Published online Oct 7, 2015. doi: 10.3748/wjg.v21.i37.10683
Peer-review started: May 11, 2015
First decision: June 19, 2015
Revised: July 1, 2015
Accepted: July 18, 2015
Article in press: July 18, 2015
Published online: October 7, 2015
Processing time: 146 Days and 6.8 Hours
AIM: To evaluate a new imaging device for colonoscopy that adds two side viewing CMOS lenses, the Third Eye® Panoramic™ cap.
METHODS: In this prospective observational feasibility study, 33 patients, 18 male and 15 female, underwent routine screening, surveillance or diagnostic colonoscopy with the new Third Eye® Panoramic™ cap clipped on to the distal tip of a high definition Fuji EC530-LS Slim Colonoscope®. All procedures were performed at the New York Presbyterian-Queens Endoscopy unit by two experienced endoscopists (Rubin M and Kim SH). Main outcome measurements included evaluation of the image quality of the Third Eye® Panoramic™ cap, adenoma detection rate, cecal intubation rate, withdrawal time and total procedure time.
RESULTS: The Third Eye® Panoramic™ cap enabled enhanced views without affecting the quality of the colonoscope’s image or its handling characteristics through the colon. Ileal intubation was accomplished in most cases, but was more challenging. The side view lenses detected polyps and diverticula hidden behind folds and in flexures not seen on the standard view. The side view lenses were easily cleaned utilizing an Endogator® Irrigation Pump (Medivators, Minneapolis, MN, United States) by angling the scope tip against the mucosa while washing. The cecum was reached in all 33 patients. Mean cecal intubation time was 8.19 ± 2.17 min, mean withdrawal time was 10.15 ± 5.56 min and mean total procedure time was 20.31 ± 5.14 min. The overall adenoma detection rate was 44%.
CONCLUSION: The Third Eye® Panoramic™ cap enables wide view colonoscopy with enhanced visualization utilizing standard forward view colonoscopes.
Core tip: In this study, we present our experience with a brand-new endoscopic device the Third Eye® Panoramic™ Cap (Avantis Medical). The cap clips onto the distal tip of a standard colonoscope and contains side viewing CMOS cameras illuminated with LED lights increasing the viewing angle to 300°. This enables visualization of the colonic mucosa behind folds and in flexures. This preliminary study presents data on the successful implementation and deployment of the cap in routine colonoscopic examinations.
- Citation: Rubin M, Lurie L, Bose K, Kim SH. Expanding the view of a standard colonoscope with the Third Eye® Panoramic™ cap. World J Gastroenterol 2015; 21(37): 10683-10687
- URL: https://www.wjgnet.com/1007-9327/full/v21/i37/10683.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i37.10683
Colorectal cancer (CRC) is the third most common cancer in males and the second most common cancer in females worldwide[1] In the United States, approximately 132700 new cases are diagnosed and 49700 deaths occur annually[2]. From 2007 to 2011, CRC cases in the United States decreased 3.6% per year and CRC deaths decreased 2.8%, mostly due to increased use of screening colonoscopy and removal of precancerous adenomas[2,3].
Recent studies have shown a direct correlation between a high adenoma detection rate (ADR) and a decreased risk of interval colon cancer, defined as colorectal cancer diagnosed after a screening or surveillance exam in which no cancer is detected, and before the date of the next recommended exam[4]. One large study showed that each 1% increase in ADR was associated with a 3% decrease in the risk of cancer[5].
Although colonoscopy remains the gold standard, tandem studies have shown miss rates of adenomas ranging from 22%-41%[5-8], and even large adenomas (measuring at least 1 cm) have a miss rate of about 12%[9-11]. Methods to improve the ADR include timed withdrawal, better preparation and the use of new devices to view lesions hidden behind folds, where 2/3 of missed adenomas are located[10].
The Third Eye® Retroscope® (TER, Avantis Medical Systems Inc., Sunnyvale, CA, United States), was the first of these technologies and was shown to increase ADR by 23.2% by providing a continuous 180° retrograde view that complemented the forward view of a standard colonoscope to allow examination of areas behind folds and flexures[5]. However, the TER was limited by its deployment through the working channel of the scope[7].
The recently introduced Fuse® Full Spectrum Endoscopy® system (EndoChoice Inc., Alpharetta, GA, United States) is a new endoscopic platform which is equipped with side-viewing cameras with LED illumination. The resulting colonoscopic image expands the view from 170° to 330°. In a tandem study comparing standard forward-viewing colonoscopy to Fuse, the adenoma miss rate was significantly lower with the Fuse system[7,8]. However, migrating to this platform requires a significant capital expenditure as well as an adjustment to different scope dynamics and optical resolution.
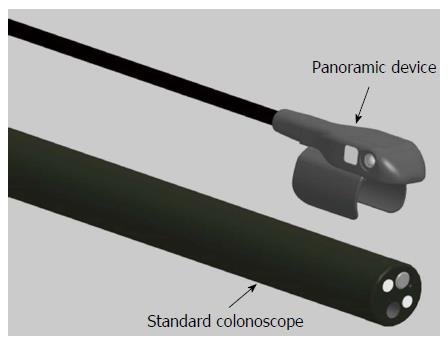
The Third Eye® Panoramic™ device (TEP, Avantis Medical Systems) is a novel plastic cap containing 2 side-viewing CMOS chips with adjacent LEDs that can be attached to the tip of any standard colonoscope. In this preliminary study, we report on the successful use of the TEP in patients undergoing colonoscopy.
Patients who were scheduled for elective outpatient screening, surveillance or diagnostic colonoscopy were asked to participate in the study. Patients were excluded if they were suspected to have a chronic stricture, active diverticulitis, toxic megacolon or history of radiation therapy. All patients underwent routine bowel preparation according to the endoscopist’s standard practice. All procedures were performed in the New York Presbyterian-Queens Endoscopy Unit by two experienced endoscopists (Rubin M and Kim SH) under Propofol sedation.
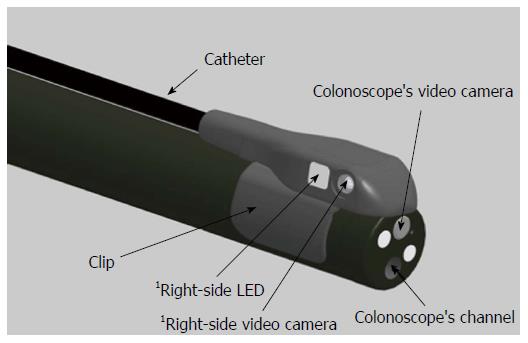
The Third Eye Panoramic cap was clipped on to the distal tip of a high definition Fuji EC530-LS Slim Colonoscope® (Fujifilm Medical Systems United States Inc., Wayne, NJ, United States) (Figures 1 and 2). A thin, flexible plastic catheter containing the video transmission wires ran along the shaft of the scope without additional attachments and was connected to an external video processor linked to the colonoscope’s high definition monitor. The result was three distinct but partially overlapping images on a single screen expanding the viewing angle to greater than 300°. The side view images were adjusted to be smaller than the center forward view image to facilitate eye focus and concentration.
We recorded ADR, cecal intubation rate, withdrawal time and total procedure time, which included time for lesion removal and intubation of the terminal ileum. All patients gave informed consent. The protocol was approved by the Institutional Review Board.
Of 34 patients enrolled, 1 was withdrawn due to a poor bowel preparation. The remaining 33 patients (18 male, 15 female) with a mean age of 60 years underwent screening, surveillance or diagnostic colonoscopy.
The cecum was reached in all 33 patients. Mean cecal intubation time was 8.1 ± 2.17 min, mean withdrawal time was 10.15 ± 5.56 min and mean total procedure time was 20.31 ± 5.14 min. The overall ADR was 44%.
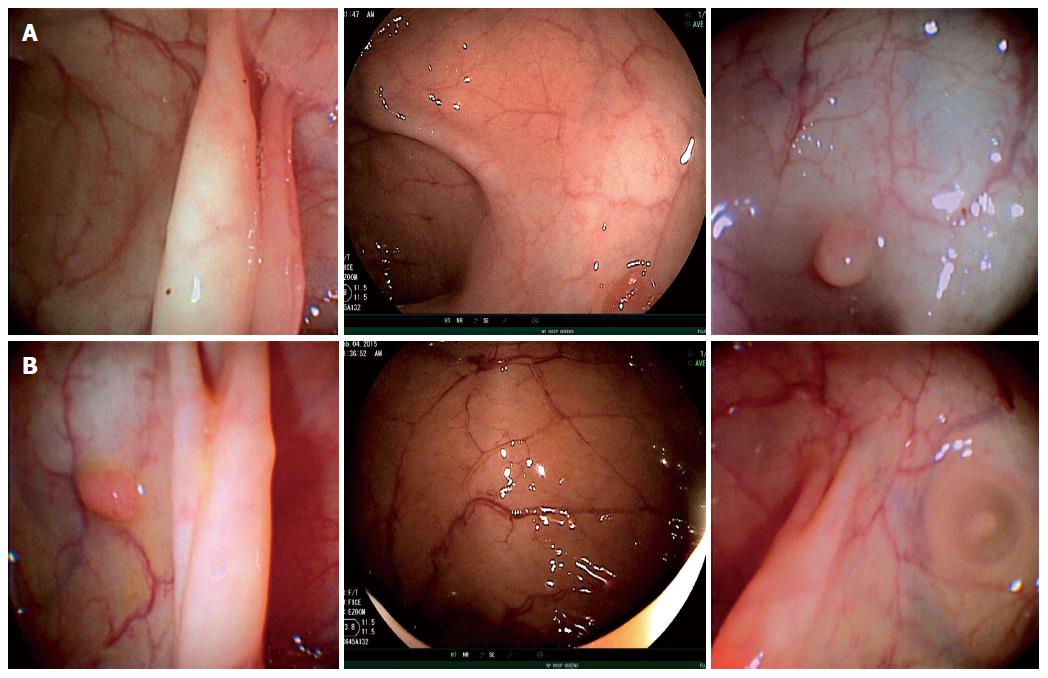
The TEP enabled enhanced views resulting in identification of polyps and diverticula that were not initially seen with the standard forward view of the colonoscope. All polyps initially detected in the lateral views were readily seen and removed following deflection of the colonoscope’s tip.
Use of the device did not affect the quality of the colonoscope’s high definition image or its handling characteristics. Specifically, there was no restriction of mobility, tip deflection or retroflexion. The terminal ileum was successfully intubated in most cases, but required additional effort in some patients. The cap only dislodged once during the study when lubricant was inadvertently placed on tip of the colonoscope prior to clipping on the cap. In all other cases, lubricant was applied after placement of the cap which fit snugly and did not move from its position. The cap did not cause damage to any scope. All devices were used once and then discarded.
Because the device does not occupy the working channel, it didn’t need to be removed to perform polypectomies, and it had no effect on suction capacity. Use of electrocautery did not affect the functioning of the cap. There were no device failures or adverse events. Telephone follow-up at 24 h found no reports of abdominal or anorectal discomfort following the procedure.
All procedures were performed with an EndoGator® Irrigation Pump (Medivators, Minneapolis, MN, United States), which successfully cleared the TEP lenses when the scope tip was angulated towards the colonic wall while washing.
Several revisions were made to the device during the study. Fasteners to hold the plastic catheter against the colonoscope’s shaft and a mechanism for cleaning lenses proved unnecessary and were eliminated. The side-viewing cameras were relocated closer to the tip of the colonoscope, improving image quality and eliminating a gap between images (Figure 3).
Our study demonstrates the successful implementation and use of the Third Eye® Panoramic™ cap fitted to a standard colonoscope. The Third Eye Panoramic device, unlike the Third Eye Retroscope, does not occupy the colonoscope channel. It accomplishes the goal of expanding the view without requiring a conversion to a new scope platform or sacrificing the high definition image quality and superior handling characteristics of standard colonoscopes. The TEP’s two additional side lenses enhanced the total viewing angle to greater than 300° without interfering with the performance of screening, surveillance or diagnostic colonoscopy. This increased viewing angle improved the ability to examine areas that would otherwise be hidden behind haustral folds and flexures. Neither the thin plastic catheter nor the cap itself affected the passage of the scope through the colon and no adjustment in technique was required. However, intubation of the ileocecal valve was more challenging. Future versions with a smaller footprint may alleviate this limitation. Although the TEP does increase the cost per case, a reusable version (currently undergoing testing) should minimize the expense. Although we found an overall adenoma detection rate of 44%, it is not known whether this device compares favorably to standard colonoscopy, cap assisted colonoscopy or other similar technologies such as the FUSE® system. Further studies comparing TEP to these other technologies and standard colonoscopy should be performed.
Colorectal cancer (CRC) is the third most common cancer in males and the second most common cancer in females worldwide From 2007 to 2011, CRC cases in the United States decreased 3.6% per year and CRC deaths decreased 2.8%, mostly due to increased use of screening colonoscopy and removal of precancerous adenomas. There is a direct correlation between a high adenoma detection rate and a decreased risk of interval colon cancer. Although colonoscopy remains the gold standard, tandem studies have shown miss rates of adenomas ranging from 22%-41%. The Third Eye® Panoramic™ Cap increases the viewing angle of a standard colonoscope enabling enhanced views behind folds and at flexures. This is the first study reporting on its successful use in patients undergoing colonoscopy.
Future studies comparing the Third Eye® Panoramic™ cap with standard colonoscopy as well as other devices now being used to improve the adenoma detection rate should be performed.
The Third Eye® Panoramic™ cap is an accessory camera system for colonoscopy that can be safely and effectively deployed utilizing existing standard high definition equipment.
Future iterations of the device will include a reusable camera system that can be disinfected in standard scope washers, a smaller footprint and the addition of high definition lenses.
This is an interesting paper describing the use of a panoramic cap allowing for wide view examination of the colon. The paper is well written and provides for proof of concept of this device.
P- Reviewer: Hsieh YH, Ji JS, Sharara A S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25536] [Article Influence: 1824.0] [Reference Citation Analysis (7)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9956] [Article Influence: 995.6] [Reference Citation Analysis (0)] |
| 3. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1463] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 4. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [PubMed] [DOI] [Full Text] |
| 5. | Leufkens AM, DeMarco DC, Rastogi A, Akerman PA, Azzouzi K, Rothstein RI, Vleggaar FP, Repici A, Rando G, Okolo PI. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest Endosc. 2011;73:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [PubMed] |
| 7. | Gralnek IM, Siersema PD, Halpern Z, Segol O, Melhem A, Suissa A, Santo E, Sloyer A, Fenster J, Moons LM. Standard forward-viewing colonoscopy versus full-spectrum endoscopy: an international, multicentre, randomised, tandem colonoscopy trial. Lancet Oncol. 2014;15:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Gralnek IM. Emerging technological advancements in colonoscopy: Third Eye® Retroscope® and Third Eye® Panoramic(TM) , Fuse® Full Spectrum Endoscopy® colonoscopy platform, Extra-Wide-Angle-View colonoscope, and NaviAid(TM) G-EYE(TM) balloon colonoscope. Dig Endosc. 2015;27:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Hewett DG, Rex DK. Miss rate of right-sided colon examination during colonoscopy defined by retroflexion: an observational study. Gastrointest Endosc. 2011;74:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352-359. [PubMed] |
| 11. | Siersema PD, Rastogi A, Leufkens AM, Akerman PA, Azzouzi K, Rothstein RI, Vleggaar FP, Repici A, Rando G, Okolo PI. Retrograde-viewing device improves adenoma detection rate in colonoscopies for surveillance and diagnostic workup. World J Gastroenterol. 2012;18:3400-3408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |