Published online Oct 14, 2015. doi: 10.3748/wjg.v21.i38.10898
Peer-review started: March 6, 2015
First decision: April 24, 2015
Revised: May 11, 2015
Accepted: August 31, 2015
Article in press: August 31, 2015
Published online: October 14, 2015
Processing time: 222 Days and 22.9 Hours
AIM: To investigate the prognostic value of preoperative lymphocyte-to-monocyte ratio (LMR) in patients with hepatocellular carcinoma (HCC) undergoing curative hepatectomy.
METHODS: Clinicopathological data of 210 hepatitis B virus (HBV)-associated HCC patients who were treated by radical hepatic resection between 2003 and 2010 were retrospectively analyzed. None of the patients received any preoperative anticancer therapy or intraoperative radiofrequency ablation. The diagnosis was confirmed by pathological examination after surgery. Absolute peripheral blood lymphocyte and monocyte counts were derived from serum complete blood cell count before surgery, and LMR was calculated by dividing lymphocyte count by monocyte count. The best cutoff was determined by receiver operating characteristics (ROC) curve analysis. Correlations between LMR levels and clinicopathological features were assessed using the χ2 test. Survival outcomes were estimated using the Kaplan-Meier method and compared by the log-rank test. Univariate and multivariate analyses were performed to evaluate the prognostic impact of LMR and other clinicopathological factors on overall survival (OS) and recurrence-free survival (RFS), using the Cox proportional hazards model.
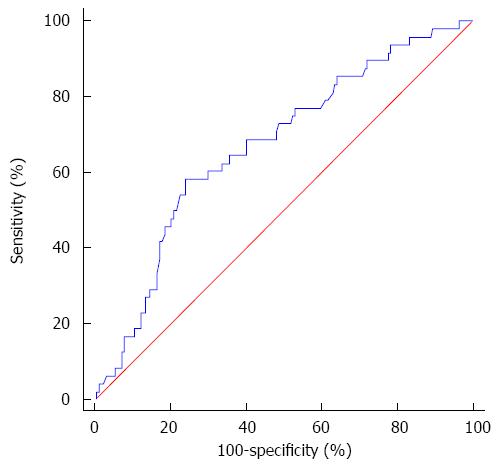
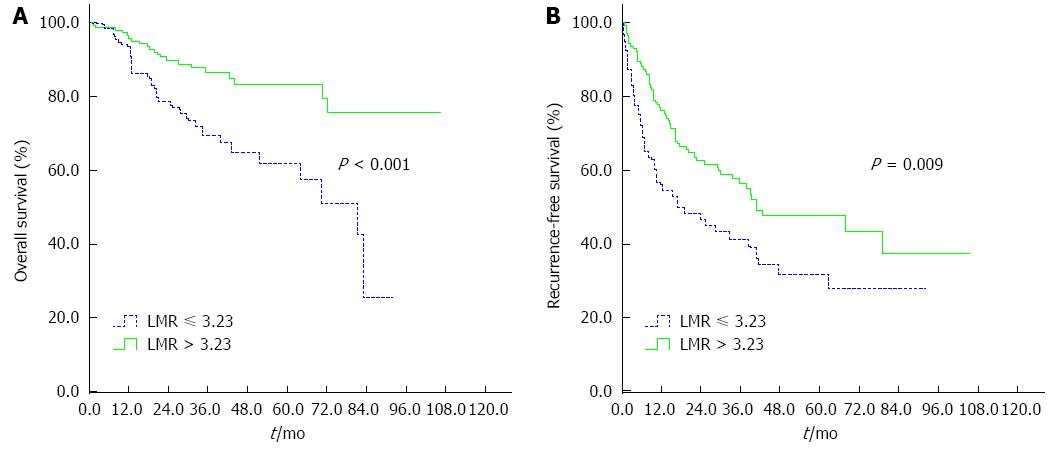
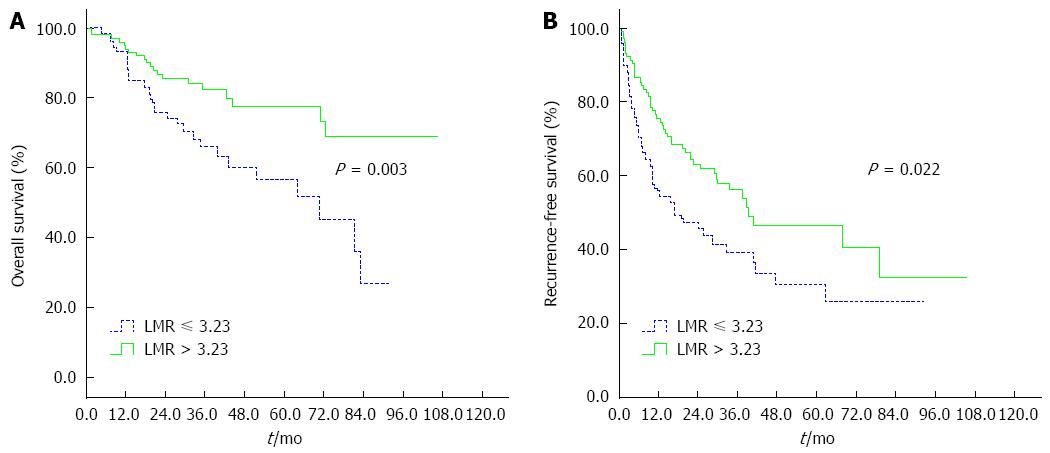
RESULTS: The optimal cutoff value of LMR for survival analysis was 3.23, which resulted in the most appropriate sensitivity of 55.3% and specificity of 74.7%, with the area under the curve (AUC) of 0.66 (95%CI: 0.593-0.725). All patients were dichotomized into either a low (≤ 3.23) LMR group (n = 66) or a high (> 3.23) LMR group (n = 144). A low preoperative LMR level was significantly correlated with the presence of cirrhosis, elevated levels of total bilirubin and larger tumor size. Patients with a low LMR level had significantly reduced 5-year OS (61.9% vs 83.2%, P < 0.001) and RFS (27.8% vs 47.6%, P = 0.009) compared to those with a high LMR level. Multivariate analyses indicated that a lower LMR level was a significantly independent predictor of inferior OS (P = 0.003) and RFS (P = 0.006). Subgroup analysis indicated that survival outcome was significantly more favorable in cirrhotic patients with LMR > 3.23. However, there were no differences between low and high LMR groups for OS and RFS in non-cirrhotic patients.
CONCLUSION: Preoperative LMR was demonstrated for the first time to serve as an independent prognostic factor in HBV-associated HCC patients after curative resection. Prospective studies with larger cohorts for validation are warranted.
Core tip: Inflammatory microenvironment plays an important role in the progression of hepatocellular carcinoma (HCC). Peripheral blood lymphocyte-to-monocyte ratio (LMR), a novel inflammatory biomarker that combines estimates of host immune homeostasis and tumor microenvironment, has been found to serve as a predictor of clinical outcomes in various malignancies. Prior to this study, there have been no reports regarding the prognostic value of LMR in HCC patients. For the first time in literature, our study identified the optimal cutoff value of LMR for survival analysis and concluded that preoperative LMR could serve as an independent prognostic factor in hepatitis B virus-associated HCC patients after curative resection.
- Citation: Lin ZX, Ruan DY, Li Y, Wu DH, Ma XK, Chen J, Chen ZH, Li X, Wang TT, Lin Q, Wen JY, Wu XY. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World J Gastroenterol 2015; 21(38): 10898-10906
- URL: https://www.wjgnet.com/1007-9327/full/v21/i38/10898.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i38.10898
Hepatocellular carcinoma (HCC) is one of the leading types of malignant tumors worldwide, which primarily develops in the setting of chronic liver inflammation[1]. In China, nearly 90% of HCC patients have underlying hepatitis B virus (HBV) infection. Hepatic resection has been established as a curative treatment for patients who have localized lesions arising in non-cirrhotic livers, or in cirrhotic livers with well-preserved hepatic function[2]. However, the long-term survival after resection remains dismal due to a high frequency of tumor recurrence[3-5]. Clinicopathological factors, such as microvascular invasion, multifocal disease, tumor size and degree of histologic differentiation, have been used to predict survival in patients with HCC after curative resection[3-6]. However, these clinical tumor parameters can only partially explain the prognostic heterogeneity of HCC.
Cumulative evidence has demonstrated that crosstalk between tumor cells and their surrounding inflammatory microenvironment plays a critical role in the initiation and progression of HCC. Inflammatory infiltrates in the tumor microenvironment can largely influence the biological behavior of HCC[7-12]. Tumor-associated macrophages (TAMs), which comprise a major proportion of leukocytes that infiltrate into the stroma, have been found to promote HCC proliferation, angiogenesis and metastasis[7,11-14]. Immunohistochemical studies have validated the association between high TAM density and unfavorable prognosis in HCC patients after curative resection[15,16]. Peripheral blood monocytes, which are precursors of TAMs[7], have also been reported to be a prognostic factor for HCC[17,18]. Tumor-infiltrating lymphocytes (TILs) are another representative component of the immune microenvironment. Specific TIL subtypes are involved in the clinical course of HCC, and TIL phenotypes are informative regarding prognosis[8-10,13].
Recently, the peripheral blood lymphocyte-to-monocyte ratio (LMR), as a simple surrogate biomarker of TILs and TAMs, has been reported to be a predictor of clinical outcomes in various malignancies[19-25]. LMR also acts as a representative biomarker by combining estimates of host immune homeostasis (i.e., absolute lymphocyte count) and tumor microenvironment (i.e., absolute monocyte count)[19,20]. To date, there have been no reports regarding the prognostic value of LMR in HCC patients. We therefore conducted this study to investigate the impact of preoperative peripheral blood LMR on long-term outcomes after curative hepatic resection for HCC.
From January 2003 to December 2010, 210 patients with HBV-associated HCC who underwent curative hepatectomy at the Third Affiliated Hospital of Sun Yat-sen University were eligible for this retrospective study. All the patients had chronic HBV infection and were negative for hepatitis C virus antibody. Preoperative diagnosis of HCC was based on typical dynamic images evaluated by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) according to the Asian Pacific Association for the Study of the Liver (APASL) guideline[26]. Pathological examination confirmed the diagnosis after surgical resection. Curative resection was defined as the complete resection of all tumor nodules with clear microscopic margins and no residual tumors as indicated by CT scan at one month after surgery. Neither preoperative anticancer therapy nor intraoperative radiofrequency ablation was performed on the patients. Antiviral therapy with oral nucleos(t)ide analogues was recommended for all the patients after liver resection.
For each patient in the group, demographic information, complete blood cell count, liver function parameters, serum alpha-fetoprotein (AFP) level, Barcelona Clinic Liver Cancer (BCLC) stage, and other tumor-related parameters were recorded. Tumor-related variables, such as maximal tumor diameter, number of tumor nodules, portal vein thrombus and histological differentiation, were obtained from pathology reports. The absolute peripheral blood lymphocyte and monocyte counts were derived from the complete blood cell count before surgery, with LMR calculated by dividing lymphocyte count by monocyte count. None of the patients exhibited clinical manifestations of acute inflammation before treatment or of coexistent hematologic disorders. The study protocol was approved by the Clinical Ethics Review Board of the Third Affiliated Hospital of Sun Yat-sen University. Informed consent was obtained according to the Declaration of Helsinki.
All patients were regularly followed for recurrence at outpatient clinics. None of the patients died within 30 d after surgery. Serum AFP test and abdominal CT scan were performed every 3 mo during the first two postoperative years and every 6 mo thereafter. If clinical recurrence was suspected, CT was performed immediately. Additional diagnostic investigation such as MRI or hepatic arterial angiography was performed in patients with suspicious lesions demonstrated by CT image. Patients with confirmed recurrence received further treatment, such as second hepatectomy, chemoembolization, radiofrequency ablation or percutaneous ethanol injection. Treatment modality after relapse varied among individuals.
Receiver operating curve (ROC) analysis was performed to determine the optimal cutoff values for preoperative absolute lymphocyte count (ALC), absolute monocyte count (AMC) and LMR as prognostic factors. The score closest to the point with both maximum sensitivity and specificity was chosen as the best cutoff value. Correlations between LMR levels and clinicopathological features were assessed using the χ2 test. Survival outcomes were estimated using the Kaplan-Meier method and compared by the log-rank test. The primary endpoint of the present study was overall survival (OS), which was calculated from the time of surgery to the date of death from any cause, or to the date of the last follow-up. The secondary endpoint was recurrence-free survival (RFS), which was defined as the duration from the date of surgery to the date of HCC recurrence, or to the date of the last follow-up. The prognostic values of ALC, AMC, LMR and other clinicopathological factors were analyzed using the Cox proportional hazards model. Significant variables identified in univariate analysis were included in the multivariate model. A P-value < 0.05 was regarded as statistically significant. All statistical analyses were performed using SPSS software (version 17.0, SPSS Inc, Chicago, IL, United States) and MedCalc statistical software (version 11.4.2.0, Broekstraat 52 Mariakerke, Belgium).
All the patients had chronic HBV infection and 161 (76.7%) patients had a histological diagnosis of cirrhosis. The median duration of follow-up was 34.8 mo (range: 1.7-106.6 mo). By the last follow-up, 110 (52.4%) patients developed tumor recurrence, 47 (22.9%) died from causes secondary to HCC progression, and one died from cerebrovascular disease. The 1-, 3-, and 5-year OS rates for all the patients in this study were 95.7%, 80.9% and 75.6%, respectively, and the 1-, 3-, and 5-year RFS rates were 69.9%, 51.7% and 42.3%, respectively.
The best cutoff points of LMR, ALC and AMC for survival outcomes were determined by ROC curve analyses, which indicated that the optimal LMR cutoff value for both OS and RFS was 3.23. The LMR cutoff point of 3.23 for OS was selected as the uniform point in survival analyses (Figure 1). The area under the curve (AUC) was recorded as 0.66 (95%CI: 0.593-0.725). Using the LMR value of 3.23 resulted in the most appropriate measures of sensitivity and specificity, which were 55.3% and 74.7%, respectively. Similarly, the most discriminative cutoff values of ALC and AMC were determined to be 1.66 × 109/L (AUC: 0.58, 95%CI: 0.511-0.648) and 0.29 × 109/L (AUC: 0.61, 95%CI: 0.542-0.678), respectively.
Based on the cutoff value, all patients were dichotomized into either a low value group or a high value group. The relationship between preoperative peripheral LMR levels and clinicopathological characteristics was summarized in Table 1. Sixty-six patients had an LMR ≤ 3.23 and one hundred and forty-four patients had an LMR > 3.23. A low LMR level was significantly correlated with ALC ≤ 1.66 (P < 0.001) and AMC > 0.29 (P < 0.001). Patients with LMR ≤ 3.23 were also prone to have liver cirrhosis (P = 0.003), elevated levels of total bilirubin (P = 0.002) and larger tumor size (P = 0.030).
| Variable | No. of patients | LMR | P value | |
| ≤ 3.23 (n = 66) | > 3.23 (n = 144) | |||
| Age (yr) | ||||
| < 60 | 165 | 52 | 113 | 0.959 |
| ≥ 60 | 45 | 14 | 31 | |
| Gender | ||||
| Female | 25 | 6 | 19 | 0.394 |
| Male | 185 | 60 | 125 | |
| Liver cirrhosis | ||||
| Absent | 49 | 7 | 42 | 0.003 |
| Present | 161 | 59 | 102 | |
| ALT (U/L) | ||||
| ≤ 75 | 172 | 51 | 121 | 0.238 |
| > 75 | 38 | 15 | 23 | |
| Total bilirubin (μmol/L) | ||||
| ≤ 34 | 197 | 57 | 140 | 0.002 |
| > 34 | 13 | 9 | 4 | |
| Albumin (g/L) | ||||
| < 35 | 15 | 7 | 8 | 0.303 |
| ≥ 35 | 195 | 59 | 136 | |
| ALP (U/L) | ||||
| ≤ 100 | 171 | 51 | 120 | 0.294 |
| > 100 | 39 | 15 | 24 | |
| AFP (ng/dL) | ||||
| ≤ 400 | 124 | 38 | 86 | 0.769 |
| > 400 | 86 | 28 | 58 | |
| Tumor size (cm) | ||||
| ≤ 5 | 157 | 43 | 114 | 0.030 |
| > 5 | 53 | 23 | 30 | |
| Tumor number | ||||
| Single | 184 | 59 | 125 | 0.597 |
| Multiple | 26 | 7 | 19 | |
| Portal vein thrombus | ||||
| Absent | 196 | 61 | 135 | 0.952 |
| Present | 14 | 5 | 9 | |
| Microvascular invasion | ||||
| Absent | 170 | 55 | 115 | 0.552 |
| Present | 40 | 11 | 29 | |
| Histological differentiation | ||||
| Poor | 22 | 8 | 14 | 0.598 |
| Well and Moderate | 188 | 58 | 130 | |
| ALC (× 109/L) | ||||
| ≤ 1.66 | 117 | 50 | 67 | < 0.001 |
| > 1.66 | 93 | 16 | 77 | |
| AMC (× 109/L) | ||||
| ≤ 0.29 | 57 | 3 | 54 | < 0.001 |
| > 0.29 | 153 | 63 | 90 | |
To identify the optimal peripheral blood immunological biomarker for patient prognosis, the impact of ALC, AMC and LMR on survival outcomes was investigated. In univariate analysis for primary endpoint of OS, ALC and AMC were shown to be significant prognostic factors, with a P-value of 0.035 for ALC (HR = 0.511, 95%CI: 0.274-0.953) and a P-value of 0.026 for AMC (HR = 2.644, 95%CI: 1.123-6.223). The association between LMR and OS was also proven to be statistically significant, with a P-value < 0.001 (HR = 0.352, 95%CI: 0.199-0.623), indicating that LMR might provide the strongest prognostic information among these three biomarkers (Table 2). With respect to RFS, significant differences were also observed between low and high LMR groups (P = 0.009, HR = 0.601, 95%CI: 0.410-0.883) (Table 3). Other significant predictors of poorer OS and RFS included a low level of serum albumin, large tumor size, the presence of portal vein thrombus, poor histological differentiation, and an advanced BCLC stage. Moreover, liver cirrhosis, an elevated level of serum alkaline phosphatase (ALP) and microvascular invasion were all associated with a shorter OS, whereas an elevated serum alanine aminotransferase (ALT) level was correlated with inferior RFS.
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr), ≥ 60 vs < 60 | 0.766 (0.410-1.433) | 0.404 | ||
| Gender, male vs female | 0.829 (0.296-2.321) | 0.721 | ||
| Liver cirrhosis, yes vs no | 7.641 (1.853-31.509) | 0.005 | 7.084 (1.694-29.614) | 0.007 |
| ALT (U/L), > 75 vs ≤ 75 | 1.513 (0.771-2.970) | 0.229 | ||
| Total bilirubin (μmol/L), > 34 vs ≤ 34 | 2.085 (0.822-5.288) | 0.122 | ||
| Albumin (g/L), ≥ 35 vs < 35 | 0.242 (0.112-0.522) | < 0.001 | ||
| ALP (U/L), > 100 vs ≤ 100 | 2.116 (1.148-3.899) | 0.016 | 2.137 (1.153-3.964) | 0.016 |
| AFP (ng/dL), > 400 vs ≤ 400 | 0.956 (0.535-1.705) | 0.878 | ||
| Tumor size (cm), > 5 vs ≤ 5 | 2.154 (1.204-3.853) | 0.010 | ||
| Tumor number, multiple vs single | 1.048 (0.444-2.477) | 0.915 | ||
| Portal vein thrombus: yes vs no | 3.348 (1.492-7.512) | 0.003 | ||
| Microvascular invasion: yes vs no | 2.121 (1.151-3.911) | 0.016 | 2.307 (1.217- 4.370) | 0.010 |
| Histological differentiation, poor vs well and moderate | 2.888 (1.467-5.684) | 0.002 | 2.375 (1.195-4.721) | 0.014 |
| BCLC stage, B + C vs 0 + A | 2.110 (1.197-3.720) | 0.010 | 2.155 (1.213-3.831) | 0.009 |
| Preoperative LMR, > 3.23 vs ≤ 3.23 | 0.352 (0.199-0.623) | < 0.001 | 0.398 (0.219-0.725) | 0.003 |
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age(yr), ≥ 60 vs < 60 | 1.319 (0.859-2.027) | 0.206 | ||
| Gender, male vs female | 0.855 (0.458-1.594) | 0.621 | ||
| Liver cirrhosis: yes vs no | 1.316 (0.831-2.086) | 0.242 | ||
| ALT (U/L), > 75 vs ≤ 75 | 1.709 (1.096-2.665) | 0.018 | 1.510 (0.960-2.375) | 0.074 |
| Total bilirubin (μmol/L), > 34 vs ≤ 34 | 1.471 (0.715-3.023) | 0.294 | ||
| Albumin (g/L), ≥ 35 vs < 35 | 0.279 (0.160-0.485) | < 0.001 | ||
| ALP (U/L), > 100 vs ≤ 100 | 1.506 (0.964-2.354) | 0.072 | ||
| AFP (ng/dL), > 400 vs ≤ 400 | 0.934 (0.636-1.373) | 0.730 | ||
| Tumor size (cm), > 5 vs ≤ 5 | 2.020 (1.354-3.012) | 0.001 | ||
| Tumor number, multiple vs single | 1.599 (0.953-2.684) | 0.075 | ||
| Portal vein thrombus, yes vs no | 2.282 (1.150-4.529) | 0.018 | ||
| Microvascular invasion, yes vs no | 1.185 (0.742-1.892) | 0.478 | ||
| Histological differentiation, poor vs well and moderate | 2.628 (1.561-4.425) | < 0.001 | 2.610 (1.542-4.416) | < 0.001 |
| BCLC stage, B + C vs 0 + A | 1.724 (1.180-2.520) | 0.005 | 1.645 (1.124-2.409) | 0.010 |
| Preoperative LMR, > 3.23 vs ≤ 3.23 | 0.601 (0.410-0.883) | 0.009 | 0.584 (0.398-0.859) | 0.006 |
Variables showing statistical significance by univariate analysis were included in the multivariate Cox proportional hazard analysis (Tables 2 and 3). As tumor size, portal vein thrombus and serum albumin level were all associated with BCLC stage, we did not enter these variables into further multivariate models so as to avoid potential bias. The results revealed that a high preoperative LMR level was an independent predictor of favorable prognostic measures, including OS (HR = 0.398; 95%CI: 0.219-0.725, P = 0.003) and RFS (HR = 0.584; 95%CI: 0.398-0.859; P = 0.006). Among the remaining factors studied, poor histological differentiation and an advanced BCLC stage were identified as independent indicators for inferior RFS and OS. In addition, cirrhotic liver parenchyma, an elevated serum ALP level and microvascular invasion were independent factors for OS.
Kaplan-Meier curve analysis revealed that a low LMR level was significantly associated with decreased OS and DFS. The 5-year OS and RFS rates were 61.9% and 27.8%, respectively, for patients with a preoperative LMR ≤ 3.23 and were statistically lower than those for patients with a LMR > 3.23 (83.2% and 47.6%, respectively; P < 0.001 and P = 0.009, respectively; Figure 2). Subgroup analysis was performed according to underlying cirrhosis status (cirrhosis, n = 161; non-cirrhosis, n = 49). In cirrhotic patients with HCC, a low preoperative LMR level was associated with inferior OS and RFS (P = 0.003 and P = 0.022, respectively; Figure 3). However, there were no differences between low and high LMR levels for OS and RFS in non-cirrhotic patients (P = 0.443 and P = 0.492, respectively).
Accumulating studies have suggested that the infiltrating inflammatory microenvironment may represent an important determinant for the clinical outcome of HCC[7-12]. The imbalance of inflammatory immune cells, such as TAMs and TILs, in the tumor microenvironment, has been proven to be an important regulator of progression in HCC[11-16]. Systemic inflammatory response can be routinely determined by traditional hematological markers, such as C-reactive protein and neutrophil-to-lymphocyte ratio, which are considered to be valuable prognostic factors in patients with HCC[27-30]. Peripheral blood LMR, as a novel inflammatory biomarker, has been recently investigated and confirmed to be a predictor of clinical outcomes in lymphoma[19,20], colon cancer[21], non-small cell lung cancer[23], nasopharyngeal carcinoma[22], breast cancer[24] and gastric cancer[25].
To the best of our knowledge, this is the first study to investigate the preoperative LMR as a prognostic marker in HCC patients initially treated by curative hepatectomy. Only HBV-related HCC was included to avoid potential confounding factors from different etiologies. An objective and reliable cutoff point for LMR was generated by employing ROC curve analysis. Univariate analysis revealed that patients with an LMR > 3.23 had significantly better OS and RFS than those with an LMR ≤ 3.23. On multivariate analysis, LMR remained an independent prognostic marker for OS and RFS throughout the cohort. These results were consistent with previous findings on other types of tumors, in which a low pretreatment level of LMR was reported as an independent unfavorable prognostic factor[19-25]. However, the cutoff values were cancer-specific in the above studies, possibly reflecting the biologic differences among these studied malignancies.
The association between decreased LMR and poor oncologic outcome is complex and remains to be elucidated. There are several possible reasons accounting for this positive correlation. First, lymphocytes are the basic components of host antitumor immunity, which are important in the destruction of residual cancer cells and related micrometastases[20-22]. They infiltrate into tumor microenvironment and manifest as TILs, both the quantity and the phenotype of which may influence the effectiveness of antitumor immune reaction[8-10]. Unitt et al[8] found that reduced lymphocyte infiltration and a low CD4+/CD8+ T cell ratio were both significant independent predictors of HCC recurrence following liver transplantation. Two additional studies demonstrated that low intratumoral cytotoxic CD8+ T and high intratumoral regulatory T cells were associated with a poorer prognosis in HCC patients after resection[9,10]. In general, peripheral blood lymphocyte count serves as a simple surrogate marker of the host immune status. In our study, an association between a low level of ALC and adverse OS was identified by univariate analysis. We also revealed that patients with a decreased LMR had relative lymphocytopenia, which might be responsible for an incompetent immune response against tumor[20-22].
Second, myeloid-lineage cells, including monocytes and their progeny, are known to have immune suppressive activity[31]. They can also promote tumor angiogenesis, tumor-cell invasion and metastasis[21,31]. Circulating monocytes are recruited to the tumor stroma and differentiate into TAMs. As a major component of tumor microenvironment in HCC, TAMs can interact with cancer cells to enhance tumor progression by producing various cytokines and chemokines[11-15]. Poor clinical outcomes associated with high infiltrations of TAMs have been indicated by Zhu et al[15] and Kong et al[16]. Peripheral blood monocytes may reflect the formation or existence of TAMs[23]. The pro-tumorigenic effect of monocytes on HCC has been associated with poor prognosis, as demonstrated by Sasaki et al[17] and Shen et al[18] and validated in the current study, which showed that monocytosis was associated with poor OS in patients with HCC after resection.
These data indicate that LMR might act as the surrogate marker which reflects the interaction between host immunity (i.e., ALC) and tumor microenvironment (i.e., AMC). The presence of preoperative lymphopenia and monocytosis both served as predictors of inferior OS in our study. However, as the combination of ALC and AMC, LMR provided a better prognostic value. A decreased LMR reflects an inflammatory status that favors tumor progression and impairs host immune surveillance, both of which are associated with poor oncologic outcome. Pretreatment LMR level was also inversely correlated with the presence of liver cirrhosis, and the poor outcome predicted by low LMR level was shown only in cirrhotic patients, not in non-cirrhotic ones. These results indicate that the association between cirrhosis and LMR may be an important mechanism for HCC progression.
LMR is a simple and easily assessable clinical biomarker for prognostic stratification of HBV-associated HCC patients after hepatectomy. However, findings of the current study should be interpreted within its possible limitations. First, formal investigations on the specific components of tumor microenvironment in this population were not performed. Second, due to the retrospective design of the study, selection bias was inevitable, which might have influenced the survival analysis. Third, as the study cohort was comprised of a small single-center sample, we were unable to divide the data set into a training set and a testing set for statistical validation.
In conclusion, our study is the first to demonstrate that preoperative LMR can serve as an independent prognostic factor for patients with HBV-associated HCC undergoing curative resection. As a simple and cost-effective biomarker, LMR could be used to identify HCC patients with a poorer survival, especially those with cirrhotic livers, which may guide postoperative treatment. Future biological studies should further correlate LMR with the tumor microenvironment. Prospective studies with larger cohorts are awaited to validate the clinical usage of LMR as a prognostic marker for HCC patients.
The authors thank the surgeons and nurses in the Department of Liver Surgery, The Third Affiliated Hospital of Sun Yat-sen University for their help in treating the patients in this study.
Cumulative evidence has suggested that the inflammatory microenvironment may represent an important determinant for the clinical outcome of hepatocellular carcinoma (HCC). Peripheral blood lymphocyte-to-monocyte ratio (LMR), which is a novel inflammatory biomarker combining estimates of host immune homeostasis and tumor microenvironment, has been demonstrated to serve as a predictor of clinical outcomes in various types of malignancies. However, the prognostic value of LMR in patients with HCC remains unknown.
The prognostic value of LMR has been widely investigated in hematological malignancies such as diffuse large B-cell lymphoma and Hodgkin’s lymphoma. However, data regarding the prognostic value of LMR in patients with solid tumors are spare. Recent published studies have shown that preoperative high level of LMR was a favorable prognostic factor in patients with operable lung cancer and colon cancer. Prior to this study, there have been no reports regarding the prognostic value of LMR in patients with HCC until now.
To date, this is the first study to investigate the preoperative LMR as a prognostic biomarker in HCC patients after curative resection. To avoid any potential confounding factors from different etiologies, the authors included only hepatitis B virus-associated HCC patients. They also calculated the optimal LMR cutoff for survival prediction. The results identified that a low LMR level (≤ 3.23) was a significantly independent predictor of inferior survival in HCC patients who were initially treated by curative hepatectomy, suggesting that preoperative LMR represents a promising prognostic marker for HCC.
The study indicated that a low preoperative LMR level was an independent unfavorable prognostic factor for HCC patients who underwent curative hepatectomy. As a simple and cost-effective biomarker, LMR can be used to identify HCC patients with a poorer survival, especially those with cirrhotic livers, which may guide postoperative treatment.
LMR is calculated by dividing the lymphocyte count by the monocyte count in peripheral blood.
This is an interesting study with sound methodology and statistical analyses, in which the authors investigated the prognostic value of preoperative LMR in HCC patients undergoing curative hepatectomy. The results suggest that a low preoperative LMR level was an independent unfavorable prognostic factor.
P- Reviewer: Montasser Iman F, Peng BG, Sturesson C S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S
| 1. | Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 2. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 3. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1234] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 4. | Gluer AM, Cocco N, Laurence JM, Johnston ES, Hollands MJ, Pleass HC, Richardson AJ, Lam VW. Systematic review of actual 10-year survival following resection for hepatocellular carcinoma. HPB (Oxford). 2012;14:285-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, Langer B, Grant DR, Greig PD, Gallinger S. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 340] [Article Influence: 17.9] [Reference Citation Analysis (1)] |
| 6. | Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 779] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 8. | Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler SL, Morris LS, Coleman N, Alexander GJ. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006;45:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 891] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 10. | Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, Zheng SS. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol. 2012;29:1817-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Peng SH, Deng H, Yang JF, Xie PP, Li C, Li H, Feng DY. Significance and relationship between infiltrating inflammatory cell and tumor angiogenesis in hepatocellular carcinoma tissues. World J Gastroenterol. 2005;11:6521-6524. [PubMed] |
| 12. | Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067-8075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 304] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 13. | Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Mano Y, Aishima S, Fujita N, Tanaka Y, Kubo Y, Motomura T, Taketomi A, Shirabe K, Maehara Y, Oda Y. Tumor-associated macrophage promotes tumor progression via STAT3 signaling in hepatocellular carcinoma. Pathobiology. 2013;80:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 461] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 16. | Kong LQ, Zhu XD, Xu HX, Zhang JB, Lu L, Wang WQ, Zhang QB, Wu WZ, Wang L, Fan J. The clinical significance of the CD163+ and CD68+ macrophages in patients with hepatocellular carcinoma. PLoS One. 2013;8:e59771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery. 2006;139:755-764. [PubMed] |
| 18. | Shen SL, Fu SJ, Huang XQ, Chen B, Kuang M, Li SQ, Hua YP, Liang LJ, Peng BG. Elevated preoperative peripheral blood monocyte count predicts poor prognosis for hepatocellular carcinoma after curative resection. BMC Cancer. 2014;14:744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Porrata LF, Ristow K, Colgan JP, Habermann TM, Witzig TE, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Nowakowski GS. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin’s lymphoma. Haematologica. 2012;97:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 20. | Li ZM, Huang JJ, Xia Y, Sun J, Huang Y, Wang Y, Zhu YJ, Li YJ, Zhao W, Wei WX. Blood lymphocyte-to-monocyte ratio identifies high-risk patients in diffuse large B-cell lymphoma treated with R-CHOP. PLoS One. 2012;7:e41658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Stotz M, Pichler M, Absenger G, Szkandera J, Arminger F, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110:435-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 259] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 22. | Li J, Jiang R, Liu WS, Liu Q, Xu M, Feng QS, Chen LZ, Bei JX, Chen MY, Zeng YX. A large cohort study reveals the association of elevated peripheral blood lymphocyte-to-monocyte ratio with favorable prognosis in nasopharyngeal carcinoma. PLoS One. 2013;8:e83069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation-based lymphocyte- monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One. 2014;9:e108062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Ni XJ, Zhang XL, Ou-Yang QW, Qian GW, Wang L, Chen S, Jiang YZ, Zuo WJ, Wu J, Hu X. An elevated peripheral blood lymphocyte-to-monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS One. 2014;9:e111886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Zhou X, Du Y, Xu J, Huang Z, Qiu T, Wang X, Qian J, Zhu W, Liu P. The preoperative lymphocyte to monocyte ratio predicts clinical outcomes in patients with stage II/III gastric cancer. Tumour Biol. 2014;35:11659-11666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 841] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 27. | Oh BS, Jang JW, Kwon JH, You CR, Chung KW, Kay CS, Jung HS, Lee S. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer. 2013;13:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, Aishima S, Ikegami T, Yoshizumi T, Yamanaka T. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 29. | Fu SJ, Shen SL, Li SQ, Hua YP, Hu WJ, Liang LJ, Peng BG. Prognostic value of preoperative peripheral neutrophil-to-lymphocyte ratio in patients with HBV-associated hepatocellular carcinoma after radical hepatectomy. Med Oncol. 2013;30:721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2285] [Cited by in RCA: 2233] [Article Influence: 203.0] [Reference Citation Analysis (0)] |
| 31. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5480] [Cited by in RCA: 5329] [Article Influence: 333.1] [Reference Citation Analysis (0)] |