Published online Aug 28, 2015. doi: 10.3748/wjg.v21.i32.9648
Peer-review started: March 15, 2015
First decision: April 13, 2015
Revised: May 28, 2015
Accepted: June 26, 2015
Article in press: June 26, 2015
Published online: August 28, 2015
Processing time: 166 Days and 6.9 Hours
AIM: To evaluate the utility of liver reserve function by acoustic radiation force impulse (ARFI) imaging in patients with liver tumors.
METHODS: Seventy-six patients with liver tumors were enrolled in this study. Serum biochemical indexes, such as aminotransferase (ALT), aspartate aminotransferase (AST), serum albumin (ALB), total bilirubin (T-Bil), and other indicators were observed. Liver stiffness (LS) was measured by ARFI imaging, measurements were repeated 10 times, and the average value of the results was taken as the final LS value. Indocyanine green (ICG) retention was performed, and ICG-K and ICG-R15 were recorded. Child-Pugh (CP) scores were carried out based on patient’s preoperative biochemical tests and physical condition. Correlations among CP scores, ICG-R15, ICG-K and LS values were observed and analyzed using either the Pearson correlation coefficient or the Spearman rank correlation coefficient. Kruskal-Wallis test was used to compare LS values of CP scores, and the receiver-operator characteristic (ROC) curve was used to analyze liver reserve function assessment accuracy.
RESULTS: LS in the ICG-R15 10%-20% group was significantly higher than in the ICG-R15 < 10% group; and the difference was statistically significant (2.19 ± 0.27 vs 1.59 ± 0.32, P < 0.01). LS in the ICG-R15 > 20% group was significantly higher than in the ICG-R15 < 10% group; and the difference was statistically significant (2.92 ± 0.29 vs 1.59 ± 0.32, P < 0.01). The LS value in patients with CP class A was lower than in patients with CP class B (1.57 ± 0.34 vs 1.86 ± 0.27, P < 0.05), while the LS value in patients with CP class B was lower than in patients with CP class C (1.86 ± 0.27 vs 2.47 ± 0.33, P < 0.01). LS was positively correlated with ICG-R15 (r = 0.617, P < 0.01) and CP score (r = 0.772, P < 0.01). Meanwhile, LS was negatively correlated with ICG-K (r = -0.673, P < 0.01). AST, ALT and T-Bil were positively correlated with LS, while ALB was negatively correlated with LS (P < 0.05). The ROC curve revealed that the when the LS value was 2.34 m/s, the Youden index was at its highest point, sensitivity was 69.2% and specificity was 92.1%.
CONCLUSION: For patients with liver tumors, ARFI imaging is a useful tool for assessing liver reserve function.
Core tip: Seventy-six patients with liver tumors were assessed by acoustic radiation force impulse (ARFI) imaging. We found that liver stiffness (LS) was positively correlated with Indocyanine green (ICG)-R15 and the Child-Pugh score, but was negatively correlated with ICG-K. Aspartate aminotransferase, aminotransferase and total bilirubin were positively correlated with LS, while albumin was negatively correlated with LS. The receiver-operator characteristic curve revealed that when LS value was 2.34 m/s, the Youden index was at its highest point. For patients with liver tumors, ARFI imaging is a useful tool to assess liver reserve function.
- Citation: Sun XL, Liang LW, Cao H, Men Q, Hou KZ, Chen Z, Zhao YE. Liver reserve function assessment by acoustic radiation force impulse imaging. World J Gastroenterol 2015; 21(32): 9648-9655
- URL: https://www.wjgnet.com/1007-9327/full/v21/i32/9648.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i32.9648
Hepatocellular carcinoma (HCC) is one of the major malignant tumors in China, and it has the third highest mortality after gastric carcinoma and lung cancer. Most HCC patients have liver fibrosis and cirrhosis[1,2]. Hepatectomy is the preferred treatment for early HCC. However, the presence of cirrhosis, results in different degrees of liver injury and low liver reserve function. Poor prognosis, such as postoperative liver failure, usually occurs[3,4]. Therefore, a good liver reserve function is a key factor for a successful surgery, and preoperative liver reserve function assessment is very important[5]. The indocyanine green (ICG) excretive test[6] and Child-Pugh (CP) scores are the main methods used to assess liver reserve function in clinical practice. However, both methods have different limitations and accuracies. In recent years, because of the development of ultrasound elastography in liver fibrosis and cirrhosis in the clinic, acoustic radiation force impulse (ARFI) imaging has become an innovative ultrasound technique that has received increasing attention[7-12]. Studies have reported[13,14] that ARFI imaging could be used for the qualitative detection of liver tumors, and that it could also be used to determine the degree of liver fibrosis by detecting liver stiffness (LS). Hence, it is hoped that this technique could evaluate liver reserve function accurately. Furthermore, ARFI imaging technology is non-invasive, simple and repeatable[15,16]. However, there have been few reports on liver reserve function assessment using ARFI imaging. Therefore, this study aimed to explore the clinical value of ARFI imaging by determining its value in assessing liver reserve function in patients with liver tumors, and providing theoretical guidance for clinical treatment.
Seventy-six patients with liver tumors combined with liver fibrosis or cirrhosis, who were admitted from October 2012 to May 2014, were enrolled into this study. All patients planned to receive hepatectomy. Among them, 42 patients were male and 34 patients were female. The patient age range was 33-75 years old, and the average age was 56.71 ± 9.32. Inclusion criteria: single tumor with a tumor diameter < 7 cm. There was neither intrahepatic metastasis, nor portal vein thrombosis or ascites by imaging examination. All patients were informed of the study and agreed to participate.
Instruments and reagents: Instruments: (1) Siemens ACUSON S2000™ ultrasound system[17,18]; and (2) DDG-3300K liver reserve function analyzer (Nihon Kohden, Tokyo, Japan)[19-22]. Reagent: Indocyanine green (ICG) reagent (Shenyang Jishi Pharmaceutical Co., Ltd.).
Detection methods: (1) Conventional serum-based liver function tests were performed including alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum albumin (ALB), total bilirubin (T-Bil) and other indicators. All data were recorded; and (2) ARFI imaging detection.
Patients underwent fasting and were kept in a left lateral position while raising the right hand. Liver right lobe tissues were detected. Patients were instructed to hold their breath. The elasticity imaging sample frame was perpendicular to the liver surface and was located 2-5 cm below the surface, avoiding nearby blood vessels. The update button was pressed to launch a high-strength low-frequency pulse. Transversal shear wave velocity (Vs, m/s) was received and recorded.
Measurements were repeated 10 times, and the average value of the Vs results was used as the LS value.
ICG examination: Patients were kept in a supine position. The environment was kept quiet and other factors (such as cell phones, contrast, etc.) were excluded. (1) Body weight (kg) was measured and the amount of ICG (0.5 mg/kg) was calculated; (2) an indwelling needle was injected and placed through the median cubital vein on one side to collect 2 mL of blood and determine the hemoglobin (Hb) content; (3) preparation of ICG solution, sterile water for injection/ICG = 5 mL: 25 mg; (4) data such as Hb, weight and ICG dosage were analyzed by a DDG-3300K analyzer; (5) a sensitive probe was fixed to the patient’s nose, and the entire ICG solution was injected to the median cubital vein via the indwelling needle in 10 s; (6) data were saved, and ICG-k and ICG-R15 were calculated; and (7) Patients were kept in a quiet environment with normal respiration at all times.
CP score: CP scores were carried out based on patient’s preoperative biochemical tests and physical condition. CP scoring criteria are shown in Table 1.
| Indexes | Score | ||
| 1 score | 2 score | 3 score | |
| Hepatic encephalopathy (class) | Without | Mild | Occasional lethargy |
| Ascites | Without | Little/controllable by diuretics | A lot |
| T-Bil (μmol/L) | < 34 | 34-51 | > 51 |
| ALB (g/L) | > 35 | 28-35 | < 28 |
| Prolonged prothrombin time (s) | < 4 | 4-6 | > 6 |
Biochemical index such as ALT, AST, ALB and T-Bil were observed. The correlations among CP scores, ICG-R15, ICG-K and LS values were observed and analyzed. A receiver-operator characteristic (ROC) curve was used to analyze LS indicators and assess the best diagnostic threshold of hepatic functional reserve.
All data were analyzed using SPSS 19.0 software. Data were expressed as the mean ± SD. To test the relationship between two variables, the Pearson correlation coefficient was used to analyze two variables if the data showed a normal distribution; while the Spearman rank correlation coefficient was used to analyze two variables if the data showed a non-normal distribution. Kruskal-Wallis test was used to compare LS values of CP scores. LS values of non-surgical patients were expressed by sensitivity, specificity and the Youden index. A ROC curve was used to analyze liver reserve function assessment accuracy. P < 0.05 means that difference was statistically significant.
Seventy-six patients with liver tumors were successfully enrolled and assessed in this study. Serum biochemical indexes such as ALT, AST, γ-glutamyl transferase (γ-GT), ALB, T-Bil and prothrombin time (PT) are shown in Table 2.
| Indexes | mean ± SD | Media, n | Minimum value | Maximum value |
| ALT (U/L) | 58.77 ± 51.13 | 57.81 | 13.74 | 127.28 |
| AST (U/L) | 64.21 ± 60.33 | 60.03 | 15.62 | 153.10 |
| ALB (g/L) | 38.19 ± 10.14 | 37.31 | 10.01 | 67.43 |
| T-Bil (μmol/L) | 26.28 ± 20.61 | 22.33 | 4.72 | 126.74 |
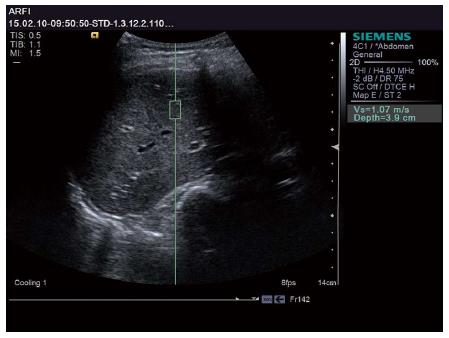
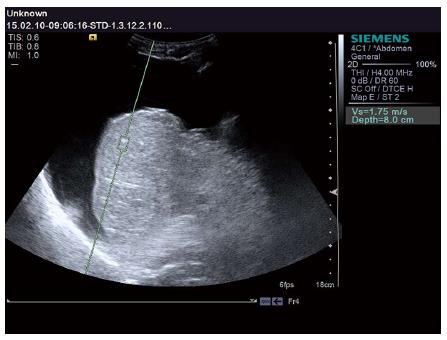
The LS values of all subjects were successfully obtained. Patients with different liver reserve functions revealed different Vs values. Representative examples are shown in Figures 1 and 2. The patient in Figure 1 had a normal liver reserve function. The Vs value was 1.07 m/s, ICG-R15 was 5.13%, and ICG-K was 0.24 L/min. The patient in Figure 2 had poor liver reserve function. The Vs value was 1.75 m/s, ICG-R15 was 16.83%, and ICG-K was 0.08 L/min.
Data from all subjects revealed that the average value of ICG-R15 was 8.13% ± 3.72% and the ICG-K average value was 0.24 ± 0.29 L/min. ICG-R15 values were divided into three groups: ICG-R15 < 10%, ICG-R15 10%-20% and ICG-R15 > 20% groups. The average LS value of the three groups is shown in Table 3. LS significantly differed among patients in the three groups (H = 25.89, P = 0.000). Kruskal-Wallis H test was further carried out. LS was significantly higher in the ICG-R15 10%-20% group than in the ICG-R15 < 10% group, and the difference was statistically significant (P < 0.01). LS was significantly higher in the ICG-R15 > 20% group than in the ICG-R15 < 10% group, and the difference was statistically significant (P < 0.01). LS was higher in the ICG-R15 >20% group than in the ICG-R15 10%-20% group, but the difference was not statistically significant (P > 0.05).
Results of different average LS, ICG-R15 and ICG-K values according to different CP scores are shown in Table 4. The average LS values were significantly different among patients with CP class A, B and C; and the difference was statistically significant (H = 33.84, P = 0.0000). Pairwise comparison results of LS among patients with CP class A, B and C showed that the LS value in patients with CP class A was lower than in patients with CP Class B; and the difference was statistically significant (P < 0.05). The LS value was lower in patients with CP class A than in patients with CP Class C, while the LS value was lower in patients with CP class B than in patients with CP class C; and the differences were statistically significant (P < 0.01).
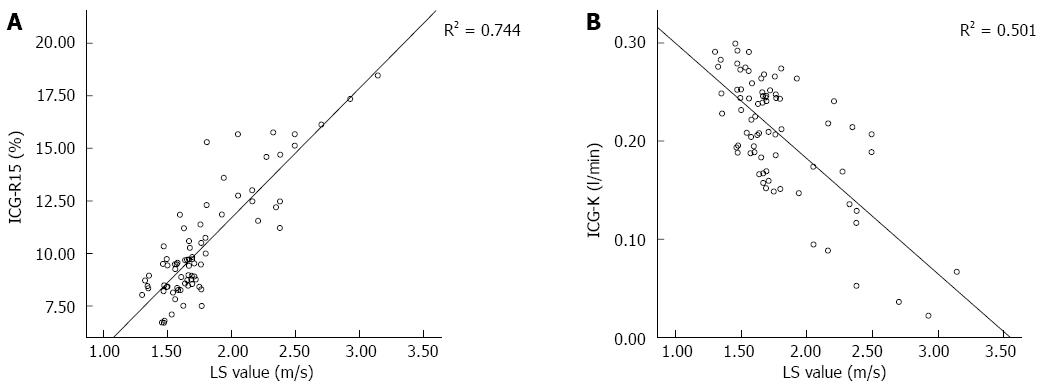
LS was positively correlated with ICG-R15 (r = 0.862, P < 0.01; Figure 3A) and CP scores (r = 0.772, P < 0.05). Meanwhile, LS was negatively correlated with ICG-K (r = -0.708, P < 0.05; Figure 3B). AST, ALT and T-Bil were positively correlated with LS, while ALB was negatively correlated with LS (P < 0.05) (Table 5, Figure 3).
| Index | r | P value |
| ICG-R15 | 0.862 | 0.000 |
| ICG-K | -0.708 | 0.000 |
| CP score | 0.772 | 0.000 |
| AST | 0.318 | 0.024 |
| ALT | 0.493 | 0.001 |
| ALB | -0.511 | 0.001 |
| T-Bil | 0.225 | 0.017 |
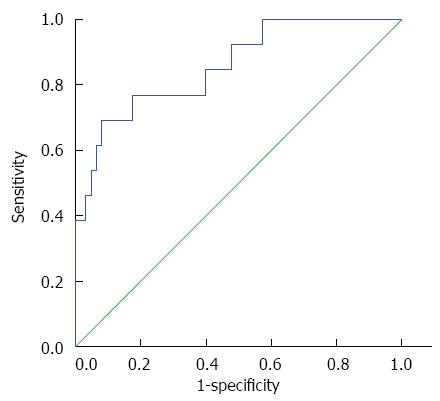
After liver reserve function assessment, 13 patients with poor liver reserve function received radiofrequency ablation (RFA) or interventional embolization, while 63 patients with normal liver reserve function received hepatectomy. The average LS of non-surgical patients was 2.61 ± 0.44 m/s, while the average LS of surgical patients was 1.57 ± 0.35 m/s. The average LS of the surgical patients was lower compared with non-surgical patients, and the difference was statistically significant (P < 0.01). The ROC curve is shown in Figure 4. When the LS value was 2.34 m/s, the Youden index was at its highest point, sensitivity was 69.2% and specificity was 92.1%.
Liver reserve function assessment is receiving increasingly attention from hepatobiliary surgeons. Preoperative evaluation of liver reserve function is very important for selecting a reasonable surgical method and a reasonable liver resection range, leading to a successful surgery and preventing postoperative hepatic failure[23]. CP scores are the main methods used to assess liver reserve function in clinical practice[24,25], and ICG excretion tests have become an important reference for surgical decision making[26]. The ICG test has been widely used to determine liver reserve function, which is an important indicator to assess surgical safety. However, ICG tests needs to be excreted through the biliary tract, which is not suitable for patients with obstructive jaundice. Hence, its application is restricted. The ARFI imaging technology applied in this study is not restricted. ARFI imaging is non-invasive, simple and repeatable, and has more extensive applications. Therefore, this study evaluated the clinical value of ARFI imaging to assess liver reserve function in patients with liver tumors, providing theoretical guidance for clinical treatment.
As liver fibrosis develops, LS increases, while liver compliance and elasticity decrease[27]. Lupsor et al[28] reported that there was a significant correlation between LS value and liver fibrosis stage. This proved that ARFI imaging could also be used to determine the degree of liver fibrosis by detecting LS, and to correctly evaluate liver reserve function. In this study, there was a strong positive correlation between LS and ICG-R15, and a negative correlation between LS and ICG-K. ICG-R15 values were divided into three groups: ICG-R15 < 10%, ICG-R15 10%-20% and ICG-R15 > 20% groups. LS significantly differed among patients in the three groups. LS was significantly higher in the ICG-R15 10%-20% group compared with the ICG-R15 < 10% and ICG-R15 > 20% groups. LS was significantly higher in the ICG-R15 > 20% group than in the ICG-R15 < 10% group. LS was higher in the ICG-R15 > 20% group than in the ICG-R15 10%-20% group, but there was no statistical significance. This implied that the LS value increases as ICG-R15 levels increase, resulting in reduced hepatic clearance and aggravated liver damage. This also demonstrated that ARFI imaging could be used to determine the degree of liver fibrosis by detecting LS, and to evaluate liver reserve function.
CP scores can reflect liver cell damage. The higher the score, the more severe the hepatic dysfunction. In this study, LS values for CP class A, B and C were 1.57 ± 0.34 m/s, 1.86 ± 0.27 m/s and 2.47 ± 0.33 m/s, respectively. Comparative results of LS values among the three CP classes revealed that: the LS value of CP class A was lower than CP class B, and the LS value of class A and B were significantly lower than CP class C. These results showed that LS values increase as CP scores increase. This outcome reflected severe liver function damage, causing LS values to increase, while the liver reserve function decreases. Therefore, this also demonstrated that LS values could reflect liver reserve function. CP scores could be used to evaluate liver reserve function based on routine liver function tests. CP scores are widely used in clinical practice because they are an easy method of detection. However, traditional biochemical indicators simply reflect the degree of liver cell damage and compensation function status; and the reserve capacity of the liver could not be accurately predicted when the body is exposed to trauma, infection, surgery and other invasions[29]. Therefore, ARFI imaging technology can supplement the CP scoring method; providing a more detailed liver reserve function assessment for predicting body endurance and permits a better choice of treatment modes.
Average LS values were lower in surgical patients compared with non-surgical patients. The ROC curve revealed that when the LS value was 2.34 m/s, the Youden index was at its highest point, sensitivity was 69.2% and specificity was 92.1%; which demonstrated that the higher the LS value, the worse the liver reserve function and prognosis after surgery. This also confirmed that LS values could react to liver reserve functions. Furthermore, an LS value greater than 2.34 m/s suggested poor liver reserve function; therefore, non-surgical treatment is recommended. Clinicians can use ARFI imaging technology to measure LS value and assess liver reserve function to determine if a patient is suitable for surgery; thereby, providing a more suitable treatment option for patients.
Although ARFI imaging is expected to provide fast, simple and accurate guidance in assessing liver reserve function in clinical practice, there are also limitations for the use of ARFI imaging. We found that ARFI imaging has high requirements from the patient. It requires patients to keep holding their breath during the course of the examination, which is difficult for elderly patients or patients in poor physical condition. The ARFI sampling frame would show deviations caused by a patient’s breathing problems, which would affect the accuracy of the measurement. To compensate for this limitation, we repeated the measurement 10 times to reduce errors. In addition, there were few subjects in this study. Seventy-six cases are not sufficient to demonstrate that LS > 2.34 m/s is the best demarcation point. Large multicenter studies are needed to obtain enough data for analysis and to provide convincing evidence.
In conclusion, ARFI could be used to determine liver reserve function by detecting LS. ARFI imaging has advantages, such as speed, simplicity, and non-invasiveness, and has a wide range of applications. It is important to determine a reasonable surgical method and liver resection range. ARFI imaging technology is a useful tool to assess liver reserve function in patients with liver tumors. It has an important clinical application and should be promoted.
Hepatocellular carcinoma (HCC) is one of the major malignant tumors in China. It has the third highest mortality after gastric carcinoma and lung cancer. Most patients with HCC have liver fibrosis and cirrhosis. Hepatectomy is the preferred treatment for early HCC. However, most HCC patients also have chronic liver diseases, such as cirrhosis, resulting in different degrees of liver injury and low liver reserve function. Poor prognosis, such as postoperative liver failure, usually occurs. Therefore, a good liver reserve function is a key factor for a successful operation, and preoperative liver reserve function assessment is of great importance.
In recent years, because of the development of ultrasound elastography in liver fibrosis and cirrhosis in clinic, acoustic radiation force impulse (ARFI) imaging has become an innovative ultrasound elastography that has received increasing attention. Studies have reported that ARFI imaging could be used for the qualitative detection of liver tumors, and could also be used to determine the degree of liver fibrosis by detecting liver stiffness (LS). Hence, it is hoped that this technique could evaluate liver reserve function accurately. Furthermore, ARFI imaging is non-invasive, simple and repeatable.
ARFI could be used to determine liver reserve function by detecting LS. ARFI imaging has advantages, such as speed, simplicity, non-invasiveness, and a wide range of applications. It is important to determine a reasonable surgical method and liver resection range. ARFI imaging technology is a useful tool to assess liver reserve function in patients with liver tumors. It has an important clinical application and should be promoted.
This study demonstrated that ARFI imaging could be used to assess liver reserve function by detecting LS, and to determine a more successful surgical option. The results showed that the higher the LS value, the worse the liver reserve function and prognosis after surgery. This also confirmed that LS values could react to liver reserve functions. Furthermore, an LS value greater than 2.34 m/s suggested poor liver reserve function; therefore, non-surgical treatment should be recommended.
This study demonstrated that ARFI imaging could be used to assess liver reserve function by detecting LS before surgery. This provides a means of determining a reasonable surgical method for patients with liver tumors. ARFI imaging has advantages. It is fast, simple, non-invasive and has a wide range of applications. Thus, ARFI imaging is a useful and valuable tool for clinical application and should be promoted.
P- Reviewer: Ciezki JP, Taylor ME S- Editor: Ma YJ L- Editor: Stewart G E- Editor: Zhang DN
| 1. | Ge PL, Du SD, Mao YL. Advances in preoperative assessment of liver function. Hepatobiliary Pancreat Dis Int. 2014;13:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Olivier AK, Gibson-Corley KN, Meyerholz DK. Animal models of gastrointestinal and liver diseases. Animal models of cystic fibrosis: gastrointestinal, pancreatic, and hepatobiliary disease and pathophysiology. Am J Physiol Gastrointest Liver Physiol. 2015;308:G459-G471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Liu F, Cai LY, Zhong L, Chen C, Xu F, Zhao ZX, Chen XM. Model for end-stage liver disease combined with serum prealbumin to predict the prognosis of patients with decompensated liver cirrhosis. J Dig Dis. 2010;11:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Maiwall R, Kumar S, Chaudhary AK, Maras J, Wani Z, Kumar C, Rastogi A, Bihari C, Vashisht C, Sarin SK. Serum ferritin predicts early mortality in patients with decompensated cirrhosis. J Hepatol. 2014;61:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Reekers M, Simon MJ, Boer F, Mooren RA, van Kleef JW, Dahan A, Vuyk J. Pulse dye densitometry and indocyanine green plasma disappearance in ASA physical status I-II patients. Anesth Analg. 2010;110:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Krenn CG, Krafft P, Schaefer B, Pokorny H, Schneider B, Pinsky MR, Steltzer H. Effects of positive end-expiratory pressure on hemodynamics and indocyanine green kinetics in patients after orthotopic liver transplantation. Crit Care Med. 2000;28:1760-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Potthoff A, Attia D, Pischke S, Kirschner J, Mederacke I, Wedemeyer H, Manns MP, Gebel MJ, Rifai K. Influence of different frequencies and insertion depths on the diagnostic accuracy of liver elastography by acoustic radiation force impulse imaging (ARFI). Eur J Radiol. 2013;82:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212-e219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 9. | Bota S, Sporea I, Sirli R, Popescu A, Danila M, Jurchis A, Gradinaru-Tascau O. Factors associated with the impossibility to obtain reliable liver stiffness measurements by means of Acoustic Radiation Force Impulse (ARFI) elastography--analysis of a cohort of 1,031 subjects. Eur J Radiol. 2014;83:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Chen SH, Li YF, Lai HC, Kao JT, Peng CY, Chuang PH, Su WP, Chiang IP. Effects of patient factors on noninvasive liver stiffness measurement using acoustic radiation force impulse elastography in patients with chronic hepatitis C. BMC Gastroenterol. 2012;12:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Litchfield IJ, Lilford RJ, Bentham LM, Greenfield SM. A qualitative exploration of the motives behind the decision to order a liver function test in primary care. Qual Prim Care. 2014;22:201-210. [PubMed] |
| 12. | Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, Gill PS, Neuberger JM, Lilford RJ, Newsome PN. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 13. | Frulio N, Laumonier H, Carteret T, Laurent C, Maire F, Balabaud C, Bioulac-Sage P, Trillaud H. Evaluation of liver tumors using acoustic radiation force impulse elastography and correlation with histologic data. J Ultrasound Med. 2013;32:121-130. [PubMed] |
| 14. | Gallotti A, D’Onofrio M, Romanini L, Cantisani V, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) ultrasound imaging of solid focal liver lesions. Eur J Radiol. 2012;81:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Stock KF, Klein BS, Cong MT, Regenbogen C, Kemmner S, Büttner M, Wagenpfeil S, Matevossian E, Renders L, Heemann U. ARFI-based tissue elasticity quantification and kidney graft dysfunction: first clinical experiences. Clin Hemorheol Microcirc. 2011;49:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Wang L, Xia P, Lv K, Han J, Dai Q, Li XM, Chen LM, Jiang YX. Assessment of renal tissue elasticity by acoustic radiation force impulse quantification with histopathological correlation: preliminary experience in chronic kidney disease. Eur Radiol. 2014;24:1694-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Clevert DA, Stock K, Klein B, Slotta-Huspenina J, Prantl L, Heemann U, Reiser M. Evaluation of Acoustic Radiation Force Impulse (ARFI) imaging and contrast-enhanced ultrasound in renal tumors of unknown etiology in comparison to histological findings. Clin Hemorheol Microcirc. 2009;43:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Goertz RS, Amann K, Heide R, Bernatik T, Neurath MF, Strobel D. An abdominal and thyroid status with Acoustic Radiation Force Impulse Elastometry--a feasibility study: Acoustic Radiation Force Impulse Elastometry of human organs. Eur J Radiol. 2011;80:e226-e230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Sheng QS, Lang R, He Q, Yang YJ, Zhao DF, Chen DZ. Indocyanine green clearance test and model for end-stage liver disease score of patients with liver cirrhosis. Hepatobiliary Pancreat Dis Int. 2009;8:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Gupta S, Chawla Y, Kaur J, Saxena R, Duseja A, Dhiman RK, Choudhary NS. Indocyanine green clearance test (using spectrophotometry) and its correlation with model for end stage liver disease (MELD) score in Indian patients with cirrhosis of liver. Trop Gastroenterol. 2012;33:129-134. [PubMed] |
| 21. | Yamazaki S, Takayama T. Reply to correlation between indocyanine green retention test and esophageal varices among patients with hepatocellular carcinoma. J Gastroenterol. 2014;49:956-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Lisotti A, Azzaroli F, Montagnani M, Porro A, Mazzella G. Correlation between Indocyanine green retention test and esophageal varices among patients with hepatocellular carcinoma. J Gastroenterol. 2014;49:954-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Pavlov CS, Casazza G, Nikolova D, Tsochatzis E, Burroughs AK, Ivashkin VT, Gluud C. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database Syst Rev. 2015;1:CD010542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 25. | Colombo S, Buonocore M, Del Poggio A, Jamoletti C, Elia S, Mattiello M, Zabbialini D, Del Poggio P. Head-to-head comparison of transient elastography (TE), real-time tissue elastography (RTE), and acoustic radiation force impulse (ARFI) imaging in the diagnosis of liver fibrosis. J Gastroenterol. 2012;47:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Sporea I, Sirli R, Bota S, Popescu A, Sendroiu M, Jurchis A. Comparative study concerning the value of acoustic radiation force impulse elastography (ARFI) in comparison with transient elastography (TE) for the assessment of liver fibrosis in patients with chronic hepatitis B and C. Ultrasound Med Biol. 2012;38:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Vergara S, Macías J, Rivero A, Gutiérrez-Valencia A, González-Serrano M, Merino D, Ríos MJ, García-García JA, Camacho A, López-Cortés L. The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis. 2007;45:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303-310. [PubMed] |
| 29. | Yamakado K, Miyayama S, Hirota S, Mizunuma K, Nakamura K, Inaba Y, Maeda H, Matsuo K, Nishida N, Aramaki T. Subgrouping of intermediate-stage (BCLC stage B) hepatocellular carcinoma based on tumor number and size and Child-Pugh grade correlated with prognosis after transarterial chemoembolization. Jpn J Radiol. 2014;32:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |