Published online Jan 21, 2015. doi: 10.3748/wjg.v21.i3.982
Peer-review started: July 16, 2014
First decision: August 15, 2014
Revised: August 22, 2014
Accepted: October 14, 2014
Article in press: October 15, 2014
Published online: January 21, 2015
Processing time: 188 Days and 23.1 Hours
AIM: To determine the laryngeal H+K+-ATPase and pharyngeal pH in patients with laryngopharyngeal reflux (LPR)-symptoms as well as to assess the symptom scores during PPI therapy.
METHODS: Endoscopy was performed to exclude neoplasia and to collect biopsies from the posterior cricoid area (immunohistochemistry and PCR analysis). Immunohistochemical staining was performed with monoclonal mouse antibodies against human H+K+-ATPase. Quantitative real-time RT-PCR for each of the H+K+-ATPase subunits was performed. The pH values were assessed in the aerosolized environment of the oropharynx (DxpH Catheter) and compared to a subsequently applied combined pH/MII measurement.
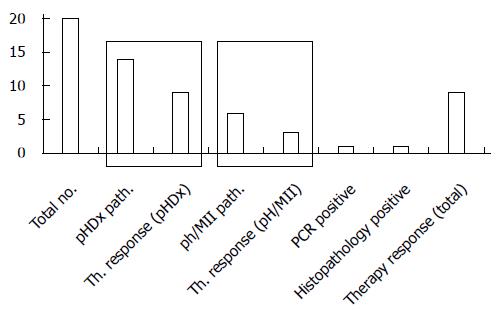
RESULTS: Twenty patients with LPR symptoms were included. In only one patient, the laryngeal H+K+-ATPase was verified by immunohistochemical staining. In another patient, real-time RT-PCR for each H+K+-ATPase subunit was positive. Fourteen out of twenty patients had pathological results in DxpH, and 6/20 patients had pathological results in pH/MII. Four patients had pathological results in both functional tests. Nine out of twenty patients responded to PPIs.
CONCLUSION: The laryngeal H+K+-ATPase can only be sporadically detected in patients with LPR symptoms and is unlikely to cause the LPR symptoms. Alternative hypotheses for the pathomechanism are needed. The role of pharyngeal pH-metry remains unclear and its use can only be recommended for patients in a research study setting.
Core tip: The pathophysiology and objective diagnosis of laryngopharyngeal reflux (LPR) is still unclear. The response to standard therapy (proton pump inhibitors) is poor. Laryngeal proton pumps (H+K+-ATPase) are often considered to be potential causes of LPR. The clinical significance of laryngeal proton pumps (H+K+-ATPase) is unclear. We present the first prospective series evaluating the laryngeal H+K+-ATPase, pharyngeal pH and symptom scores in patients with LPR symptoms. Laryngeal H+K+-ATPases can only be sporadically detected, and they are unlikely to cause LPR symptoms. The role of pharyngeal pH-metry remains unclear and its use can only be recommended for patients in the research study setting.
- Citation: Becker V, Drabner R, Graf S, Schlag C, Nennstiel S, Buchberger AM, Schmid RM, Saur D, Bajbouj M. New aspects in the pathomechanism and diagnosis of the laryngopharyngeal reflux-clinical impact of laryngeal proton pumps and pharyngeal pH metry in extraesophageal gastroesophageal reflux disease. World J Gastroenterol 2015; 21(3): 982-987
- URL: https://www.wjgnet.com/1007-9327/full/v21/i3/982.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i3.982
The incidence of laryngopharyngeal reflux (LPR) has dramatically grown in recent years[1]. LPR includes numerous clinically relevant symptoms, such as chronic cough, chronic globus sensations, hoarseness, asthma, sinusitis, subglottic stenosis, laryngospasm, and halitosis[2,3]. These symptoms remain a diagnostic and therapeutic challenge for the involved physicians.
The pathomechanism of LPR is still unclear. The most commonly discussed theory is that LPR symptoms are a result of direct alteration of the laryngeal mucosa by gastric fluids due to gastroesophageal reflux disease (GERD). Multichannel impedance monitoring in combination with pH-metry (pH/MII) and 2-channel pH-metry are safe and reliable tools to objectify gastroesophageal reflux events as source of LPR symptoms[4,5]. Based on the actual hypothesis of the LPR pathomechanism, standard therapy consists of high dose proton pump inhibitor therapy for up to 6 mo[6]. However, in randomized trials, there is insufficient evidence to conclude that treatment with PPIs is superior to placebo[7]. Nevertheless, there are data suggesting that LPR-patients might benefit from antireflux surgery[8]. However, the correlation between GERD, LPR symptoms and the response to PPI is comparatively poor, and an interventional antireflux therapy (e.g., Fundoplication) might harbor significant risks. In a recently published study, we were able to demonstrate that a pathological acidic environment in the oropharynx in LPR-patients was not correlated to objectified gastroesophageal reflux episodes[9]. These results were reconfirmed in another study with the same design[10]. Based on these data, the generally accepted pathomechanism of the direct alteration of the laryngeal epithelium by gastric contents, resulting in LPR-symptoms, needs to be reconsidered and alternative mechanisms should be discussed.
There are data supporting that LPR symptoms can also be associated with acid production[11] by laryngeal H+/K+-ATPase proton-pumps. H+K+-ATPase proton pumps were identified by immunohistochemistry in pathologic specimens of the larynx. Hence, local laryngeal acid production might be responsible for LPR symptoms because the laryngeal area is very sensitive to acid[10]. To objectively evaluate the laryngeal acid levels, selective pH values in the aerosolized environment can continuously be assessed with a pH measurement system. In this study, the pH-antimon probe is positioned in the oropharynx above the upper sphincter of the esophagus (DxpH, Restech, San Diego, United States). The special shape of the catheter keeps liquids out of the pH-sensor. Only the aerosol pH values are detected, and reference values are available.
The aim of this study was to correlate the laryngeal H+K+-ATPase expression, results of the pharyngeal pH metry, pH/MII and symptom response to PPI therapy, evaluating the laryngeal acid production as a potential alternative cause of LPR symptoms
Between June 2011 and December 2012, a total of 20 consecutive patients (male = 11; 40-78 years old) with oropharyngeal symptoms suspicious of atypical GERD were included. The study was approved by the Ethics Committee of the Technical University of Munich (Study Number 5024/11). All authors had access to the study data and reviewed and approved the final manuscript. Before study inclusion, PPIs had to be stopped for at least 14 d. Informed consent to participate in the study and evaluate the data was obtained from all patients. To exclude neoplasia or erosive reflux diseases, an upper endoscopic examination was performed under conscious sedation with propofol in accordance with German medical practice regulations[12]. Standard biopsies (Radial Jaw® 4 Biopsy Forceps, Boston Scientific) were collected from the post cricoid area during the same session under the direct supervision of an experienced otolaryngologist (SG) (two biopsies for PCR analysis and two biopsies for immunohistochemistry) (Figure 1).
The Dx-pH measurement was performed standardized as established previously[13-15]. Meals and body position were documented. Duration of measurement was minimum 22 h. Criteria for pathological results were Ryan Score > 9.4 in an upright position (pH < 5.5) or > 6.8 in a supine position (pH < 5.0)[13].
Combined pH/ MII monitoring (Tecnomatix ZAN S 61 C 01 E, Sandhill Scientific, Highlands Ranch, United States) was also performed as established previously[16,17]. Duration of measurement was minimum 22 h. Criteria for pathological results were pH level < 4 for more than 4% of the examination period and/or more than 73 mixed and/or fluid reflux events in impedance monitoring[17]. Both measurements (DxpH and pH/MII) were performed during the same time period.
RNA was isolated from tissue biopsies using the RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s advice. To remove genomic DNA, DNAse (RNase Free DNase Set Qiagen, Hilden, Germany) was used. Isolated RNA was transcribed reverse into complementary DNA (cDNA) with random hexamere primers as described previously[18]. Quantitative mRNA analysis was performed using real-time PCR with SYBR ®Green dye (APPLIED BIOSYSTEMS®, LIFE TECHNOLOGIES™, Darmstadt, Germany) and standard curves were generated as previously described[19,20]. As a housekeeping gene, Cyclophilin was used for normalization. The following primers were used for amplification of the α and β subunits of the human H+K+-ATPase: alpha subunit forward primer: CTTTGCCATCCAGGCTAGTGA and reverse primer: GGTGACGACAACCACAGCAAT; beta subunit forward primer: CCAGGTGGGTGTGGATCAG and reverse primer: GAGGCACAGGGCGAAGAG (http://www.eurofinsdna.com).
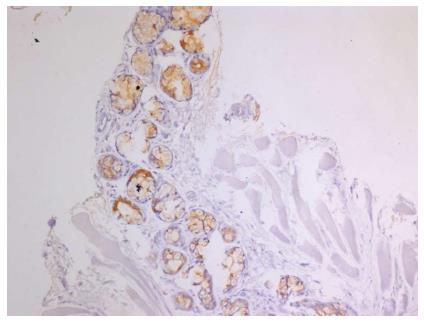
For histopathological analysis (Figure 2), tissue was fixed in 4% buffered formalin. After embedment in paraffin, tissue was sectioned (2.5 μm thick) and stained with hematoxylin and eosin as previously described[21]. For immunodetection, formalin-fixed paraffin-embedded tissue sections were deparaffinized in Histo-Clear (Roti®-Histol, Carl Roth GmbH, Karlsruhe, Germany) and ethanol. To recover antigens, sections were incubated in antigen unmasking solution (pH = 9, Vector Laboratories, Burlingame, CA) and placed in a microwave for 15 minutes at 360 watts. The following primary antibodies were used for immunostaining: Anti-Proton pump/H+K+-ATPase α subunit (1:285; D031-3, Clone 1H9) and H+K+-ATPase β subunit (1:285; D032-3, Clone 1B6; both from MBL® international corporation, Woburn, MA). Following primary antibodies, samples were treated with secondary antibodies conjugated to biotin (Vector Laboratories). Peroxidase conjugated streptavidin and 3,3’-diaminobenzidine tetrahydrochloride (DAB, Sigma-Aldrich, Munich, Germany) were used as a chromogen for detection as previously described[22]. Sections were counterstained with hematoxylin. As positive controls, biopsies from human corpus mucosa were used.
No complications or technical problems were documented during all procedures. Upper endoscopic examination did not reveal any relevant pathology, such as neoplasia or severe erosive esophagitis.
Twenty patients with LPR symptoms were included (Table 1). Fourteen out of twenty patients had pathological results in DxpH; 6/20 patients had pathological results in pH/MII. Four patients had pathological results in both functional tests. In one patient, laryngeal H+K+-ATPase expression was verified by immunohistochemical staining. In this patient, DxpH and pH/MII showed pathological results, and the PPI response was reported. In another patient, real-time RT-PCR for each of the H+K+-ATPase subunits was positive. Pathological results were assessed with DxpH; there were regular results in the pH/MII measurements and the PPI response was noted as positive (Figure 3).
| No | Gender | Age | pHDx | Rayn | Rayn | Impedance | deMeester | Epithelium /Glands | Immuno-histo | PCR | Therapy response | Symptom-score |
| + path | > 9.4 | > 6.8 | + path | Score | + pos | + pos | + pos | + pos | before/after therapy | |||
| upright position | supine position | < 22.0 | ||||||||||
| 1 | M | 74 | + | 23.92 | 2.17 | + | 29.9 | - | - | - | + | 7/4 |
| 2 | F | 49 | + | 17.84 | 2.17 | - | 7.2 | - | - | - | - | 6/6 |
| 3 | M | 78 | + | 20.88 | 11.31 | + | 38.2 | - | - | - | - | 7/6 |
| 4 | M | 57 | - | 4.04 | 2.17 | - | 7.1 | - | - | - | - | 6/5 |
| 5 | M | 46 | + | 19.35 | 2.17 | - | 3.7 | - | - | - | + | 6/3 |
| 6 | F | 59 | + | 115.85 | 7.99 | + | 25.4 | + | + | - | + | 8/5 |
| 7 | M | 53 | - | 2.12 | 2.17 | - | 1.5 | - | - | - | - | 8/8 |
| 8 | F | 40 | - | 2.12 | 2.17 | - | 11.0 | - | - | - | - | 5/3 |
| 9 | M | 52 | + | 53.3 | 2.17 | + | 24.1 | - | - | - | + | 5/1 |
| 10 | M | 46 | + | 52.39 | 2.17 | - | 0.9 | - | - | - | - | 6/5 |
| 11 | F | 62 | + | 2.12 | 7.57 | - | 3.2 | - | - | + | + | 8/5 |
| 12 | F | 77 | + | 37.01 | 2.17 | - | 14.2 | - | - | - | - | 7/7 |
| 13 | F | 63 | - | 2.12 | 2.17 | - | 7.2 | - | - | - | - | 8/8 |
| 14 | M | 51 | + | 124.43 | 9.26 | - | 7.1 | - | - | - | - | 4/4 |
| 15 | F | 64 | + | 201.59 | 2.17 | - | 9.4 | - | - | - | + | 6/3 |
| 16 | F | 75 | + | 38.98 | 9.33 | - | 1.3 | - | - | - | + | 7/4 |
| 17 | M | 55 | - | 2.12 | 2.17 | + | 37.0 | - | - | - | - | 6/6 |
| 18 | M | 53 | + | 23.23 | 2.17 | - | 0.9 | - | - | - | + | 8/4 |
| 19 | F | 75 | + | 41.12 | 2.17 | - | 12.8 | - | - | - | + | 8/5 |
| 20 | M | 65 | - | 4.15 | 2.17 | + | 28.8 | - | - | - | - | 7/6 |
Symptom relief (at least reduction of three points on a ten point scale) with PPI was reported in 9 of 20 patients. In patients with pathological DxpH, 9 of 14 patients reported significant symptom relief. Seventy percent of the patients had pathological results in DxpH, and only 30% of the patients were pathological in pH/MII.
The primary aim of the study was to correlate the laryngeal H+K+-ATPase expression, results of the pharyngeal pH-metry, pH/MII and symptom response to PPI therapy and evaluate laryngeal acid production as a potential alternative pathomechanism for LPR symptoms.
Since the identification of laryngeal H+K+-ATPase proton pumps in pathologic specimens by immunohistochemistry, the clinical significance of laryngeal H+K+-ATPase proton pumps has been controversial[11]. We verified a pathologic, acidic environment in the oropharynx in most of the examined LPR-patients without any correlation with the objectified gastroesophageal reflux episodes[9]. This finding supports the theory of laryngeal acid production. However, the pharyngeal acid levels were only documented with the Dx-pH system. As reported in previous trials, Dx-pH more often detects pathological acid pH levels than the standard pH/MII. On the other hand, the pharyngeal system regularly misses proximal pH exposure that is documented in regular pH/MII[9,10]. Hence, the clinical significance of this Dx-pH system is not clear. In the current study, Dx-pH revealed pathological values in 70% of the patients, whereas 30% of the patients had pathological values in pH/MII.
As mentioned previously, the Dx-pH system potentially detects a high number of false-positive patients; as a result, the pH/MII is the gold standard. However, the evidence of involvement of laryngeal H+K+-ATPase proton pumps is much higher in patients with LPR symptoms, which might explain the pathological acid environment of the oropharynx in patients without gastroesophageal reflux. The lack of detection of H+K+-ATPase could be explained in different ways. First, H+K+-ATPases are only located in the seromucinous glands of the human larynx and not in the squamous epithelium. Due to endoscopic sampling error with random biopsies during standard endoscopy, submucosal glands with H+K+-ATPase proton pumps can easily be missed, which was previously described in Barrett’s esophagus[23]. In our study, the submucosal glands were only detected in one patient. In this patient, the immunohistochemistry was positive. Second, the idea of an “activated” or “inducible” state of the proton pumps needs to be discussed[24]. Inflammation, infection or gastroesophageal reflux might “activate” H+K+-ATPase proton pumps, resulting in increased proton secretion[24,25]. Determining whether both laryngeal H+K+-ATPase proton pumps and gastric H+K+-ATPase proton pumps respond to PPI therapy might be of clinical significance. As confirmed by the Altman group, the α and β-subunits of the H+K+-ATPase proton pumps have identical components as those found in the stomach[11]. Therefore, PPI therapy should be an effective therapy for LPR patients. However, the concentration of the laryngeal H+K+-ATPase proton pumps is much lower than the concentration in the stomach[26]. Compared to the distal esophagus, relatively low acid levels might result in relevant symptoms because the larynx area is extremely sensitive to pH alterations[10]. This might explain the need for high dose, long-term PPI therapy in LPR patients to terminate proton secretion and resolve symptoms.
However, the detection of only one patient with histopathological evidence of H+K+-ATPase and one patient with positive PCR challenges the theory that laryngeal H+K+-ATPase proton pumps cause LPR symptoms. We conclude that laryngeal H+K+-ATPase proton pumps lack clinical relevance in patients with LPR symptoms. Alternative pathomechanisms must be discussed, including that LPR patients can have a pathologically acidic environment in the oropharynx without being correlated to the number of gastroesophageal reflux episodes. Still, it is important to note that this is a feasibility study with limitations, including the small number of patients and single-center setting. Both patients with detectable laryngeal H+K+ATPase proton pumps responded to PPI therapy, and nine of fourteen (64%) Dx-pH positive patients responded to therapy, which is a remarkable number of patients with LPR symptoms.
As previously mentioned, the results and clinical significance of pharyngeal pH testing are controversial[27]. Recently, a study group of a retrospective chart review reported that patients with atypical reflux symptoms have better symptom relief after surgical antireflux procedures in the group with pathological pharyngeal pH levels than the study group with pathological result esophageal pH levels. The median follow up was 18 mo[28]. However, the study has several limitations. Symptom relief was only judged as a symptomatic parameter, and no objective data with the Dx-pH system were analyzed. Objective data would have been very interesting for the pathomechanism of LPR. Furthermore, only patients with previously performed esophageal and pharyngeal pH testing were included (retrospectively), which leads to a relevant patient selection. Hence, the impact of the study results is unclear. To objectively evaluate gastroesophageal reflux episodes leading to LPR symptoms, pH/MII is the most accurate diagnostic instrument[4]. However, simultaneous measurements of combined pH/MII and Dx-pH were not correlated[10]. Furthermore, the pharyngeal probe missed almost 90% of all proximal reflux episodes detected by MII, but the data are reproducible[9]. Therefore, it is unclear what the pharyngeal pH probe is actually measuring. Based on these data, the results of pharyngeal pH metry should not be used to establish the diagnosis of laryngopharyngeal reflux or to guide therapy, including surgical anti-reflux procedures.
In conclusion, laryngeal H+K+-ATPases can only be sporadically detected in patients with LPR symptoms, and they are unlikely to cause LPR symptoms. Alternative pathomechanisms must be discussed. The role of pharyngeal pH-metry remains unclear, and its use can only be recommended for patients in the research study setting.
The pathophysiology and objective diagnosis of laryngopharyngeal reflux (LPR) is still unclear. Correlation between gastroesophageal reflux disease (GERD), LPR-symptoms and the response to proton pump inhibitors (PPI) is poor. Recently, laryngeal proton pumps (H+K+-ATPases) were identified and linked as potential causative agents for LPR. However, their clinical significance is unclear. Pharyngeal pH-metry was introduced for use in clinical procedures even though its role in the diagnosis of atypical GERD has questionable impact.
The study aim was to evaluate laryngeal H+K+-ATPases and pharyngeal pH in patients with LPR-symptoms as well as to assess the symptom scores and PPI therapy response.
Previous studies have identified laryngeal H+K+-ATPase proton pumps in pathologic specimens by immunohistochemistry. Furthermore, the acidic environment in the oropharynx can be verified without gastroesophageal reflux episodes. Therefore, acid production in the laryngeal epithelium is increasingly discussed. This is the first study to correlate pharyngeal pH metry results with laryngeal H+K+-ATPase proton pumps, subjective evaluation and therapy response.
For patients with LPR symptoms, a positive correlation and therapeutic effect of PPI therapy might offer a new diagnostic and therapeutic approach.
LPR has dramatically grown in recent years and includes numerous clinically relevant symptoms such as chronic cough, globus sensations in the throat, asthma, chronic sinusitis, subglottic stenosis, laryngospasm, halitosis, hoarseness and dysphonia.
This paper is a well written study about new aspects of LPR. The study is well designed and the diverse results are well discussed.
P- Reviewer: Dormann AJ, Pehl C S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Hicks DM, Ours TM, Abelson TI, Vaezi MF, Richter JE. The prevalence of hypopharynx findings associated with gastroesophageal reflux in normal volunteers. J Voice. 2002;16:564-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA. 2005;294:1534-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 332] [Article Influence: 16.6] [Reference Citation Analysis (35)] |
| 3. | Rohof WO, Hirsch DP, Boeckxstaens GE. Pathophysiology and management of gastroesophageal reflux disease. Minerva Gastroenterol Dietol. 2009;55:289-300. [PubMed] |
| 4. | Bredenoord AJ. Impedance-pH monitoring: new standard for measuring gastro-oesophageal reflux. Neurogastroenterol Motil. 2008;20:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Bajbouj M, Becker V, Neuber M, Schmid RM, Meining A. Combined pH-metry/impedance monitoring increases the diagnostic yield in patients with atypical gastroesophageal reflux symptoms. Digestion. 2007;76:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Dore MP, Pedroni A, Pes GM, Maragkoudakis E, Tadeu V, Pirina P, Realdi G, Delitala G, Malaty HM. Effect of antisecretory therapy on atypical symptoms in gastroesophageal reflux disease. Dig Dis Sci. 2007;52:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (2)] |
| 7. | Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev. 2011;CD004823. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Koch OO, Antoniou SA, Kaindlstorfer A, Asche KU, Granderath FA, Pointner R. Effectiveness of laparoscopic total and partial fundoplication on extraesophageal manifestations of gastroesophageal reflux disease: a randomized study. Surg Laparosc Endosc Percutan Tech. 2012;22:387-391. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Becker V, Graf S, Schlag C, Schuster T, Feussner H, Schmid RM, Bajbouj M. First agreement analysis and day-to-day comparison of pharyngeal pH monitoring with pH/impedance monitoring in patients with suspected laryngopharyngeal reflux. J Gastrointest Surg. 2012;16:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Ummarino D, Vandermeulen L, Roosens B, Urbain D, Hauser B, Vandenplas Y. Gastroesophageal reflux evaluation in patients affected by chronic cough: Restech versus multichannel intraluminal impedance/pH metry. Laryngoscope. 2013;123:980-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Altman KW, Haines GK 3rd, Hammer ND, Radosevich JA. The H /K -ATPase (proton) pump is expressed in human laryngeal submucosal glands. Laryngoscope. 2003;113:1927. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Riphaus A, Wehrmann T, Weber B, Arnold J, Beilenhoff U, Bitter H, von Delius S, Domagk D, Ehlers AF, Faiss S. [S3-guidelines--sedation in gastrointestinal endoscopy]. Z Gastroenterol. 2008;46:1298-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Ayazi S, Hagen JA, Zehetner J, Oezcelik A, Abate E, Kohn GP, Sohn HJ, Lipham JC, Demeester SR, Demeester TR. Proximal esophageal pH monitoring: improved definition of normal values and determination of a composite pH score. J Am Coll Surg. 2010;210:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Ayazi S, Lipham JC, Hagen JA, Tang AL, Zehetner J, Leers JM, Oezcelik A, Abate E, Banki F, DeMeester SR. A new technique for measurement of pharyngeal pH: normal values and discriminating pH threshold. J Gastrointest Surg. 2009;13:1422-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Sifrim D, Holloway R, Silny J, Xin Z, Tack J, Lerut A, Janssens J. Acid, nonacid, and gas reflux in patients with gastroesophageal reflux disease during ambulatory 24-hour pH-impedance recordings. Gastroenterology. 2001;120:1588-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 270] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Becker V, Bajbouj M, Waller K, Schmid RM, Meining A. Clinical trial: persistent gastro-oesophageal reflux symptoms despite standard therapy with proton pump inhibitors - a follow-up study of intraluminal-impedance guided therapy. Aliment Pharmacol Ther. 2007;26:1355-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Shay S, Tutuian R, Sifrim D, Vela M, Wise J, Balaji N, Zhang X, Adhami T, Murray J, Peters J. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 383] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 18. | Saur D, Paehge H, Schusdziarra V, Allescher HD. Distinct expression of splice variants of neuronal nitric oxide synthase in the human gastrointestinal tract. Gastroenterology. 2000;118:849-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Saur D, Seidler B, Schneider G, Algül H, Beck R, Senekowitsch-Schmidtke R, Schwaiger M, Schmid RM. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology. 2005;129:1237-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | von Burstin J, Eser S, Paul MC, Seidler B, Brandl M, Messer M, von Werder A, Schmidt A, Mages J, Pagel P. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology. 2009;137, 361-371, 371 e361-e365. |
| 21. | Seidler B, Schmidt A, Mayr U, Nakhai H, Schmid RM, Schneider G, Saur D. A Cre-loxP-based mouse model for conditional somatic gene expression and knockdown in vivo by using avian retroviral vectors. Proc Natl Acad Sci USA. 2008;105:10137-10142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Eser S, Messer M, Eser P, von Werder A, Seidler B, Bajbouj M, Vogelmann R, Meining A, von Burstin J, Algül H. In vivo diagnosis of murine pancreatic intraepithelial neoplasia and early-stage pancreatic cancer by molecular imaging. Proc Natl Acad Sci USA. 2011;108:9945-9950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Gatenby PA, Ramus JR, Caygill CP, Shepherd NA, Watson A. Relevance of the detection of intestinal metaplasia in non-dysplastic columnar-lined oesophagus. Scand J Gastroenterol. 2008;43:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Roussa E, Thévenod F, Sabolic I, Herak-Kramberger CM, Nastainczyk W, Bock R, Schulz I. Immunolocalization of vacuolar-type H+-ATPase in rat submandibular gland and adaptive changes induced by acid-base disturbances. J Histochem Cytochem. 1998;46:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Layden TJ, Agnone LM, Schmidt LN, Hakim B, Goldstein JL. Rabbit esophageal cells possess an Na+,H+ antiport. Gastroenterology. 1990;99:909-917. [PubMed] |
| 26. | Herrmann M, Selige J, Raffael S, Sachs G, Brambilla A, Klein T. Systematic expression profiling of the gastric H+/K+ ATPase in human tissue. Scand J Gastroenterol. 2007;42:1275-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Merati AL, Lim HJ, Ulualp SO, Toohill RJ. Meta-analysis of upper probe measurements in normal subjects and patients with laryngopharyngeal reflux. Ann Otol Rhinol Laryngol. 2005;114:177-182. [PubMed] |
| 28. | Worrell SG, DeMeester SR, Greene CL, Oh DS, Hagen JA. Pharyngeal pH monitoring better predicts a successful outcome for extraesophageal reflux symptoms after antireflux surgery. Surg Endosc. 2013;27:4113-4118. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |