic stem cells), increased serum hepatocyte growth factor and proliferation of hepatic progenitor cells in day 7 liver biopsy specimens. However, there was no change in liver function compared to the control group[82]. A randomised open label trial of G-CSF treatment of Acute on Chronic Liver Failure (of which 57% had alcoholic hepatitis as the underlying aetiology) demonstrated increased hepatic CD34+ cells after 28 d and significantly improved 60 d survival[83]. A recent open label RCT of 5 d of G-CSF vs standard care (including pentoxifylline) in the treatment of patients with AAH resulted in a greater number of serum CD34+ cells and improved 3 mo survival compared to standard care[84].
These trials have demonstrated the potential benefit of G-CSF in the treatment of AAH but confirmation of its benefit in a large double-blind RCT is needed. Further study is required to elucidate the mechanisms of G-CSF action and evaluate its benefit in the context of steroid resistant disease.
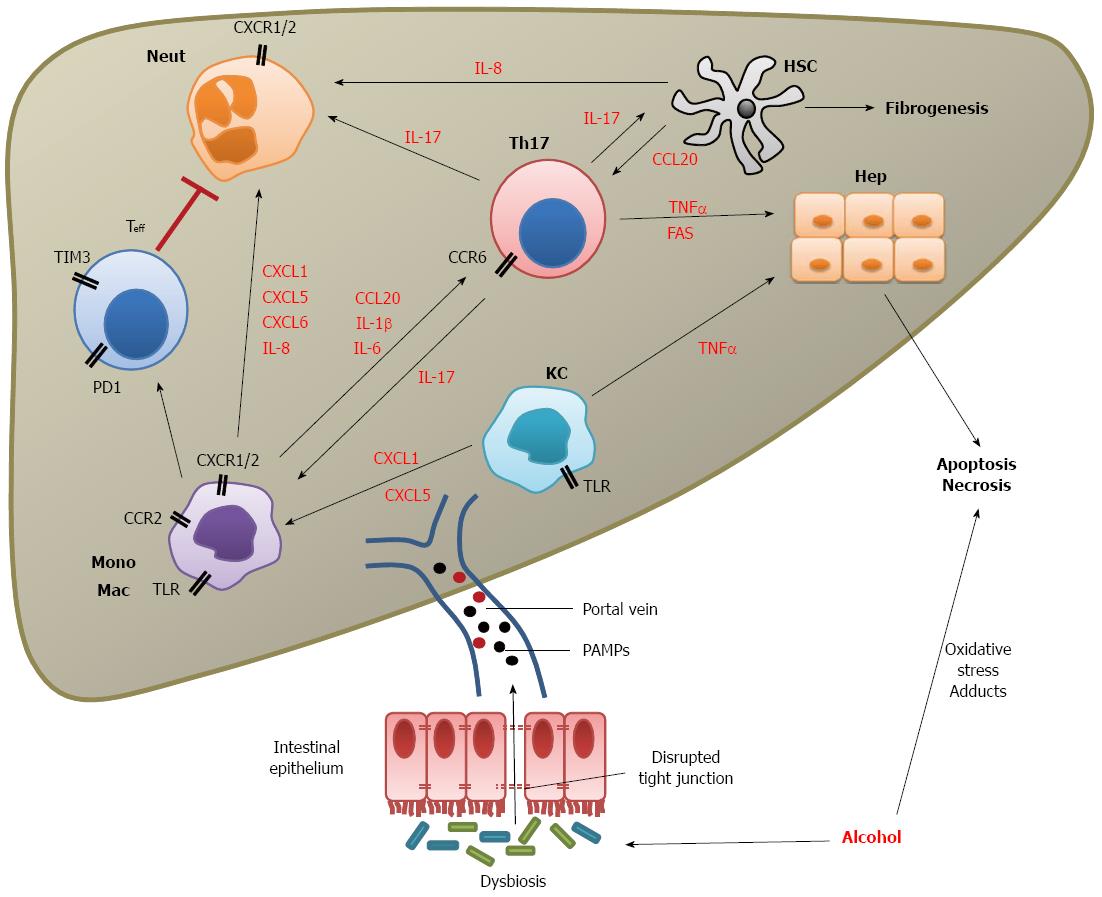
Evidence drawn from studies on patients with AAH and chronic ALD as well as animal models has enhanced our understanding of the immune mechanisms that occur in AAH. In summary, chronic alcohol consumption leads to gut dysbiosis, disrupting the gut epithelial integrity and allowing the presentation of PAMPs to intrahepatic cells via the portal circulation. This in turn causes activation of a network of cells resulting in the alteration of surface molecular patterns and the release of a plethora of soluble mediators, which co-ordinates the influx of immune cells into the liver. In the context of AAH, these immune cell subsets cause direct damage to hepatocytes and stimulate HSCs to produce fibrogenic molecules leading to liver cell death and fibrosis. Additionally, there is evidence of immune paresis with poor innate cell responses and increased T cell exhaustion resulting in a reduced ability to prevent bacterial infection (Figure 1).
Many of the mechanisms of pathogenesis require confirmation and testing in patients with AAH but have the potential to yield new therapeutic targets in the future. Here, we have highlighted the gut microbiome, the expression of TLRs on different myeloid cell subsets, the chemokine pathway, the steroid responsiveness of Th17 cells, T cell exhaustion and G-CSF therapy as priority areas for further research.
P- Reviewer: Akyuz F, Kasztelan-Szczerbinska B S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Williams R, Aspinall R, Bellis M, Camps-Walsh G, Cramp M, Dhawan A, Ferguson J, Forton D, Foster G, Gilmore I. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384:1953-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 447] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 2. | Leon DA, McCambridge J. Liver cirrhosis mortality rates in Britain from 1950 to 2002: an analysis of routine data. Lancet. 2006;367:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | NHS Health and Social Care Information Centre. : NHS 2014; . |
| 4. | World Health Organsiation WHO. Global status report on alcohol and health - 2014 edition. : World Health Organisation 2014; . |
| 5. | Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcoholic hepatitis in Denmark 1999-2008: a nationwide population based cohort study. J Hepatol. 2011;54:760-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 693] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 7. | Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis--a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther. 2008;27:1167-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Dhanda AD, Collins PL, McCune CA. Is liver biopsy necessary in the management of alcoholic hepatitis? World J Gastroenterol. 2013;19:7825-7829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Mookerjee RP, Lackner C, Stauber R, Stadlbauer V, Deheragoda M, Aigelsreiter A, Jalan R. The role of liver biopsy in the diagnosis and prognosis of patients with acute deterioration of alcoholic cirrhosis. J Hepatol. 2011;55:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Wells JT, Said A, Agni R, Tome S, Hughes S, Dureja P, Lucey MR. The impact of acute alcoholic hepatitis in the explanted recipient liver on outcome after liver transplantation. Liver Transpl. 2007;13:1728-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 12. | Gustot T, Maillart E, Bocci M, Surin R, Trépo E, Degré D, Lucidi V, Taccone FS, Delforge ML, Vincent JL. Invasive aspergillosis in patients with severe alcoholic hepatitis. J Hepatol. 2014;60:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, Davion T, Oberti F, Broët P, Emilie D. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 322] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 14. | Parker R, Armstrong MJ, Corbett C, Rowe IA, Houlihan DD. Systematic review: pentoxifylline for the treatment of severe alcoholic hepatitis. Aliment Pharmacol Ther. 2013;37:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Tilg H, Jalan R, Kaser A, Davies NA, Offner FA, Hodges SJ, Ludwiczek O, Shawcross D, Zoller H, Alisa A. Anti-tumor necrosis factor-alpha monoclonal antibody therapy in severe alcoholic hepatitis. J Hepatol. 2003;38:419-425. [PubMed] |
| 16. | Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, Gores GJ, Harmsen WS, McClain CJ. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 255] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Sidhu SS, Goyal O, Singla P, Gupta D, Sood A, Chhina RS, Soni RK. Corticosteroid plus pentoxifylline is not better than corticosteroid alone for improving survival in severe alcoholic hepatitis (COPE trial). Dig Dis Sci. 2012;57:1664-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Mathurin P, Louvet A, Duhamel A, Nahon P, Carbonell N, Boursier J, Anty R, Diaz E, Thabut D, Moirand R. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013;310:1033-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 19. | Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 558] [Article Influence: 55.8] [Reference Citation Analysis (1)] |
| 20. | Mathurin P, O’Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, Ramond MJ, Naveau S, Maddrey WC, Morgan TR. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 21. | Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva-Delcambre V, Deltenre P, Mathurin P. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 22. | European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 456] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 23. | Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol. 2014;20:17756-17772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 286] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (5)] |
| 24. | Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev. 2010;3:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 25. | Thurman RG, Bradford BU, Iimuro Y, Frankenberg MV, Knecht KT, Connor HD, Adachi Y, Wall C, Arteel GE, Raleigh JA. Mechanisms of alcohol-induced hepatotoxicity: studies in rats. Front Biosci. 1999;4:e42-e46. [PubMed] |
| 26. | Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 638] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 27. | Bode JC, Bode C, Heidelbach R, Dürr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30-34. [PubMed] |
| 28. | Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 388] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 29. | Lata J, Novotný I, Príbramská V, Juránková J, Fric P, Kroupa R, Stibůrek O. The effect of probiotics on gut flora, level of endotoxin and Child-Pugh score in cirrhotic patients: results of a double-blind randomized study. Eur J Gastroenterol Hepatol. 2007;19:1111-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 31. | Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966-G978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 595] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 32. | Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218-224. [PubMed] |
| 33. | Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276:G965-G974. [PubMed] |
| 34. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 2710] [Article Influence: 169.4] [Reference Citation Analysis (0)] |
| 35. | Wang Y, Kirpich I, Liu Y, Ma Z, Barve S, McClain CJ, Feng W. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am J Pathol. 2011;179:2866-2875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 36. | Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 37. | Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742-747. [PubMed] |
| 38. | Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321-1329. [PubMed] |
| 39. | Leclercq S, Cani PD, Neyrinck AM, Stärkel P, Jamar F, Mikolajczak M, Delzenne NM, de Timary P. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun. 2012;26:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 40. | Leclercq S; Clinicaltrials. gov. (accessed 24 Mar 2015). United States National Institutes of Health. Available from: http://clinicaltrials.gov/ct2/show/NCT01501162. |
| 41. | West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 534] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 42. | Ostuni R, Zanoni I, Granucci F. Deciphering the complexity of Toll-like receptor signaling. Cell Mol Life Sci. 2010;67:4109-4134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 43. | Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Devière J, Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 44. | Stadlbauer V, Mookerjee RP, Wright GA, Davies NA, Jürgens G, Hallström S, Jalan R. Role of Toll-like receptors 2, 4, and 9 in mediating neutrophil dysfunction in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G15-G22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 395] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 46. | Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15 Suppl:D20-D25. [PubMed] |
| 47. | Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509-1518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 48. | Wang Y, Hu Y, Chao C, Yuksel M, Colle I, Flavell RA, Ma Y, Yan H, Wen L. Role of IRAK-M in alcohol induced liver injury. PLoS One. 2013;8:e57085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Bird GL, Sheron N, Goka AK, Alexander GJ, Williams RS. Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann Intern Med. 1990;112:917-920. [PubMed] |
| 50. | Hill DB, Marsano LS, McClain CJ. Increased plasma interleukin-8 concentrations in alcoholic hepatitis. Hepatology. 1993;18:576-580. [PubMed] |
| 51. | Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267-276. [PubMed] |
| 52. | Pastorino JG, Hoek JB. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology. 2000;31:1141-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 53. | Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 54. | Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589-3593. [PubMed] |
| 55. | Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577-594.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 628] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 56. | Maltby J, Wright S, Bird G, Sheron N. Chemokine levels in human liver homogenates: associations between GRO alpha and histopathological evidence of alcoholic hepatitis. Hepatology. 1996;24:1156-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Bajt ML, Farhood A, Jaeschke H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1188-G1195. [PubMed] |
| 58. | Affò S, Dominguez M, Lozano JJ, Sancho-Bru P, Rodrigo-Torres D, Morales-Ibanez O, Moreno M, Millán C, Loaeza-del-Castillo A, Altamirano J. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut. 2013;62:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 59. | Gao B, Xu M. Chemokines and alcoholic hepatitis: are chemokines good therapeutic targets? Gut. 2014;63:1683-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1385] [Cited by in RCA: 1574] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 61. | Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 1164] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 62. | Gao B, Waisman A. Th17 cells regulate liver fibrosis by targeting multiple cell types: many birds with one stone. Gastroenterology. 2012;143:536-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Lemmers A, Moreno C, Gustot T, Maréchal R, Degré D, Demetter P, de Nadai P, Geerts A, Quertinmont E, Vercruysse V. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 64. | Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 1566] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 65. | Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849-1861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1399] [Cited by in RCA: 1480] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 66. | Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803-2812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 726] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 67. | Affò S, Morales-Ibanez O, Rodrigo-Torres D, Altamirano J, Blaya D, Dapito DH, Millán C, Coll M, Caviglia JM, Arroyo V. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut. 2014;63:1782-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 68. | Støy S, Sandahl TD, Dige AK, Agnholt J, Rasmussen TK, Grønbæk H, Deleuran B, Vilstrup H. Highest frequencies of interleukin-22-producing T helper cells in alcoholic hepatitis patients with a favourable short-term course. PLoS One. 2013;8:e55101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 70. | Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, McCauley JL, Abreu MT, Unutmaz D, Sundrud MS. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med. 2014;211:89-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 71. | McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089-4097. [PubMed] |
| 72. | Schewitz-Bowers LP, Lait PJ, Copland DA, Chen P, Wu W, Dhanda AD, Vistica BP, Williams EL, Liu B, Jawad S. Glucocorticoid-resistant Th17 cells are selectively attenuated by cyclosporine A. Proc Natl Acad Sci USA. 2015;112:4080-4085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Hawthorne AB. Ciclosporin and refractory colitis. Eur J Gastroenterol Hepatol. 2003;15:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | Dhanda AD, di Mambro AJ, Hunt VL, McCune CA, Dayan CM, Dick AD, Lee RW, Collins PL. Long-term outcome in patients with severe alcoholic hepatitis can be reliably determined using an in vitro measure of steroid sensitivity. Hepatology. 2015;61:1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | Linsley PS. Distinct roles for CD28 and cytotoxic T lymphocyte-associated molecule-4 receptors during T cell activation? J Exp Med. 1995;182:289-292. [PubMed] |
| 76. | Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541-547. [PubMed] |
| 77. | Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3785] [Cited by in RCA: 4140] [Article Influence: 243.5] [Reference Citation Analysis (0)] |
| 78. | Han G, Chen G, Shen B, Li Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol. 2013;4:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 79. | Markwick LJ, Riva A, Ryan JM, Cooksley H, Palma E, Tranah TH, Manakkat Vijay GK, Vergis N, Thursz M, Evans A. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology. 2015;148:590-602.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 80. | Thorgeirsson SS, Grisham JW. Hematopoietic cells as hepatocyte stem cells: a critical review of the evidence. Hepatology. 2006;43:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 81. | Roilides E, Walsh TJ, Pizzo PA, Rubin M. Granulocyte colony-stimulating factor enhances the phagocytic and bactericidal activity of normal and defective human neutrophils. J Infect Dis. 1991;163:579-583. [PubMed] |
| 82. | Spahr L, Lambert JF, Rubbia-Brandt L, Chalandon Y, Frossard JL, Giostra E, Hadengue A. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 83. | Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sakhuja P, Sarin SK. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505-512.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 84. | Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol. 2014;109:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |