Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1243
Peer-review started: May 26, 2014
First decision: June 18, 2014
Revised: July 6, 2014
Accepted: September 5, 2014
Article in press: September 5, 2014
Published online: January 28, 2015
Processing time: 246 Days and 4.2 Hours
AIM: To investigate the prognostic significance of estrogen receptor 1 (ER1) and vascular endothelial growth factor A (VEGF-A) expression in primary gallbladder carcinoma (GBC) to identify new prognostic markers for this malignancy.
METHODS: Using immunohistochemistry, we investigated ER1 and VEGF-A expression in 78 GBC and 78 cholelithiasis (CS) tissues. The results were correlated with clinicopathological features. Univariate and multivariate analyses were performed to evaluate the relationship between ER1 and VEGF-A expression and patients’ prognosis. Further Kaplan-Meier survival analysis was also performed.
RESULTS: ER1 and VEGF-A expression was significantly higher in GBC compared with CS (47/78 vs 28/78, P < 0.05; 51/78 vs 33/78, P < 0.05). ER1 expression was correlated with gender (P < 0.05) and VEGF-A expression was correlated with tumor differentiation in GBC patients (P < 0.05). In univariate analysis, age and tumor node metastasis (TNM) stage were factors associated with GBC prognosis (P < 0.05). Although there was no statistical difference between the expression of ER1 or VEGF-A and overall survival, the high expression of ER1 combined with VEGF-A predicted a poor prognosis for GBC patients (16.30 ± 1.87 vs 24.97 ± 2.09, log-rank P < 0.05). In multivariate analysis, combined expression of ER1 and VEGF-A and TNM stage were independent prognostic factors for GBC patients (P < 0.05).
CONCLUSION: Combined expression of ER1 and VEGF-A is a potential prognostic marker for GBC patients. Clinical detection of ER1 and VEGF-A in surgically resected GBC tissues would provide an important reference for decision-making of postoperative treatment programs.
Core tip: Gallbladder carcinoma (GBC) is a serious threat to public health for its poor prognosis. The authors found that estrogen receptor 1 (ER1) and vascular endothelial growth factor A (VEGF-A) expression was significantly higher in GBC than in cholelithiasis tissues, and high expression of ER1 combined with VEGF-A conferred a poor prognosis in GBC patients after surgery. Combined expression of ER1 and VEGF-A was an independent factor associated with GBC prognosis. Clinical detection of ER1 and VEGF-A may guide postoperative clinical treatment of GBC patients.
- Citation: Zhang LQ, Xu XS, Wan Y, Song SD, Wang RT, Chen W, Wang ZX, Chang HL, Wei JC, Dong YF, Liu C. Prognostic implications of estrogen receptor 1 and vascular endothelial growth factor A expression in primary gallbladder carcinoma. World J Gastroenterol 2015; 21(4): 1243-1250
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1243.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1243
Primary gallbladder carcinoma (GBC), originating from the bile duct epithelium, is characterized by poor prognosis[1,2]. Most of GBC patients were asymptomatic until the disease has progressed to an advanced and non-curative stage. According to epidemiological investigations, the 5-year survival rate for GBC patients was less than 10%, with the overall mean survival time of 6 mo. In clinical practice, the tumor node metastasis (TNM) staging system sometimes could not predict GBC patients’ prognosis accurately. In spite of this, except for the TNM staging system, there were no other molecular markers available to facilitate the evaluation of GBC prognosis. Therefore, it is imperative to explore new predictive factors to guide the postoperative treatments for GBC patients.
Due to the female predominance in GBC incidence, it is speculated that estrogen may play important roles in the genesis and progression of GBC[3-5]. Estrogen executes its biological functions by binding to estrogen receptor (ER), and a number of studies have reported that ER was associated with carcinogenesis[6-10]. ER includes two subtypes, ER1 (or ER-α) and ER2 (or ER-β). In spite of similar molecular structure, ER1 and ER2 exhibited an antagonistic effect in some biological processes. As far as our knowledge, ER1 is able to promote tumor development and indicates poor prognosis, while ER2 usually suppresses tumor progression and prefigures good survival[11-13]. Therefore, some researchers assumed that ER1 possibly keep a subtle balance with ER2 in normal conditions[14]. Sumi et al[13] have reported the relationship between ER2 and GBC prognosis. However, although ER1 has been detected in GBC samples, its clinical significance is still equivocal.
Angiogenesis is essential for cancer growth, invasion and metastasis. It is well known that vascular endothelial growth factor (VEGF) is a potent vascular active molecule which directly stimulates the proliferation of vascular endothelial cells[15]. Accumulating evidence suggested that VEGF plays important roles in many kinds of tumors by inducing neoangiogenesis. In human cholangiocarcinoma, VEGF-A was positively expressed and was considered to mediate the proliferative effects of estrogen[16]. Similar to other tumors, adequate blood supply and sufficient angiogenesis are fundamental requirements for the growth of GBC. In GBC, the VEGF-A single nucleotide polymorphisms were implicated in GBC risk[17]. There was also investigations indicating that VEGF-A was highly expressed in GBC and was correlated with a poor prognosis[18]. Nevertheless, Giatromanolaki et al[19] reported that VEGF was not associated with GBC patient survival, but combined VEGF and thymidine phosphorylase expression was considered an unfavorable prognostic factor. Therefore, it is still controversial with regards to the prognostic significance of VEGF in GBC.
ER1 and VEGF-A play important roles in GBC. Estrogen can modulate VEGF expression[20-23]. However, there have been no relevant reports about the prognostic significance of ER1 and VEGF-A in GBC. Hence, we investigated the expression status of ER1 and VEGF-A in resected human GBC tissues, and to evaluate their prognostic value in GBC.
In the present study, tissue specimens were collected from 156 patients who had undergone surgical resection at the First Affiliated Hospital of Medical College, Xi’an Jiaotong University (Xi’an, China) between October 2009 and October 2010, including 78 patients with GBC confirmed by postoperative pathological diagnosis, and 78 patients with cholelithiasis (CS) who underwent cholecystectomy. None of them received any preoperative radiochemotherapy. The two groups were matched in age and gender. The clinicopathological information was obtained from the hospital’s medical records. The following data of each patient was included: age, gender, gallstone status, tumor differentiation, and TNM stage. All GBC patients were closely followed after surgery for 4 to 53 mo, and we defined that GBC patient’s death was the only positive outcome in our study.
The streptavidin-peroxidase (SP) method was performed using rabbit polyclonal antibody to ER1 and VEGF-A obtained from Santa Cruz Biotechnology to detect the expression of ER1 and VEGF-A in GBC and CS tissues. Formalin-fixed and paraffin-embedded specimens were cut into 4-μm sections, mounted onto slides treated with poly-L-lysine, deparaffinized, and rehydrated. The slides were heated at 96-98 °C in a microwave for 15 min in a citrate buffer solution at pH 6.0 and cooled for 30 min at room temperature to retrieve antigen. To quench the endogenous peroxidase activity, sections were treated with 0.3% H2O2 for 30 min. Subsequently, the sections were treated with 5% normal goat serum in phosphate-buffered saline for 1 h to block nonspecific sites. All sections in a humidified box were incubated overnight at 4 °C with specific antibodies detecting ER1 and VEGF-A, and then incubated with biotinylated anti-rabbit IgG and avidin-biotin-peroxidase complex, respectively. Antibody binding was visualized by exposure to diaminobenzidine. Hematoxylin was used to weakly counterstain sections. The sections were dehydrated in graded alcohol and cleared in xylene. Finally, all sections were mounted with neutral gum.
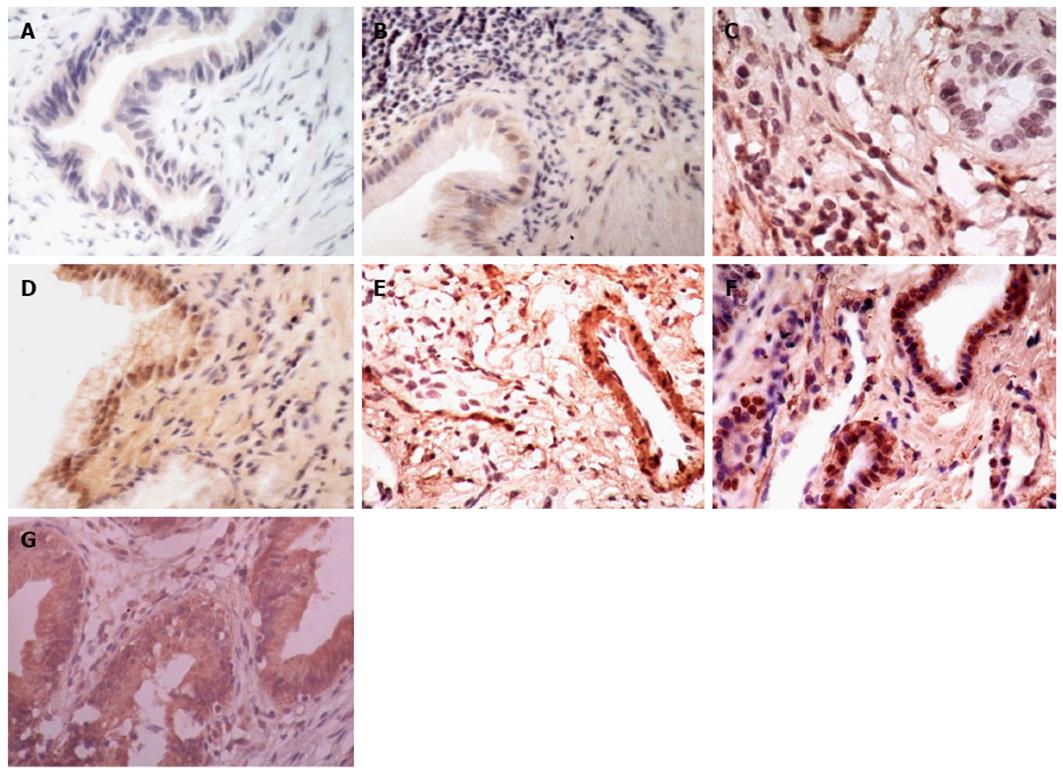
According to the previous literature[18,24,25], a semiquantitative manner was used to evaluate the staining of ER1 and VEGF-A. All of the sections were assessed independently by two investigators in a blind manner under a transmission light microscope. The intensity of staining (IS) and the percentage of positively stained (PS) cells were evaluated. The IS was scored as 0 (absent), l (weak), 2 (moderate), and 3 (strong). The percentage of PS cells was scored as 0 (none), 1 (1%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%). Five fields per case and 100 tumor cells per × 40 field were examined. The mean value obtained was the final score for each case. A final score (FS) was calculated using the formula: FS = IS + PS. Finally, all the sections were defined as “low” expression if FS was 0-4 or “high” expression if FS was 5-7 for assessment of ER1 and VFGF-A staining. The typical histology corresponding to each histological score used in this study is shown in Figure 1.
Fisher’s exact test or χ2 test as appropriate was performed to assess the associations between the ER1 and VEGF-A expression and clinicopathological variables. Kaplan-Meier method was used to plot survival curves, and the log-rank test was used to determine statistical differences. Multivariate analysis was performed using Cox proportional hazard model. A P-value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 13.0 program.
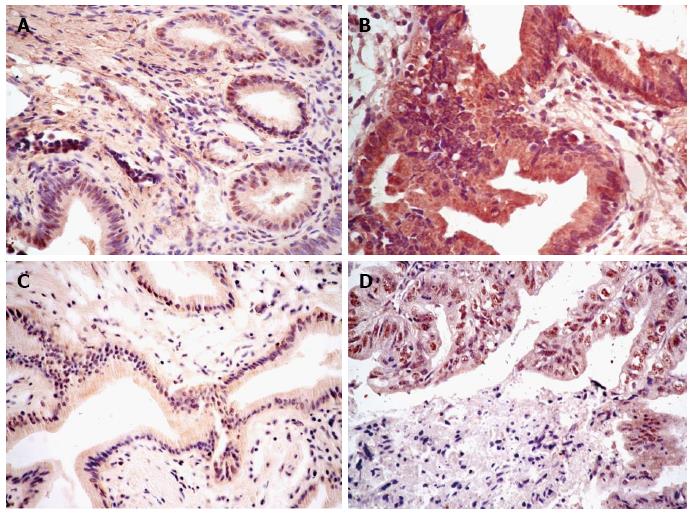
The expression status of ER1 and VEGF-A is shown in Figure 2. VEGF-A was expressed in the cytoplasmic compartment, and ER1 was expressed in the nucleus. The expression of both ER1 and VEGF-A was significantly higher in GBC compared with CS (Table 1). Higher ER1 expression was observed in more GBC (47/78, 60.3%) than in CS tissues (28/78, 35.9%) (P = 0.002). Similarly, higher expression of VEGF-A was observed in more GBC (51/78, 65.4%) than in CS tissues (33/78, 42.3%) (P = 0.004). In GBC patients, there was no statistical significance between the histological scores of ER1 and VEGF-A (r = 0.176, P = 0.124).
| Group | ER1 expression | P-value | VEGF-A expression | P-value | ||
| High | Low | High | Low | |||
| GBC | 47 | 31 | 0.002 | 51 | 27 | 0.004 |
| CS | 28 | 50 | 33 | 45 | ||
ER1 expression was associated with gender. ER1 expression was more frequent in females than males (P = 0.022). In addition, VEGF-A expression was correlated with tumor differentiation (P = 0.01). No significant difference was found between the expression of ER1 and VEGF-A and other clinicopathological factors (Table 2).
| Characteristic | ER1 expression | P-value | VEGF-A expression | P-value | ||
| High | Low | High | Low | |||
| Gender | 0.022 | 0.099 | ||||
| Male | 15 | 18 | 25 | 8 | ||
| Female | 32 | 13 | 26 | 19 | ||
| Age (yr) | 0.151 | 0.095 | ||||
| ≤ 55 | 18 | 17 | 23 | 12 | ||
| > 55 | 29 | 14 | 28 | 15 | ||
| Gallstones | 0.370 | 0.056 | ||||
| Present | 32 | 24 | 33 | 23 | ||
| Absent | 15 | 7 | 18 | 4 | ||
| TNM stage | 0.177 | 0.781 | ||||
| II | 17 | 16 | 21 | 12 | ||
| III/IV | 30 | 15 | 30 | 15 | ||
| Differentiation | 0.205 | 0.010 | ||||
| Well | 16 | 15 | 15 | 16 | ||
| Moderate/poor | 31 | 16 | 36 | 11 | ||
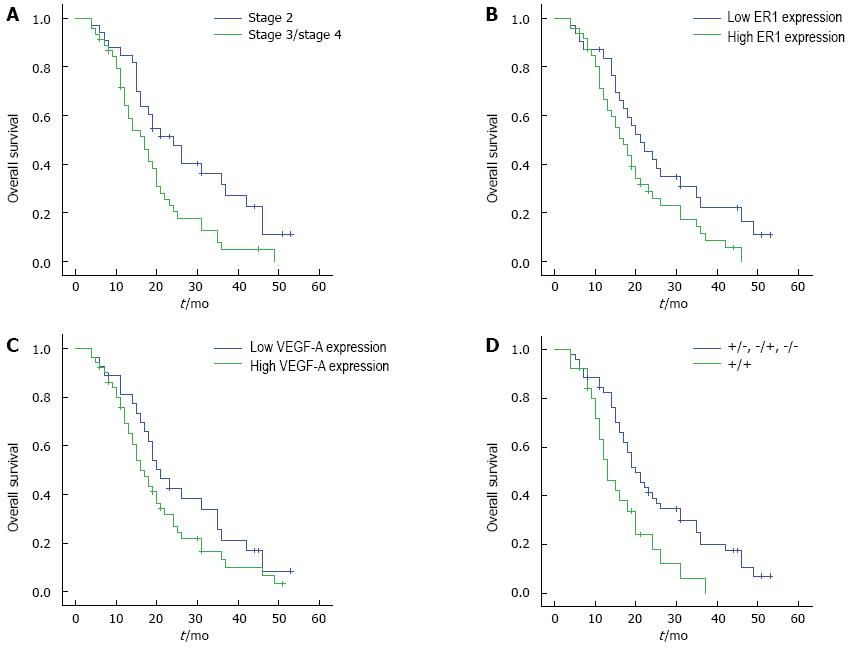
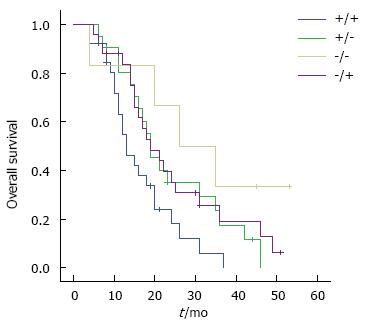
Univariate analysis (Table 3) revealed that age and TNM stage were significantly associated with GBC prognosis (P < 0.05). Patients with stage 2 GBC had a better survival than those with stages 3 and 4 disease (Figure 3A). Although there was no statistical difference between ER1 or VEGF-A expression status and GBC prognosis (Figure 3B and 3C, P > 0.05), combined expression of ER1 and VEGF-A was correlated with postoperative survival of GBC patients (Figures 3D and 4, P < 0.05). GBC patients with simultaneous high expression of ER1 and VEGF-A had a poorer prognosis. By multivariate analysis, TNM stage and combined ER1 and VEGF-A expression were identified as independent prognostic factors (P < 0.05) (Table 4). There was no statistical significance between ER1 and VEGF-A expression and GBC recurrence (P > 0.05).
| Risk factor | Survival time(month) (mean ± SE) | P-value (Log-rank test) |
| Gender | 0.682 | |
| Male | 23.24 ± 4.09 | |
| Female | 21.97 ± 1.72 | |
| Age (yr) | 0.015 | |
| ≤ 55 | 26.34 ± 2.71 | |
| > 55 | 18.77 ± 1.71 | |
| Gallstones | 0.068 | |
| Present | 17.69 ± 2.06 | |
| Absent | 23.96 ± 2.02 | |
| TNM stage | 0.007 | |
| II | 27.16 ± 2.77 | |
| III/IV | 18.59 ± 1.74 | |
| Differentiation | 0.685 | |
| Well | 23.19 ± 2.94 | |
| Moderate/poor | 21.65 ± 1.86 | |
| ER1 level | 0.053 | |
| High | 19.81 ± 1.79 | |
| Low | 25.85 ± 2.86 | |
| VEGF-A level | 0.155 | |
| High | 20.35 ± 1.87 | |
| Low | 25.65 ± 2.86 | |
| ER1 combined with VEGF-A | 0.007 | |
| +/+ | 16.30 ± 1.87 | |
| +/-, -/+, -/- | 24.97 ± 2.09 |
| Item | Hazard ratio | 95% CI | P-value |
| Age, yr | 0.076 | ||
| ≤ 55 vs > 55 | 0.615 | 0.359-1.053 | |
| TNM stage | 0.031 | ||
| III/IV vs II | 1.781 | 1.054-3.011 | |
| ER1 combined with VEGF-A | 0.042 | ||
| +/+ vs +/-, -/+, -/- | 1.773 | 1.021-3.080 |
The present study examined the expression of ER1 and VEGF-A in resected human GBC and CS tissues. The main findings are: (1) ER1 and VEGF-A expression were significantly higher in GBC than in CS tissues, ER1 expression was significantly associated with gender, and VEGF-A expression was associated with tumor differentiation; and (2) high expression of ER1 combined with VEGF-A in GBC predicted a poor prognosis. This is the first study to report prognostic significance of expression of ER1 combined with VEGF-A in GBC.
The poor prognosis of GBC has caused wide public attentions. Despite rapid improvement in medical technology over past decades, the survival time of GBC patients are far from satisfactory. Based on many clinical and molecular investigations about GBC, we speculated that the dismal prognosis of GBC patients may be attributed to the following aspects: (1) early diagnosis is difficult and most GBC cases are diagnosed at an advanced stage and have lost the best surgical chance; (2) GBC is relatively resistant to chemotherapy and radiation; apart from surgical resection, other effective measures are lacking; and (3) postoperative therapy for GBC patients should be selected according to patients’ prognosis. Despite a number of studies have been conducted about the molecular mechanisms of GBC, there have been no effective prognostic biomarkers for GBC to guide postoperative treatment. The present study exhibited that combined ER1 VEGF-A expression was associated with GBC prognosis, which would favor postoperative treatment.
ER1 as a promising prognostic factor has been investigated in several tumors. In ER-negative breast cancer, ER1 expression was necessary and sufficient in the bone marrow-derived cells themselves to promote tumor formation in response to estrogen[12]. In biliary tract cancers (including tumors of the gallbladder, bile duct and ampulla of Vater), the single nucleotide polymorphisms of the gene coding ER1 were correlated with risks of these tumors[26]. In our study, the results showed that ER1 expression was significantly higher in GBC compared with CS. This indicated that ER1 probably plays an important role in GBC, despite that the exact mechanisms are unclear at present. In addition, ER1 expression in GBC tissue exhibited a female predominance. It is well known that the overall level of estrogen in females is obviously higher than in males. It is likely that estrogen induces ER overexpression in females. Thus, our findings may partially explain why GBC is more frequent in females. Nevertheless, there was no significant correlation between ER1 expression and postoperative survival.
VEGF-A, a classic biological molecule in angiogenesis, has been investigated in various kinds of cancer. In human intra-hepatic cholangiocarcinoma, VEGF-A mediated the proliferative effect of estrogen to promote cholangiocarcinoma growth[16]. As to VEGF-A and GBC, there have been many reports[17,18,24,25]. Recently, a study revealed that VEGF-A was highly expressed in GBC and correlated with poor prognosis[18]. Additionally, another study showed that VEGF-A expression in GBC tissues is correlated with histologic differentiation and is an independent prognostic factor[24]. Our results were inconsistent with these previous investigations. Nevertheless, of note in our results, the high expression of ER1 combined with VEGF-A in GBC tissues predicted a poor prognosis. Based on this finding, we speculate that there were potentially synergistic effects between VEGF-A and ER1 in GBC progression. From the perspective of biological significance, this assumption is possible. Estrogen binding to ER can promote production of VEGF as mentioned before. Increasing VEGF can induce angiogenesis to provide plenty of oxygen and nutrients, and thus promote GBC growth, invasion and metastasis, finally leading to a poor survival. Of course, this assumption needs to be confirmed by further investigations.
Some limitations of this study should be taken into account. The sample size of this study was small. In addition, our study was not mechanistic, and there was very little information about molecular mechanisms.
In conclusion, our study suggested that expression of ER1 combined with VEGF-A confers a particularly poor postoperative survival outcome, and represents a potential prognostic biomarker for GBC. Clinical detection of ER1 and VEGF-A in surgically resected GBC tissues may provide a reference for decision-making of postoperative treatment programs. GBC patients having high expression of ER1 and VEGF-A deserve a close surveillance to reduce postoperative mortality.
Although ER1 and VEGF-A have been considered to be involved in progression of many kinds of tumors, their roles in GBC development have not been reported. Further investigations are required to explore the potential roles of ER1 and VEGF-A in GBC progression to clarify the molecular mechanism of GBC. In addition, ER1 and VEGF-A may represent potential therapeutic targets and adjuvant endocrine therapy may be new approaches for GBC.
Primary gallbladder carcinoma (GBC) is characterized by poor prognosis. In clinical practice, there has been no effective biomarker to predict the prognosis of GBC patients. Estrogen receptor 1 (ER1) and vascular endothelial growth factor A (VEGF-A) are involved in the progression of several kinds of malignancies. However, the prognostic significance of ER1 and VEGF-A in GBC is controversial, and needs further investigation.
According to epidemiology, the 5-year postoperative survival of GBC patients is less than 10%. Therefore, it is a current hotspot that exploring effective prognostic markers to guide postoperative treatment for GBC patients so as to improve survival after surgery.
Clinical detection of ER1 and VEGF-A expression can predict prognosis of GBC patients, and provides a reference for making-decision of postoperative treatment programs. In addition, the identification of ER1 and VEGF-A expression in human GBC tissues would help to investigate the molecular mechanisms of GBC.
ER1 also named estrogen receptor alpha, is a ligand-regulated transcription factor and mediates biological actions of estrogen. ER1 is implicated in several kinds of tumors. VEGF-A (vascular endothelial growth factor A) can promote physiological and pathological angiogenesis, and is believed to play an important role in various tumors.
The authors reported that expression of ER1 combined with VEGF-A assessed by immunohistochemistry was a potential prognostic marker for GBC patients after surgery. Their findings were useful for the postoperative clinical treatment of GBC patients.
P- Reviewer: Miyoshi E S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Lazcano-Ponce EC, Miquel JF, Muñoz N, Herrero R, Ferrecio C, Wistuba II, Alonso de Ruiz P, Aristi Urista G, Nervi F. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 558] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 2. | Boutros C, Gary M, Baldwin K, Somasundar P. Gallbladder cancer: past, present and an uncertain future. Surg Oncol. 2012;21:e183-e191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Alvaro D, Alpini G, Onori P, Perego L, Svegliata Baroni G, Franchitto A, Baiocchi L, Glaser SS, Le Sage G, Folli F. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology. 2000;119:1681-1691. [PubMed] |
| 4. | Alvaro D, Onori P, Metalli VD, Svegliati-Baroni G, Folli F, Franchitto A, Alpini G, Mancino MG, Attili AF, Gaudio E. Intracellular pathways mediating estrogen-induced cholangiocyte proliferation in the rat. Hepatology. 2002;36:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | DeMorrow S. Cholangiocarcinoma: estrogen-induced autocrine effects of VEGF on cell proliferation. Dig Liver Dis. 2009;41:164-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Sica V, Nola E, Contieri E, Bova R, Masucci MT, Medici N, Petrillo A, Weisz A, Molinari AM, Puca GA. Estradiol and progesterone receptors in malignant gastrointestinal tumors. Cancer Res. 1984;44:4670-4674. [PubMed] |
| 7. | Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 526] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 8. | Fisher RI, Neifeld JP, Lippman ME. Oestrogen receptors in human malignant melanoma. Lancet. 1976;2:337-339. [PubMed] |
| 9. | Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26:3727-3734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Park SK, Andreotti G, Sakoda LC, Gao YT, Rashid A, Chen J, Chen BE, Rosenberg PS, Shen MC, Wang BS. Variants in hormone-related genes and the risk of biliary tract cancers and stones: a population-based study in China. Carcinogenesis. 2009;30:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, Price RH, Pestell RG, Kushner PJ. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem. 2002;277:24353-24360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 333] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 12. | Iyer V, Klebba I, McCready J, Arendt LM, Betancur-Boissel M, Wu MF, Zhang X, Lewis MT, Kuperwasser C. Estrogen promotes ER-negative tumor growth and angiogenesis through mobilization of bone marrow-derived monocytes. Cancer Res. 2012;72:2705-2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Sumi K, Matsuyama S, Kitajima Y, Miyazaki K. Loss of estrogen receptor beta expression at cancer front correlates with tumor progression and poor prognosis of gallbladder cancer. Oncol Rep. 2004;12:979-984. [PubMed] |
| 14. | Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 570] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 15. | Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2348] [Cited by in RCA: 2485] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 16. | Mancino A, Mancino MG, Glaser SS, Alpini G, Bolognese A, Izzo L, Francis H, Onori P, Franchitto A, Ginanni-Corradini S. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis. 2009;41:156-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Mishra K, Behari A, Kapoor VK, Khan MS, Prakash S, Agrawal S. Vascular endothelial growth factor single-nucleotide polymorphism in gallbladder cancer. J Gastroenterol Hepatol. 2013;28:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Letelier P, Garcia P, Leal P, Ili C, Buchegger K, Riquelme I, Sandoval A, Tapia O, Roa JC. Immunohistochemical expression of vascular endothelial growth factor A in advanced gallbladder carcinoma. Appl Immunohistochem Mol Morphol. 2014;22:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Giatromanolaki A, Koukourakis MI, Simopoulos C, Polychronidis A, Sivridis E. Vascular endothelial growth factor (VEGF) expression in operable gallbladder carcinomas. Eur J Surg Oncol. 2003;29:879-883. [PubMed] |
| 20. | Buteau-Lozano H, Ancelin M, Lardeux B, Milanini J, Perrot-Applanat M. Transcriptional regulation of vascular endothelial growth factor by estradiol and tamoxifen in breast cancer cells: a complex interplay between estrogen receptors alpha and beta. Cancer Res. 2002;62:4977-4984. [PubMed] |
| 21. | Losordo DW, Isner JM. Estrogen and angiogenesis: A review. Arterioscler Thromb Vasc Biol. 2001;21:6-12. [PubMed] |
| 22. | Hyder SM, Huang JC, Nawaz Z, Boettger-Tong H, Mäkelä S, Chiappetta C, Stancel GM. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect. 2000;108 Suppl 5:785-790. [PubMed] |
| 23. | Buteau-Lozano H, Velasco G, Cristofari M, Balaguer P, Perrot-Applanat M. Xenoestrogens modulate vascular endothelial growth factor secretion in breast cancer cells through an estrogen receptor-dependent mechanism. J Endocrinol. 2008;196:399-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Sun XN, Cao WG, Wang X, Wang Q, Gu BX, Yang QC, Hu JB, Liu H, Zheng S. Prognostic impact of vascular endothelial growth factor-A expression in resected gallbladder carcinoma. Tumour Biol. 2011;32:1183-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Alvarez H, Corvalan A, Roa JC, Argani P, Murillo F, Edwards J, Beaty R, Feldmann G, Hong SM, Mullendore M. Serial analysis of gene expression identifies connective tissue growth factor expression as a prognostic biomarker in gallbladder cancer. Clin Cancer Res. 2008;14:2631-2638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Park SK, Andreotti G, Rashid A, Chen J, Rosenberg PS, Yu K, Olsen J, Gao YT, Deng J, Sakoda LC. Polymorphisms of estrogen receptors and risk of biliary tract cancers and gallstones: a population-based study in Shanghai, China. Carcinogenesis. 2010;31:842-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |