Published online Sep 28, 2015. doi: 10.3748/wjg.v21.i36.10443
Peer-review started: May 5, 2015
First decision: June 3, 2015
Revised: July 13, 2015
Accepted: August 31, 2015
Article in press: August 31, 2015
Published online: September 28, 2015
Processing time: 146 Days and 23.4 Hours
AIM: To compare the efficacy and safety of chemoembolization alone or chemoembolization combined with hepatic arterial infusion chemotherapy (HAIC), including oxaliplatin (OXA), 5-fluorouracil (5-FU) and folinic acid (CF), in inoperable hepatocellular carcinoma (HCC) without distant metastasis.
METHODS: Eighty-four inoperable HCC patients were enrolled. Thirty-nine patients underwent chemoembolization alone, and the other 45 patients underwent chemoembolization + HAIC (OXA/5-FU/CF) treatment non-randomly. The progression free survival (PFS), objective response rate (ORR), disease control rate (DCR) and adverse reactions were compared between the two groups.
RESULTS: A significant difference in the ORR was observed between the chemoembolization alone and chemoembolization + HAIC groups. There was no statistically significant difference in DCR between the two groups. The median PFS (mPFS) showed a significant difference between the two groups. For patients with BCLC stage A/B disease, with or without vessel invasion, the chemoembolization + HAIC group showed better mPFS when compared to chemoembolization alone, but no significant difference was found in patients with BCLC stage C disease. The parameter of pain (grade III-IV) in the chemoembolization + HAIC group was increased statistically.
CONCLUSION: Chemoembolization combined with HAIC with OXA/5-FU/CF may be safe and more effective than chemoembolization alone for inoperable HCC patients without distant metastasis.
Core tip: Eighty-four inoperable hepatocellular carcinoma (HCC) patients were enrolled, 39 patients underwent chemoembolization alone, and the other 45 patients underwent chemoembolization + hepatic arterial infusion chemotherapy (HAIC) [oxaliplatin (OXA)/5-fluorouracil (5-FU)/folinic acid (CF)] treatment non-randomly. The progression free survival (PFS), objective response rate (ORR), disease control rate (DCR) and adverse reactions were compared between the two groups. A significant difference in the ORR was observed between the two groups. There was no statistically significant difference in DCR between the two groups. The median PFS (mPFS) showed a significant difference between the two groups. For patients with BCLC stage A/B disease, with or without vessel invasion, the chemoembolization + HAIC group showed better mPFS when compared to chemoembolization alone, but no significant difference was found in patients with BCLC stage C disease. The parameter of pain (grade III-IV) in the chemoembolization + HAIC group was increased statistically. Chemoembolization combined with HAIC with OXA/5-FU/CF may be safe and more effective than chemoembolization alone for inoperable HCC patients without distant metastases.
-
Citation: Gao S, Zhang PJ, Guo JH, Chen H, Xu HF, Liu P, Yang RJ, Zhu X. Chemoembolization alone
vs combined chemoembolization and hepatic arterial infusion chemotherapy in inoperable hepatocellular carcinoma patients. World J Gastroenterol 2015; 21(36): 10443-10452 - URL: https://www.wjgnet.com/1007-9327/full/v21/i36/10443.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i36.10443
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide[1]. Chemoembolization is an effective treatment that has been widely used to treat unresectable HCC[2,3]. Combined with chemoembolization, treatments such as radiofrequency ablation (RFA), microwave ablation, absolute alcohol injection, sorafenib and hepatic artery infusion chemotherapy (HAIC) have been the recent focus of HCC interventional clinical studies[4].
Because of the multidrug resistance mechanisms of HCC, traditional systemic chemotherapeutic drugs are generally not helpful in eliminating HCC cells. Recently, some new anti-tumor drugs and formulations, such as oxaliplatin (OXA), capecitabine and gemcitabine, have been investigated in HCC chemotherapy clinical studies. In addition, the treatment containing OXA demonstrated better safety and efficacy[5,6]. Chemotherapy for unresectable cases of middle- and late-stage HCC again garnered attention. In 2010, an open, randomized, controlled, multicenter phase III clinical study (EACH test) of the use of FOLFOX4 venous chemotherapy to treat inoperable HCC showed that the FOLFOX 4 protocol had significant advantages in terms of overall survival (OS) and tumor progression (TTP); in terms of toxicities and side effects, this regimen was well tolerated[7].
Compared to systemic chemotherapy, chemotherapeutic drugs are directly infused into the blood supply through the hepatic artery to directly expose HCC cells to high-concentration drugs, which may reduce the systemic side effects caused by excessively high concentrations of drugs in the peripheral blood during systemic chemotherapy[8]. A number of clinical studies of HAIC with OXA used to treat hepatic metastasis for colon or rectal cancer have illustrated its good curative effect and safety[9-11]. Rathore et al[12] reported a phase I study in which the single drug OXA was perfused in a dose-escalation test through the hepatic artery to treat HCC and a curative effect was observed; the administration of OXA (150 mg/m2) during HAIC treatment for middle- and late-stage HCC was safe and effective. However, few high-level evidence-based clinical studies of HAIC with OXA for the treatment of HCC have been reported[13].
In our study, based on our previous results[14,15], we aimed to conduct a phase II, prospective, non-randomized clinical study in inoperable HCC patients without extra-hepatic metastasis. The curative effect and safety of the combination of chemoembolization and HAIC with OXA/5-fluorouracil (5-FU)/folinic acid (CF) were investigated.
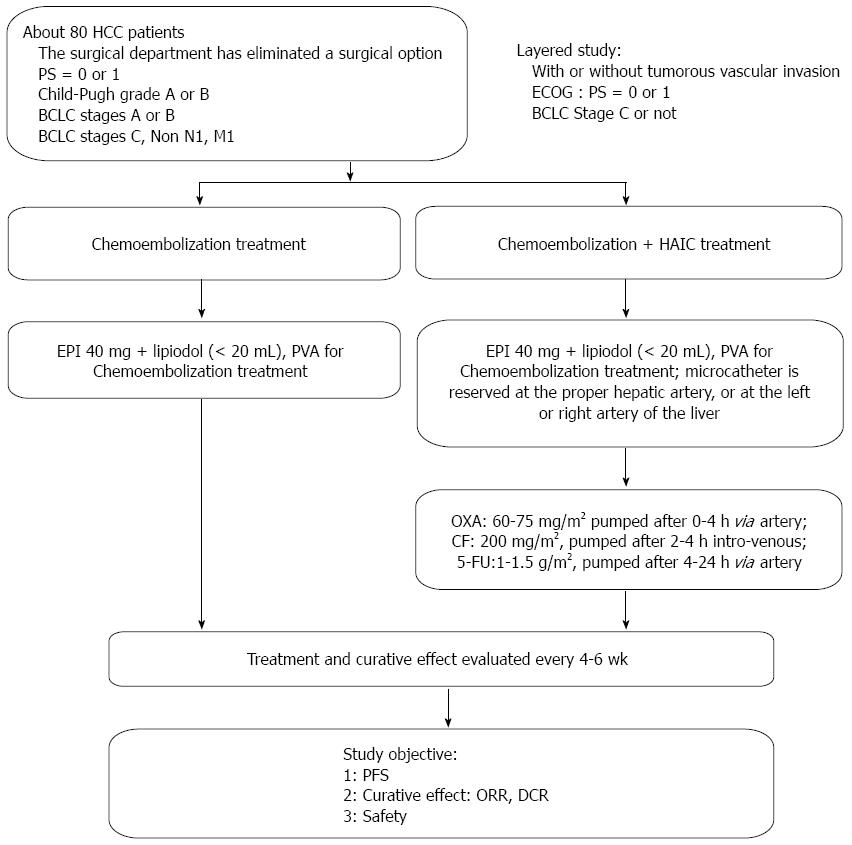
From December 2010 to August 2011, patients who had been diagnosed with HCC based on clinical and pathologic manifestations [using the American Association for the Study of Liver Diseases (AASLD) criteria] at the Department of Interventional Treatment in our hospital were enrolled[16]. A total of 84 HCC patients were enrolled, including 39 patients who were treated with chemoembolization alone and 45 patients who were treated with chemoembolization combined with HAIC containing OXA/5-FU/CF. Our study was approved by the local ethics committee and conducted according to the standards of the Declaration of Helsinki, and informed written consent was obtained from all patients. The patients treated with chemoembolization were defined as the control group (group A). Patients who were treated with the combination of chemoembolization and OXA/5-FU/CF-containing HAIC were defined as group B. The curative effect (modified RECIST)[17], progression free survival (PFS) rate and safety were compared between the two groups. Two groups of patients were enrolled on a non-randomized basis; approximately 40 patients were enrolled in each group.
The following inclusion criteria were applied: (1) male or female patients 18-80 years of age; (2) inoperable HCC patients (i.e., the surgical department eliminated a surgical option) without extrahepatic metastasis, including patients who had undergone surgery but suffered recurrence; (3) Child-Pugh grade A or B for liver function; (4) Barcelona staging (BCLC staging) of hepatic lymph node metastasis (N1) and distant metastasis (M1), except for patients in stage A, B or C; (5) an Eastern Cooperative Oncology Group (ECOG) patient state (PS) grade of 0 or 1; (6) patients who had enough reserve functions in the liver, kidney and medulla ossium; and (7) and an estimated survival time ≥ 12 wk.
The following exclusion criteria were applied: (1) the development or simultaneous development of other histological tumors; (2) patients who had undergone liver transplant surgery or received any other prior anti-tumor treatments, including interferon (IFN-α), systemic chemotherapy, and sorafenib; (3) patients who had developed severe coronary heart disease, severe arrhythmia requiring treatment with medicines other than a β receptor blocking agent or digoxin, severe active infection (> grade 2, NCI - CTCAE v3.0 criteria), combined HIV infection, renal insufficiency [creatinine (Cr) level > 2 mg/dL], unconsciousness (including patients with a history of epilepsy), severe allergic constitution, or allergy to contrast media; (4) women who were pregnant or lactating at the time of enrollment; (5) ECOG grading, PS > 2; (6) Child-Pugh grade C for liver function; (7) BCLC staging, stage 0 or D; (8) the tumor volume accounted for > 70% of the liver volume; and (9) portal vein thrombosis with no obvious collateral circulation established.
As shown in Figure 1, the chemoembolization treatment for all enrolled patients was performed with a digital subtraction angiography (DSA) machine (Innova 4100 IQ, GE Corporation, United States) in our hospital. Group A received the chemoembolization treatment alone using the right femoral approach; DSA of the celiac, superior mesenteric and splenic arteries was performed to evaluate the hepatic arterial anatomy and tumor blood supply. The portal venous system was evaluated in the portal venous phases of the superior mesenteric or splenic angiograms. A 2.7 F microcatheter (Progreat, Terumo, Japan) was advanced coaxially through the outer catheter for selective catheterization of the arteries supplying the tumor. A total of 40 mg of emulsified epirubicin (EPI) and lipiodol (total volume < 20 mL) was administered for embolization. For some large tumors, polyvinyl alcohol particles (PVA) embolization was performed. If there was extrahepatic parasitic blood supply to the tumor (e.g., from the right phrenic artery, left gastric artery, or right inferior adrenal artery), chemoembolization was recommended and performed after selective catheterization through the parasitic artery.
For patients in group B, the chemoembolization treatment was performed as described above. If the microcatheter head was near the gastroduodenal artery or the right gastric artery, microcoil embolization was performed; to protect the normal gastrointestinal tract, the microcatheter was reserved at the proper hepatic artery or at the left or right hepatic artery. After the patient returned to the ward, the microcatheter was externally connected to the artery infusion pump (Model LP 2000-P2) to administer the following HAIC treatment: OXA [60-75 mg/m2 (Child-Pugh A, 75 mg/m2 and Child-Pugh B, 60 mg/m2)] intra-arterially administered for 0-4 h; CF (200 mg/m2) intravenously administered for 2-4 h; and 5-FU [1-1.5 g/m2 (Child-Pugh A, 1.5 g/m2 and Child-Pugh B, 1 g/m2)] intra-arterially administered for 4-24 h.
After the treatment was completed in both groups, the indwelling catheter in the artery was removed, and the pressure hemostasis was regulated at the puncture point. All patients were given a fluid infusion to support and protect the liver treatment.
During and after the arterial chemotherapy treatment, any development of pain, fever, nausea, vomiting and anxiety was recorded. Then, 5-7 d later, laboratory tests were performed, including a routine blood test and thrombotest, blood ammonia and liver and kidney function tests. These tests were repeated after 4-6 wk to observe any adverse reactions in the patients. Contrast enhanced magnetic resonance imaging (MRI) or computed tomography (CT) examinations were performed every 4-6 wk to evaluate the treatment (mRECIST) efficacy.
The original treatment protocol was stopped, and the PFS time was recorded if any of the following developments occurred: (1) the patient died or there were extrahepatic metastases or intrahepatic lesion development; (2) there were intolerable grade IV adverse reactions (NCI-CTCAE v3.0 criteria); or (3) a Child-Pugh class of C and an ECOG > 2 were recorded.
The following supplementary descriptions were also recorded in our study: (1) if the patients demonstrated stable conditions with complete response (CR), an alpha-fetoprotein (AFP) recheck was performed every 3 mo at the clinic or ward, and contrast enhanced MRI or CT evaluations were conducted; (2) if there were any new tumor nodes (> 5 mm) in the liver, the progressive disease (PD) was evaluated, and the PFS was recorded. The original protocol could be administered for continuous treatment. When PD was identified twice consecutively, the patient was excluded from the study and received regular follow-ups; and (3) the HCC patients who received stage-II resection or ablation after these interventional treatments were excluded. Only the curative effect and safety were evaluated; no PFS rates were recorded.
All patients received chemoembolization alone or combined treatment at least once; then, the efficacy and safety of the two protocols were evaluated: (1) The curative effect, according to the mRECIST, was analyzed and evaluated, including the objective response rate (ORR) of the tumor/disease control rate (DCR): ORR = CR + partial response (PR); DCR = CR + PR + C (SD); and (2) The median progression free survival (mPFS) was analyzed. Any adverse reactions were recorded, and the safety of this treatment was evaluated (NCI-CTCAE v3.0; Levi special grading standard for sensory nerve toxicity)[18].
All data were analyzed using SPSS Version 15.0 (SPSS Inc., 2006, Chicago IL, United States). The end point of this clinical study was mPFS. When the patients died, or if the tumors progressed, the PFS time was recorded for the survival analysis. If the treatment was terminated due to the loss of follow-up information, if the patient underwent surgery after the interventional treatment, if the patient’s general condition deteriorated (PS > 2), if the patient’s liver function deteriorated (Child-Pugh C), or if the patient experienced intolerable adverse reactions, then the last examination and evaluation time or the resection time of the patient was recorded and designated as censored data for the survival analysis. The curative effect, ORR and DCR of the two groups were compared using the Pearson correlation coefficient and the Chi-squared test. The mPFS time (Kaplan-Meier method) was assessed for all enrolled HCC patients. Log-rank tests were used to perform the univariate analysis for the PS status, liver function (Child-Pugh grading)[19], vascular invasion, AFP value, total bilirubin value, tumor quantity and the diameter of the tumorous target lesion. Multivariate analysis was performed using the Cox proportional hazards regression model. The Pearson method or the Fisher’s exact test and the Chi-square test were used to compare the rate of adverse reactions in the two groups, with P < 0.05 indicating statistical significance.
As shown in Table 1, the baseline clinical characteristics of the enrolled patients showed no significant difference between the two groups. Clinical characteristics, including age, gender, hepatitis conditions, physical condition grading, ascites, Child-Pugh grade, diameter of tumor target lesion, number of tumors, blood vessel invasion, Barcelona stage and follow-up time, showed no significant differences between the two groups (P > 0.05). The blood test indicators, including white blood cell count, hemoglobin value, platelet count, glutamate pyruvate transaminase, glutamic-oxaloacetic transaminase, total bilirubin, serum albumin, prothrombin time, creatinine and AFP, also showed no significant difference between the two groups (P > 0.05), as shown in Supplementary Table 1.
| Chemoembolization group (n = 39) | Chemoembolization + HAIC group (n = 45) | P-value | |
| Age (yr) | 59.69 ± 13.13 | 57.16 ± 10.34 | 0.325 |
| Gender | 0.832 | ||
| Male | 35 (89.7) | 41 (91.1) | |
| Female | 4 (10.3) | 4 (8.9) | |
| Hepatitis condition | 0.309 | ||
| Hepatitis B | 32 (82.1) | 39 (86.7) | |
| Hepatitis C | 3 (7.7) | 3 (6.7) | |
| Hepatitis B + hepatitis C | 1 (2.6) | 3 (6.7) | |
| Non-hepatitis | 3 (7.7) | 0 | |
| Grading of physical condition | 0.393 | ||
| PS = 0 | 18 (46.2) | 24 (53.3) | |
| PS = 1 | 21 (53.8) | 21 (46.7) | |
| Ascites | 0.900 | ||
| Yes | 10 (25.6) | 11 (24.4) | |
| No | 29 (82.4) | 34 (75.6) | |
| Liver function Child-Pugh | 0.075 | ||
| Grade A | 36 (92.3) | 41 (91.1) | |
| Grade B | 3 (7.7) | 4 (8.9) | |
| Diameter of tumor target lesion (cm) | 0.347 | ||
| ≤ 10 | 19 (48.7) | 29 (64.4) | |
| > 10 | 20 (51.3) | 16 (35.6) | |
| Number of tumor | 0.149 | ||
| > 3 | 22 (56.4) | 31 (68.9) | |
| ≤ 3 | 17 (43.6) | 14 (31.1) | |
| Blood vessel invasion | 1.000 | ||
| No | 26 (66.7) | 30 (66.7) | |
| Yes | 13 (33.3) | 15 (33.3) | |
| BCLC stage | 0.860 | ||
| Stage A | 4 (10.3) | 3 (6.7) | |
| Stage B | 9 (23.1) | 15 (33.3) | |
| Stage C | 22 (56.4) | 21 (44.1) | |
| Relapse after surgical resection | 4 (10.3) | 6 (13.3) | |
| Follow-up time, median (mo) | 7.2 (1.3-15.2) | 9.3 (3.8-14.6) | 0.169 |
As shown in Table 2, excluding the two patients in the chemoembolization treatment group who were lost to follow-up, the ORR was 45.9%. The ORR in the chemoembolization + HAIC treatment group was 68.9%. There were statistically significant differences between the two groups (P = 0.036). In the chemoembolization + HAIC treatment group, the DCR was 86.7%, which was higher than that in the chemoembolization group; however, there was no statistically significant difference between the two groups (P = 0.068).
| Chemoembolizationgroup (n = 39) | Chemoembolization + HAICgroup (n = 45) | P-value | |
| CR | 5 | 12 | |
| PR | 12 | 19 | |
| SD | 9 | 8 | |
| PD | 11 | 6 | |
| Not evaluated | 2 (lost) | 0 | |
| ORR | 17/37 (45.9%) | 31/45 (68.9%) | 0.036 |
| DCR | 26/37 (70.3%) | 39/45 (86.7%) | 0.068 |
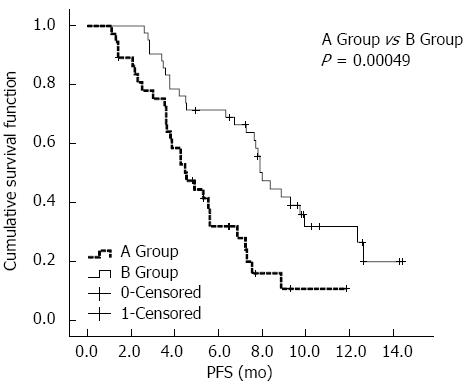
As shown in Table 3, two patients were excluded from group A: one patient experienced a reduced lesion size after treatment, and based on the PR benchmark, this patient received surgical resection; and one patient received the combined RF treatment after three chemoembolization treatments. Three patients were excluded from group B: one patient received resection after experiencing a lesion size reduction and reduced tumor thrombosis located in the portal vein left branch, based on the PR benchmark; and two patients received the combined RF treatment after chemoembolization + HAIC treatment. After Kaplan-Meier univariate analysis, the following conditions, which showed significant differences in terms of mPFS between the two groups, were associated with a better prognosis and longer mPFS: chemoembolization combined with HAIC (P = 0.00049), ECOG grading PS = 0 (P = 0.00014), BCLC stage A or B (P = 0.00051), no vascular tumor invasion (P = 0.00047), AFP < 400 ng/mL (P = 0.03), a target lesion diameter sum ≤ 10 cm (P = 0.00011), and albumin (Alb) ≥ 35 g/L (P = 0.027). The other factors showed no significant differences in terms of MPFS between the two groups, as shown in Supplementary Table 2.
| Correlative factor | Number of cases | Kaplan-Meier univariate analysis | Cox multivariate analysis | ||||
| mPFS (mo) | 95%CI | P-value | Sig | EXP(B) | 95%CI | ||
| Interventional therapy | 0.00049 | 0.00048 | 0.26 | 0.174-0.612 | |||
| Chemoembolization | 37 | 4.5 | 3.3-5.7 | ||||
| Chemoembolization combined with HAIC | 42 | 8.0 | 7.2-8.8 | ||||
| Grading of physical condition | 0.00014 | 0.312 | 1.525 | 0.674-3.452 | |||
| PS = 0 | 40 | 8.9 | 6.2-11.5 | ||||
| PS = 1 | 39 | 4.5 | 3.7-5.2 | ||||
| Vascular invasion1 | 0.00047 | 0.050 | 1.963 | 0.989-3.898 | |||
| No | 52 | 7.9 | 7.3-8.5 | ||||
| Yes | 27 | 3.8 | 2.7-4.8 | ||||
| BCLC Stage2 | 0.00051 | 0.003 | 3.083 | 1.479-6.426 | |||
| Stage C | 30 | 3.8 | 3.0-4.6 | ||||
| Stage A or B | 39 | 9.3 | 6.7-11.9 | ||||
| AFP (ng/mL) | 0.03 | 0.488 | 1.255 | 0.660-2.386 | |||
| < 400 | 52 | 7.3 | 5.8-8.8 | ||||
| ≥ 400 | 27 | 4.5 | 2.1-7.0 | ||||
| Sum of tumor target lesion diameter (cm) | 0.00011 | 0.326 | 1.449 | 0.691-3.338 | |||
| ≤ 10 | 45 | 7.8 | 6.1-9.5 | ||||
| > 10 | 34 | 4.3 | 3.2-5.3 | ||||
| Serum albumin (g/L) | 0.027 | 0.27 | 0.628 | 0.275-1.434 | |||
| < 35 | 7 | 4.3 | 3.0-5.6 | ||||
| ≥ 35 | 72 | 7.3 | 5.9-8.6 | ||||
Then, a Cox proportional hazards regression model was used to conduct the multivariate analysis of the patients in terms of interventional therapy proposal, ECOG grade, BCLC stage, vascular tumor invasion, AFP, sum of tumor target lesion diameter and Alb. The model demonstrated that the chemoembolization + HAIC treatment (P = 0.00048) was an independent good prognostic factor, but BCLC stage C (P = 0.003) and vascular tumor invasion (P = 0.050) were independent poor prognostic factors for inoperable HCC patients.
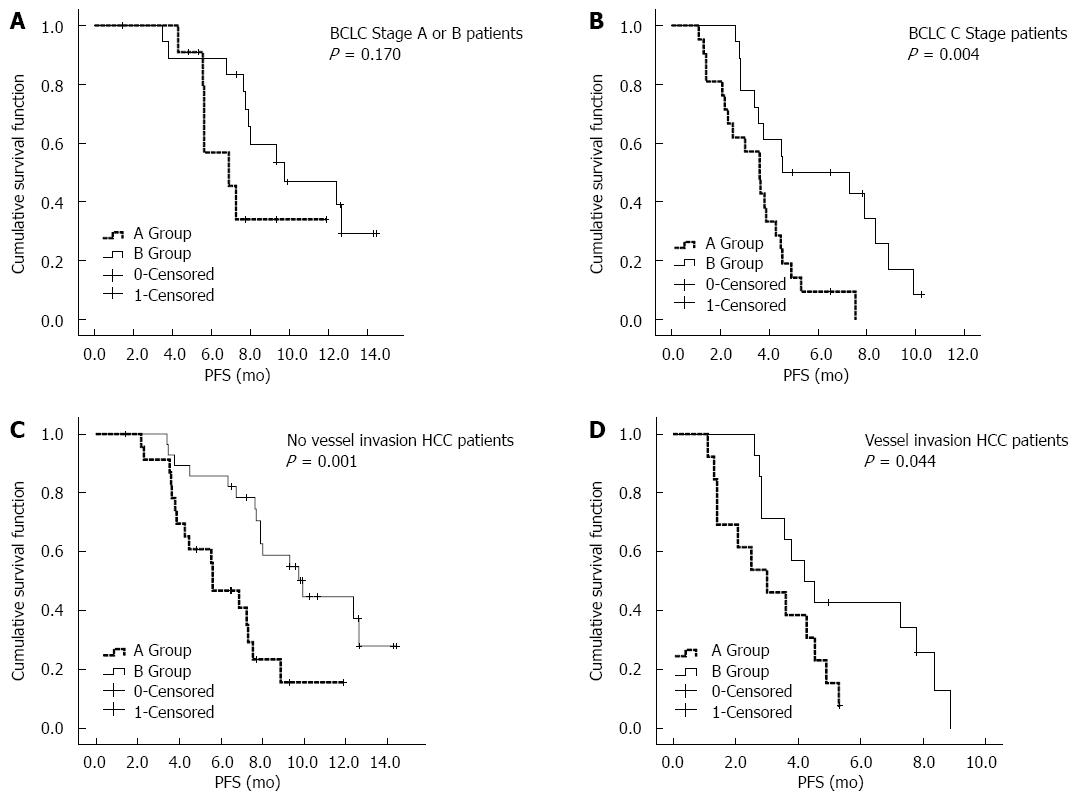
After the univariate and multivariate analyses, a survival analysis of the three factors was performed, including the interventional therapy proposal, BCLC stage, and vascular tumor invasion. As shown in Figure 2, the mPFS in the chemoembolization + HAIC treatment group (8.0 mo, 95%CI: 7.2-8.8 mo) was significantly better than that in the chemoembolization-only treatment group (4.5 mo, 95%CI: 3.3-5.7 mo). As shown in Figure 3A, for patients with BCLC stage A/B disease, the mPFS was prolonged in group B (9.7 mo, 95%CI: 4.6-14.9 mo) compared with group A (6.9 mo, 95%CI: 4.5-9.2 mo); however, there were no statistically significant differences between the two groups (P = 0.170). As shown in Figure 3B, for the BCLC Stage C patients, group B (4.5 mo, 95%CI: 0.1-9.6 mo) had better mPFS than group A (3.6 mo, 95%CI: 2.6-4.5 mo), and there was a statistically significant difference between the two groups (P = 0.004). As shown in Figure 3C, for patients with no vessel invasion, the mPFS rate in group B (9.9 mo, 95%CI: 7.1-12.7 mo) was significantly better compared with that of patients in group A (5.6 mo, 95%CI: 3.8-7.4 mo) (P = 0.001). As shown in Figure 3D, for patients with vessel invasion, group B (4.2 mo, 95%CI: 2.8-5.6 mo) also had significantly better mPFS than group A (3.0 mo, 95%CI: 1.2-4.8 mo) (P = 0.044).
The patients in group A received a total of 92 treatments, and the patients in group B received a total of 137 treatments. As shown in Table 4, when grades I and II adverse reactions were compared between the two groups (χ2 test), we found that the chemoembolization group had fewer reactions. The adverse reactions, including low platelets (P = 0.001), nausea (P = 0.030), and vomiting (P = 0.025) demonstrated significant differences. When grades III and IV adverse reactions were compared between the two groups, there was a significant difference in pain (P = 0.008).
| Adverse reaction | Chemoembolization (n = 92) | Chemoembolization + HAIC (n = 137) | ||||
| I + II | III + IV | I + II | III + IV | P1 | P2 | |
| Hematologic toxicity | ||||||
| Reduction of white cells | 13 (14.1) | 0 | 21 (15.3) | 2 (1.4) | 0.803 | - |
| Reduction of hemoglobin | 17 (18.5) | 3 (3.3) | 21 (15.3) | 2 (1.4) | 0.530 | 0.393 |
| Reduction of thrombocyte | 19 (20.7) | 7 (7.6) | 57 (41.6) | 5 (3.6) | 0.001 | 0.231 |
| Non-hematologic toxicity | ||||||
| Increase of total bilirubin | 38 (41.3) | 4 (4.3) | 74 (54.0) | 5 (3.6) | 0.059 | 0.744 |
| Increase of glutamate pyruvate transaminase | 55 (59.8) | 5 (5.4) | 78 (56.9) | 11 (8.0) | 0.668 | 0.450 |
| Increase of glutamic-oxaloacetic transaminase | 50 (54.3) | 8 (8.7) | 82 (59.8) | 9 (6.5) | 0.408 | 0.547 |
| Fever | 55 (59.8) | 11 (12.0) | 64 (46.7) | 12 (8.8) | 0.052 | 0.430 |
| Pain1 | 40 (43.5) | 3 (3.3) | 63 (46.0) | 19 (13.9) | 0.708 | 0.008 |
| Nausea | 47 (51.1) | 3 (3.3) | 91 (66.4) | 5 (3.6) | 0.030 | 0.875 |
| Vomit | 19 (20.7) | 0 | 47 (34.3) | 3 (2.2) | 0.025 | - |
| Diarrhea | 1 (1.1) | 0 | 4 (3.0) | 0 | 0.768 | - |
| Constipation | 18 (19.6) | 1 (1.1) | 29 (21.2) | 0 | 0.6513 | - |
| Neurotoxicity2 | - | - | 13 (6.8) | 0 | - | - |
According to clinical meta-analysis for a number of international HCC interventional treatments, chemoembolization has not demonstrated a better curative effect or greater survival benefits compared with embolization treatment[20-23], and the effect of perfusion chemotherapy through the hepatic artery is questionable[16]. This finding may be related to the following factors: there are significant differences in terms of the enrolled patients and treatment methods for chemoembolization in each clinical study; there is no standard for the selection of chemotherapeutic drugs and doses; and no high-level evidence-based studies have been proposed[21]. In addition, the perfusion time through the hepatic artery is short and does not consider the metabolism of the chemotherapeutic drug in the liver and tumor.
In our study, we selected a small dose of OXA (60-75 mg/m2) combined with 5-FU for the continuous HAIC treatment administered to the patients. CF was given to increase the response rate of 5-FU through chemical biological tuning. Compared to the previous studies, we designated the chemoembolization treatment group as the control group to ensure that we could better observe the effects of HAIC. The mRECIST criteria that introduced the concept of the “active lesion” were used for the analysis, which may more objectively and accurately reflect the curative effect compared with the original RECIST criteria for HCC[24-26]. Our study results demonstrated that chemoembolization + HAIC treatment is significantly better than chemoembolization treatment alone in terms of DCR, and our findings also support the use of ORR compared with chemoembolization treatment alone. Data from the integrated study show that the chemoembolization + HAIC combined treatment may significantly increase the short-term effect of the treatment for the HCC patients who have no metastasis or unresectable tumors, and it may extend the mPFS.
In addition, the chemoembolization + HAIC treatment demonstrated a better prognosis than the chemoembolization treatment alone. The survival analysis results also showed that for patients with or without vascular invasion, the mPFS between the chemoembolization + HAIC treatment and chemoembolization treatment showed a statistically significant difference. For the BCLC stage C patients, the mPFS for the chemoembolization + HAIC group was significantly prolonged, with a better curative effect. The following may explain this improvement: chemoembolization caused tumor ischemia, and the induced transmembrane ion pump failed, which, to some extent, had a synergistic effect combined with the high-concentration chemotherapy and enabled the tumor cells to absorb more chemotherapeutic drugs[27]; and after the chemoembolization treatment, HAIC had a therapeutic effect on the portal vein, hepatic vein tumor thrombosis, and the tumor, evidenced by the lack of obvious feeding vessels in the angiography[28,29]. For the HCC patients with BCLC stage A or B disease, the mPFS rate was prolonged in the chemoembolization + HAIC treatment compared with that in the chemoembolization-only treatment group, although there was no statistically significant difference between the two groups, which may be related to the small number of enrolled patients. Although the tumor vessels were already embolized with chemoembolization, the vessels may recur and the HAIC treatment may have a therapeutic effect on the tumor mass if it was completely embolized.
In terms of adverse reactions, our study results showed that the chemoembolization + HAIC treatment demonstrated good safety and tolerance. The chemoembolization + HAIC treatment increased the occurrence of adverse reactions, including grades I-II low platelet count, nausea and vomiting. However, in terms of grades III-IV adverse reactions, statistically significant differences were only found for pain. Meanwhile, during the chemoembolization + HAIC treatment in this study, no bleeding, thrombus, infection or other related complications caused by the indwelling catheter were found. Note that in group B, OXA caused sharp pain (VAS: Grade 8-10) in six different patients at the midsection during the administration of HAIC (most of which occurred in 2-4 hours after the chemotherapy). The HAIC treatment was complemented with lidocaine given at intervals during the infusion treatment. The specific cause and mechanism of the sharp pain experienced by the patient may be related to the local administration of the high-concentration chemotherapy drugs[30]. This side effect should be further observed in future clinical studies.
There were also some limitations to our study. First, the patients were not enrolled on a randomized basis, and the data may be affected by a selection bias. Most of the enrolled HCC patients had Child-Pugh grade A, PS = 0 or 1, and whether the patients with Child-Pugh grade B and PS = 2 can benefit from chemoembolization + HAIC treatment must be assessed in our future study. Second, the therapy endpoints of chemoembolization were not completely consistent. Some patients were treated with PVA particles, which may affect the study result. Third, the number of enrolled patients for this study was small, and a multi-center analysis is needed.
In conclusion, chemoembolization combined with HAIC with OXA/5-FU/CF may be a generally safe treatment, and it is more effective than chemoembolization alone for patients with unresectable HCC without distant metastasis. Based on these promising results, a large-scale, multicenter, randomized, controlled study should be conducted in the future.
Clinical studies of hepatic arterial infusion chemotherapy (HAIC) with oxaliplatin (OXA) used to treat hepatic metastasis for colon or rectal cancer have illustrated its good curative effects, however, few high-level evidence-based clinical studies of HAIC with OXA for the treatment of hepatocellular carcinoma (HCC) have been reported.
We conducted a phase II, prospective, non-randomized clinical study in inoperable HCC patients without extra-hepatic metastases. The curative effect and safety of the combination of chemoembolization and HAIC with OXA/5-fluorouracil (5-Fu)/folinic acid (CF) were investigated.
Chemoembolization combined with HAIC with OXA/5-Fu/CF may be a generally safe treatment.
Chemoembolization combined with HAIC with OXA/5-Fu/CF may be more effective than chemoembolization alone for patients with unresectable HCC without distant metastasis.
Chemoembolization combined with HAIC with OXA/5-Fu/CF showed be taken into consideration for the therapy for patients with unresectable HCC without distant metastasis, and more evidence should be used to demonstrate the therapy effect.
This study found that chemoembolization combined with HAIC with OXA/5-Fu/CF may be safe and more effective than chemoembolization alone for inoperable HCC patients without distant metastasis. Over all, this study is well designed, and the manuscript is interesting.
P- Reviewer: Sharma M S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2651] [Cited by in RCA: 2599] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 2. | Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009;10:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (32)] |
| 3. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2272] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 4. | Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29:3960-3967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 5. | Uhm JE, Park JO, Lee J, Park YS, Park SH, Yoo BC, Paik SW, Koh KC, Kang WK, Lim HY. A phase II study of oxaliplatin in combination with doxorubicin as first-line systemic chemotherapy in patients with inoperable hepatocellular carcinoma. Cancer Chemother Pharmacol. 2009;63:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Yen Y, Lim DW, Chung V, Morgan RJ, Leong LA, Shibata SI, Wagman LD, Marx H, Chu PG, Longmate JA. Phase II study of oxaliplatin in patients with unresectable, metastatic, or recurrent hepatocellular cancer: a California Cancer Consortium Trial. Am J Clin Oncol. 2008;31:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, Yang TS, Bhudhisawasdi V, Kang WK, Zhou Y. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 8. | Kim HY, Kim JD, Bae SH, Park JY, Han KH, Woo HY, Choi JY, Yoon SK, Jang BK, Hwang JS. A comparative study of high-dose hepatic arterial infusion chemotherapy and transarterial chemoembolization using doxorubicin for intractable, advanced hepatocellular carcinoma. Korean J Hepatol. 2010;16:355-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Kerr DJ, McArdle CS, Ledermann J, Taylor I, Sherlock DJ, Schlag PM, Buckels J, Mayer D, Cain D, Stephens RJ. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet. 2003;361:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, Weeks JC, Sigurdson ER, Herndon JE, Zhang C. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 286] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 11. | Del Freo A, Fiorentini G, Sanguinetti F, Muttini MP, Pennucci C, Mambrini A, Pacetti P, Della Seta R, Lombardi M, Torri T. Hepatic arterial chemotherapy with oxaliplatin, folinic acid and 5-fluorouracil in pre-treated patients with liver metastases from colorectal cancer. In Vivo. 2006;20:743-746. [PubMed] |
| 12. | Rathore R, Safran H, Soares G, Dubel G, McNulty B, Ahn S, Iannitti D, Kennedy T. Phase I study of hepatic arterial infusion of oxaliplatin in advanced hepatocellular cancer: a brown university oncology group study. Am J Clin Oncol. 2010;33:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Si Y, Hu X, Du H, Lou W, Zhang H, Cao F, Yu W, Wang W, Jin K. Transarterial chemoembolization for patients with unresectable hepatocellular carcinoma: a retrospective study of a 5-year experience in a single institution. Hepatogastroenterology. 2013;60:1405-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Zhu L, Zhong ZX, Yang RJ. Sensitivity of different chemotherapy drugs on the liver cancer cell lines under environment of intervention therapy. Zhongguo Shiyan Zhen Duan Xue. 2011;15:1861-1866. |
| 15. | Gao XZ, Yang RJ. TACE combined with Oxaliplatin/5-FU/LV for the transarterial chemotherapy for Hepatocellular Cancer. Jieru Fangshexue Zazhi. 2012;21:377-383. |
| 16. | Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 728] [Article Influence: 42.8] [Reference Citation Analysis (1)] |
| 17. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3303] [Article Influence: 220.2] [Reference Citation Analysis (36)] |
| 18. | Lévi F, Misset JL, Brienza S, Adam R, Metzger G, Itzakhi M, Caussanel JP, Kunstlinger F, Lecouturier S, Descorps-Declère A. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumor effectiveness against metastatic colorectal cancer. Cancer. 1992;69:893-900. [PubMed] |
| 19. | Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK. Systematic review: The model for end-stage liver disease--should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079-1089. [PubMed] |
| 20. | Ikeda M, Maeda S, Shibata J, Muta R, Ashihara H, Tanaka M, Fujiyama S, Tomita K. Transcatheter arterial chemotherapy with and without embolization in patients with hepatocellular carcinoma. Oncology. 2004;66:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 618] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 22. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [PubMed] |
| 23. | Maeda S, Fujiyama S, Tanaka M, Ashihara H, Hirata R, Tomita K. Survival and local recurrence rates of hepatocellular carcinoma patients treated by transarterial chemolipiodolization with and without embolization. Hepatol Res. 2002;23:202-210. [PubMed] |
| 24. | Di Maio M, Daniele B, Gallo C, Perrone F. Re: Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:1557; author reply 1557-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Liu Q, Li A, Sun S, Luo R, Chen F. The true role of mRECIST guideline: does it really estimate viable tumor or merely improve accuracy in hepatocellular carcinoma response evaluation? J BUON. 2014;19:398-405. [PubMed] |
| 26. | Kim DJ, Clark PJ, Heimbach J, Rosen C, Sanchez W, Watt K, Charlton MR. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transplant. 2014;14:1383-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Trevisani F, De Notariis S, Rossi C, Bernardi M. Randomized control trials on chemoembolization for hepatocellular carcinoma: is there room for new studies? J Clin Gastroenterol. 2001;32:383-389. [PubMed] |
| 28. | Lai YC, Shih CY, Jeng CM, Yang SS, Hu JT, Sung YC, Liu HT, Hou SM, Wu CH, Chen TK. Hepatic arterial infusion chemotherapy for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2003;9:2666-2670. [PubMed] |
| 29. | Kudo M. Treatment of advanced hepatocellular carcinoma with emphasis on hepatic arterial infusion chemotherapy and molecular targeted therapy. Liver Cancer. 2012;1:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373:614-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |