Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8653
Peer-review started: January 30, 2015
First decision: March 10, 2015
Revised: March 26, 2015
Accepted: May 2, 2015
Article in press: May 4, 2015
Published online: July 28, 2015
Processing time: 181 Days and 13.4 Hours
AIM: To investigate the virological relapse rate in hepatitis B e antigen (HBeAg)-negative patients after antiviral therapy discontinuation and analyze the factors associated with virological relapse.
METHODS: Among patients diagnosed with chronic hepatitis B infection between May 2005 and July 2010, 204 were eligible for analysis. The Kaplan-Meier method and log-rank test were used to calculate the cumulative rate of relapse and compare cumulative relapse rates between groups. The Cox proportional hazards regression model was used to evaluate the predictive factor of virological relapse.
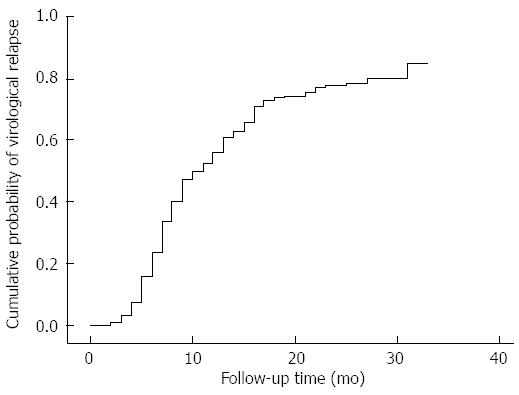
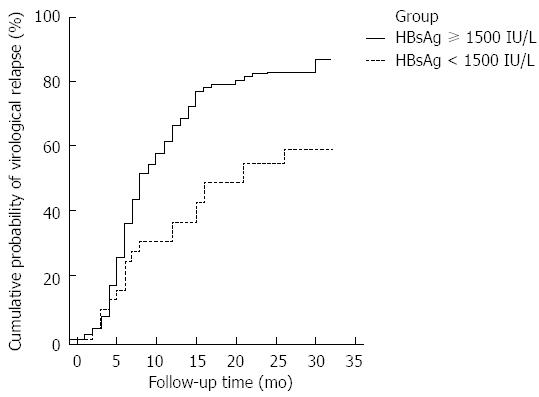
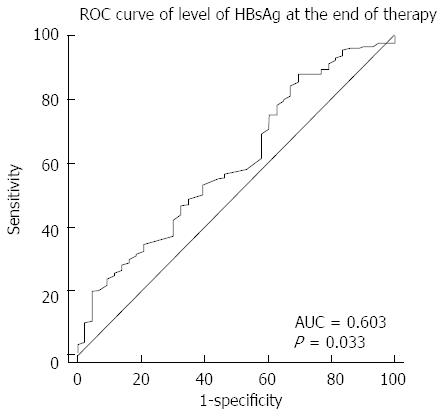
RESULTS: The 2 and 1 year cumulative risks of virological relapse after antiviral therapy discontinuation were 79.41% (162/204) and 43.82% (71/162), respectively. Multivariate analysis revealed that only post treatment hepatitis B surface antigen (HBsAg) level was associated with virological relapse (P = 0.011). The cumulative risk of virological relapse was higher in the patients with HBsAg levels ≥ 1500 IU/L than in those with HBsAg levels < 1500 IU/L (P = 0.0013). The area under the curve was 0.603 (P = 0.033). The cutoff HBsAg value for predicting virological relapse was 1443 IU/L.
CONCLUSION: We found that the virological relapse rate remained high after antiviral therapy discontinuation in the HBeAg-negative patients and that the post treatment HBsAg levels predicted virological relapse.
Core tip: To study the rate of virological relapse in hepatitis B e antigen (HBeAg)-negative patients after stopping antiviral therapy. Two hundred and four patients were eligible for this analysis. The cumulative probability of virological relapse after stopping antiviral therapy was 79.41% (162/204) in 2 years and 43.82% (71/162) in 1 year. Cumulative probability of virological relapse in levels of the hepatitis B surface antigen (HBsAg) ≥ 1500IU/L group was higher than that of levels of the HBsAg < 1500 IU/L group. The cutoff value for predicting virological relapse was 1443 IU/L. We found that the virological relapse rate maintained a high level after stopping NUCs in HBeAg-negative patients and HBsAg levels of cessation of nucleos(t)ide analogs predicted virological relapse.
- Citation: Ge GH, Ye Y, Zhou XB, Chen L, He C, Wen DF, Tan YW. Hepatitis B surface antigen levels of cessation of nucleos(t)ide analogs associated with virological relapse in hepatitis B surface antigen-negative chronic hepatitis B patients. World J Gastroenterol 2015; 21(28): 8653-8659
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8653.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8653
Hepatitis B virus (HBV) infection is a global public health problem. About 350 million people worldwide are chronic carriers of HBV and have a 15% to 25% mortality risk from HBV-related liver disease[1]. In China, a seroepidemiological survey of HBV infection cases in 2006 showed that the prevalence of hepatitis B surface antigen (HBsAg) positivity was 7.18%. The estimated number of HBV carriers was 93 million, of whom 30 million had chronic hepatitis B (CHB) infection. CHB may progress to cirrhosis and hepatocellular carcinoma[2].
The natural history of CHB infection is a dynamic process and is divided into five phases, according to the European Association for the Study of the Liver Clinical Practice Guidelines (2012)[1]. In the hepatitis B e antigen (HBeAg)-negative phases, it is characterized by recurrent fluctuations in HBV DNA expression level and repeatedly elevated levels of aminotransferases. Patients with this type of CHB have a higher risk of liver fibrosis, cirrhosis and liver cancer[3,4]. The treatment goal in HBeAg-negative patients is sustained suppression of HBV replication[5]. The ideal end point is sustained loss of HBsAg expression after therapy and a satisfactory end point is sustained virological and biochemical responses after therapy[1]. However, the ideal end point is difficult to achieve. A 5 year antiviral therapy with nucleos(t)ide reportedly resulted in a reduced HBsAg expression level usually lower than 5% in HBeAg-negative patients[6,7].
A survey showed that among 124 gastroenterologists and liver disease experts from 12 Asia-Pacific countries, 60% would recommend that patients discontinue treatment according to the Asia-Pacific Guide[8] owing to the high cost of antiviral drugs. Moreover, approximately 25% to 50% of hepatitis cases relapse after antiviral therapy discontinuation, even if it were according to the recommendation of the Asian Pacific Association for the Study of the Liver (APASL)[9-11].
In this study, we aimed to investigate the virological relapse rate in HBeAg-negative patients after antiviral therapy discontinuation and analyze the factors associated with virological relapse.
This was a retrospective study with CHB patients diagnosed between January 2005 and June 2010 at the Liver Clinic and Department of Hepatosis, The Third Hospital of Zhenjiang Affiliated Jiangsu University. The patients were followed up at least every 3 to 6 mo, or more often if clinically indicated. At each visit, liver biochemical and HBV serological tests were performed, including those for HBsAg, HBeAg, anti-HBe, HBV DNA and genotypes. The inclusion criteria were as follows: (1) HBsAg positivity ≥ 6 mo; (2) HBeAg negativity; (3) HBV DNA load ≥ 104 copies/mL; (4) time of abnormal alanine aminotransferase (ALT) ≥ 6 mo; and (5) good treatment compliance. The exclusion criteria were as follows[12]: (1) other hepatitis virus infections such as hepatitis A, C, D or E infection or human immunodeficiency virus coinfection; (2) evidence of liver disease with other etiologies such as use of hepatotoxic drugs or regular alcohol consumption; (3) pregnancy or lactation; and (4) complication of variceal bleeding, ascites and encephalopathy.
Patients were recruited from May 2005 to July 2010 after the last patient follow-up. During the study period, 424 consecutive CHB patients who were HBeAg-negative discontinued outpatient antiviral therapy. After excluding 107 patients who did not meet the APASL treatment cessation criteria, 78 who were lost to follow-up, and 35 who were followed up for < 24 mo without relapse, 204 patients were eligible for this analysis. The CHB patients were treated with adefovir (ADV) at 10 mg/d, entecavir (ETV) at 0.5 mg/d, lamivudine (LAM) at 100 mg/d, telbivudine (LDT) at 600 mg/d, and LAM100 mg/d plus ADV at 10 mg/d. The types of antivirals used were selected according to the economic condition of the patients and the advice of the physicians. The baseline follow-up of the treatment-naive patients was conducted at the start of antiviral therapy. All of the patients who had regular follow-up with ALT, HBV serological and HBV DNA tests were monitored every 3 mo. Only patients with good compliance to medication and follow-up were analyzed in this study.
The criterion for antiviral therapy discontinuation recommended by the 2008 APASL[13] was undetectable HBV DNA expression on 3 separate occasions 6 mo apart. After antiviral therapy discontinuation, HBV DNA, HBV serological and ALT tests were performed every month within the first 6 mo and then every 1-3 mo thereafter. All of the patients were followed up for 24 mo. Virological relapse was defined as a serum HBV DNA level > 1000 copies/mL. Sustained response (no virological relapse) was defined as undetectable HBV DNA expression (< 1000 copies/mL) after antiviral therapy discontinuation for 24 mo. Finally, 204 patients were recruited in the study, of whom 162 had and 42 had no virological relapse until the last follow-up visit.
HBV DNA was measured by performing a real-time polymerase chain reaction (PCR) assay (Shanghai Kehua Bioengineering Co., Ltd, Shanghai, China) with a lower limit of quantification at 1000 copies/mL. Serum HBsAg, HBeAg and anti-HBe levels were measured by time-resolved fluorescence immunoassay kits (Sum-Bio Lifescience, Shanghai, China). The post treatment HBsAg level was quantified by using the Architect chemiluminescent microparticle immunoassay (Abbott Laboratories, Abbott Park, IL, United States), at a sensitivity ranging from 0.05 to 250 IU/mL. Samples with HBsAg titers higher than 250 IU/mL were retested after dilution to 1:500-1000 to bring the reading to the range of the calibration curve.
Data were expressed as mean ± SD or median. The demographic data and clinical features of CHB patients were compared by using χ2 tests for categorical variables. Independent sample t test with analysis of variance was used for group comparisons of parametric quantitative data. The cumulative relapse rate was calculated by using the Kaplan-Meier method in order to compare the cumulative relapse rate between the groups by using the log-rank test. The Cox proportional hazards regression model was used to evaluate the relevant factors of virological relapse among various variables, including age, sex, history of vertical transmission of infection, time to undetectable HBV DNA expression, sustained time of antiviral therapy, drugs used, post treatment HBsAg level and genotype. These statistical analyses were conducted by using the Statistical Package for the Social Sciences (SPSS) version 21.0 software (SPSS Inc., Chicago, IL, United States). Receiver operating characteristic curve (ROC curve) was used to calculate the cutoff value of the factor of virological relapse. The ROC analysis was performed by using the software 21 MedCalc (Version 10.4.7.0; MedCalc, Mariakerke, Belgium). All P values were two-sided.
Of the 204 patients, 162 had a virological relapse at 8.99 ± 9.38 mo (range, 1-20 mo) after therapy cessation. The 2 and 1 year cumulative risks of virological relapse after antiviral therapy discontinuation were 79.41% (162/204) and 43.82% (71/162), respectively (Figure 1). Among the patients, 5 showed reappearance of HBeAg, 26 had elevated ALT levels to more than twice the upper limit of normal (235.2 ± 139.1 IU/L), and 1 patient developed liver failure. In 1 of the 42 sustained responders, the HBsAg expression disappeared. The 42 patients were followed up for 25.71 ± 2.17 mo (range, 24-36 mo). The characteristics of CHB patients with and of those without virological relapse are shown in Table 1.
| Factors | Virological relapse (n = 162) | No virological relapse (n = 42) | P value |
| Age (yr) | 36.83 ± 10.51 | 35.19 ± 9.10 | 0.357 |
| Gender | |||
| Male | 95 (58.64) | 26 (61.9) | 0.332 |
| Female | 67 (41.36) | 16 (38.1) | |
| Vertical transmission of infection history | |||
| Yes | 80 (49.38) | 21 (50) | 0.936 |
| No | 82 (50.62) | 21 (50) | |
| Time to undetectable HBV DNA | 5.01 ± 2.11 | 4.93 ± 2.96 | 0.819 |
| Sustained time of antiviral | 44.62 ± 8.17 | 41.21 ± 6.20 | 0.110 |
| Drugs used | |||
| Lamivudine | 29 (17.9) | 10 (23.81) | 0.736 |
| Adefovir | 36 (22.22) | 11 (26.19) | |
| Telbivudine | 25 (12.35) | 4 (9.52) | |
| Entecavir | 44 (27.16) | 10 (23.81) | |
| Lamivudine + Adefovir | 28 (17.28) | 7 (16.67) | |
| Follow-up time of cessation, mo | 8.99 ± 9.38 | 25.71 ± 2.17 | < 0.0001 |
| Level of HBsAg at the end of therapy (IU/L) | 4334.179 ± 2493.99 | 3290.163 ± 2019.79 | 0.013 |
| Genotype | |||
| B | 54 (33.33) | 12 (28.57) | 0.557 |
| C | 108 (66.67) | 30 (71.43) |
The univariate Cox regression analysis revealed that sustained time of antiviral therapy and post treatment HBsAg level were associated with virological relapse in the HBeAg-negative CHB patients. Meanwhile, the stepwise multivariate analysis revealed that only post treatment HBsAg level was associated with virological relapse (RR = 1.971; 95%CI: 1.172-3.316; P = 0.011; Table 2).
| Factors | Univariate analysis | Multivariate analysis1 | ||||||
| RR | Wald | 95%CI | P value | RR | Wald | 95%CI | P value | |
| Age | 0.996 | 0.285 | 0.981-1.011 | 0.592 | 0.996 | 0.231 | 0.980-1.012 | 0.996 |
| Gender | ||||||||
| Male | 1 | 1 | ||||||
| Female | 0.914 | 0.298 | 0.662-1.262 | 0.585 | 0.956 | 0.061 | 0.067-1.364 | 0.805 |
| Vertical transmission of infection history | ||||||||
| Yes | 1 | 1 | ||||||
| No | 1.064 | 0.155 | 0.782-1.448 | 0.694 | 1.063 | 0.139 | 0.769-1.470 | 0.71 |
| Time to undetectable HBV DNA | 1.041 | 1.162 | 0.967-1.121 | 0.281 | 1.069 | 1.644 | 0.965-1.184 | 0.2 |
| Sustained time of antiviral | 1.022 | 4.671 | 1.002-1.042 | 0.031 | 1.017 | 2.17 | 0.995-1.04 | 0.141 |
| Drugs used | 1.807 | 0.771 | 2.018 | 0.733 | ||||
| Lamivudine | 1 | 1 | ||||||
| Adefovir | 1.011 | 0.002 | 0.614-1.666 | 0.964 | 0.834 | 0.427 | 0.485-1.436 | 0.514 |
| Telbivudine | 1.333 | 1.109 | 0.781-2.278 | 0.292 | 1.233 | 0.494 | 0.687-2.212 | 0.482 |
| Entecavir | 0.985 | 0.004 | 0.620-1.565 | 0.949 | 1.112 | 0.153 | 0.652-1.898 | 0.696 |
| Lamivudine + Adefovir | 0.991 | 0.001 | 0.589-1.667 | 0.973 | 0.872 | 0.228 | 0.496-1.531 | 0.633 |
| Level of HBsAg at the end of therapy (LgIU/L) | 1.971 | 6.542 | 1.172-3.316 | 0.011 | 2.041 | 5.303 | 1.098-3.194 | 0.021 |
| Genotype | ||||||||
| B | 1 | 1 | ||||||
| C | 0.942 | 0.142 | 0.690-1.285 | 0.706 | 0.981 | 0.014 | 0.712-1.348 | 0.905 |
We divided the patients into 2 groups according to post treatment HBsAg level (≥ 1500 and < 1500 IU/L). The cumulative risk of virological relapse was higher in the HBsAg level ≥ 1500 IU/L group than in the HBsAg level < 1500 IU/L group (HR = 2.0730; 95%CI: 1.4247-3.0164 and HR= 0.4824; 95%CI: 0.3315-0.7019, respectively; P = 0.0013; Figure 2).
The ROC curve of the post treatment HBsAg serum levels for predicting virological relapse is shown in Figure 3. The area under the curve was 0.603 (95%CI: 0.508-0.699, P = 0.033). The cutoff HBsAg value for predicting virological relapse was 1443 IU/L, with a sensitivity of 88.2% and specificity of 30.2% (Figure 3).
In China, more than 4 kinds of nucleos(t)ide analogues (NUCs), such as LAM, ADV, LDT and ETV, are used as treatment agents for chronic HBV infection and are considered for partial reimbursement by the national health insurance system. However, these drugs are expensive for most Chinese people and have uncertain efficacies in long-term treatment[14-16]. Most CHB patients cannot afford long-term antiretroviral therapy and most hepatologists recommend NUC therapy discontinuation when it reaches the withdrawal standard indicated in the guidelines in China. In our report, the 2 and 1 year cumulative risks of virological relapse after antiviral therapy discontinuation were 79.41% and 43.82% (71/162), respectively, similar to those reported by Liang et al[17] who reported a 1 year cumulative risk of virological relapse of 47% in HBeAg-negative patients. However, the follow-up period in this study was only 1 year and the total number of observed cases was only 43. Nevertheless, we found an increasing virological relapse rate with longer follow-up time. Pan et al[12] recruited 162 HBeAg-positive CHB patients and reported that the 6 and 48 mo cumulative relapse rates after therapy discontinuation were 29.2% and 82.5%, respectively. In another report[18], among 61 HBeAg-negative patients who had received lamivudine for at least 2 years (HBV DNA level > 10000 copies/mL), 37% and 56% had a relapse in 1 year and 5 years, respectively. These studies showed comparable results that indicate an increasing virological relapse rate with longer follow-up.
Some factors have been reported to be associated with the risk of relapse after NUC therapy discontinuation in HBeAg-positive patients. Yeh et al and Fung et al found that the pretreatment HBV-DNA level was the only factor of virological relapse. Age is also related to sustained response. Some studies demonstrated that patients younger than 36 years old have a high sustained response to lamivudine. Other studies found that age younger than 40 years old is significantly associated with sustained response and higher HBeAg seroconversion. This can be explained by the level of immune activity in relation to age. However, we did not find any significant association of age to virological relapse in the HBeAg-negative patients. Genotype is another factor associated with sustained virological response. This fact was demonstrated by Kao et al who followed up 146 Taiwanese adult HBeAg-positive hepatitis B carriers for a mean of 52 mo and tested them for HBV genotype by using a molecular method. During the follow-up period, genotype C patients had a significantly lower rate of spontaneous HBeAg seroconversion than genotype B patients. Another report from Japan[19] demonstrated that compared with genotype C patients, genotype B patients had a lower rate of HBeAg positivity. However, we did not find any significant association of HBV genotype to virological relapse in the HBeAg-negative patients.
Serum HBsAg level has been used as a hot biomarker since serum HBsAg level was proven to be associated with the amount of covalently closed circular DNA in the liver[20,21]. In studies of the natural history of HBV, lower serum HBsAg levels were associated with improved immunity to eradicate the virus[22,23]. Moreover, in this open-label multicenter study, 379 nucleos(t)ide-naive patients with HBeAg-positive or HBeAg-negative CHB were randomized and treated with daily ETV or in combination with tenofovir for 100 wk. Greater decline in HBsAg level within 96 wk of treatment was observed in the HBeAg-positive patients, especially in those who achieved subsequent HBeAg loss[24]. A recent report[25] showed that long-term ETV treatment achieved only a slow decline in serum HBsAg level, in contrast to the profound HBV DNA suppression.
In this study, we showed that serum HBsAg levels after antiviral therapy discontinuation were associated with virological relapse. Our findings were congruent with a finding that among 53 HBeAg-negative patients who discontinued lamivudine treatment, the 5 year cumulative probabilities of sustained response and HBsAg clearance among the patients with HBsAg levels ≤ 2 log IU/mL were higher than among those with HBsAg levels > 2 log IU/mL[26].
In conclusion, we investigated the virological relapse rate and analyzed the factors of recurrence after NUC therapy discontinuation in HBeAg-negative patients. As a result, we found that post treatment HBsAg levels predict virological relapse.
This goal of treatment in hepatitis B e antigen (HBeAg)-negative patients is suppressed hepatitis B virus (HBV) replication in a sustained manner. The ideal end point is hepatitis B surface antigen (HBsAg) loss sustained off-therapy in HBeAg-negative patients and a satisfactory end point is sustained off-therapy virological and biochemical response. However, the ideal end point is difficult to achieve. In this study, they aimed to study the rate of virological relapse in HBeAg-negative patients after stopping antiviral therapy.
Owing to the expensive antiviral drugs in China, there is approximately a 25% to 50% rate of hepatitis relapse after stopping antiviral therapy, even if according to the APASL recommendation.
They showed that serum HBsAg levels at the end of stopping antiviral therapy were associated with virological relapse.
Serum HBsAg levels at the end of stopping antiviral therapy were associated with virological relapse.
Virological relapse was defined as serum HBV DNA > 1000 copies/mL. Sustained response (no virological relapse) was defined as undetectable HBV DNA (< 1000 copies/mL) after stopping antiviral therapy for 24 mo.
The authors have performed a good study to analyze the factors associated with virological relapse in HBeAg-negative patients after stopping antiviral therapy. The manuscript is presented in a well-organized and easy to read manner.
P- Reviewer: Wang DR S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1280] [Cited by in RCA: 1603] [Article Influence: 123.3] [Reference Citation Analysis (1)] |
| 2. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Semin Liver Dis. 2004;24 Suppl 1:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | de Ruiter CJ, Van Leeuwen D, Heijblom A, Bobbert MF, de Haan A. Fast unilateral isometric knee extension torque development and bilateral jump height. Med Sci Sports Exerc. 2006;38:1843-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Brunetto MR, Oliveri F, Coco B, Leandro G, Colombatto P, Gorin JM, Bonino F. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J Hepatol. 2002;36:263-270. [PubMed] |
| 5. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 6. | Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, Ng KY, Nicholls GJ, Dent JC, Leung NW. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004;19:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 681] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 8. | Sung JJ, Amarapurkar D, Chan HL, Cheng J, Kao JH, Han KH, Piratvisuth T. Treatment of chronic hepatitis B in Asia-Pacific countries: is the Asia-Pacific consensus statement being followed? Antivir Ther. 2010;15:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Sohn HR, Min BY, Song JC, Seong MH, Lee SS, Jang ES, Shin CM, Park YS, Hwang JH, Jeong SH. Off-treatment virologic relapse and outcomes of re-treatment in chronic hepatitis B patients who achieved complete viral suppression with oral nucleos(t)ide analogs. BMC Infect Dis. 2014;14:439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Chen CH, Lu SN, Hung CH, Wang JH, Hu TH, Changchien CS, Lee CM. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol. 2014;61:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Fontaine H, Kahi S, Chazallon C, Bourgine M, Varaut A, Buffet C, Godon O, Meritet JF, Saïdi Y, Michel ML. Anti-HBV DNA vaccination does not prevent relapse after discontinuation of analogues in the treatment of chronic hepatitis B: a randomised trial--ANRS HB02 VAC-ADN. Gut. 2015;64:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Pan X, Zhang K, Yang X, Liang J, Sun H, Li X, Zou Y, Xu Q, An G, Li G. Relapse rate and associated-factor of recurrence after stopping NUCs therapy with different prolonged consolidation therapy in HBeAg positive CHB patients. PLoS One. 2013;8:e68568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 14. | Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-1722. [PubMed] |
| 15. | Minde Z, Yimin M, Guangbi Y, JinLin H, Hao W, Hong R, Yuming W, Xiaqiu Z, Daozhen X, Yagang C. Five years of treatment with adefovir dipivoxil in Chinese patients with HBeAg-positive chronic hepatitis B. Liver Int. 2012;32:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Heathcote EJ, Marcellin P, Buti M, Gane E, De Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 17. | Liang Y, Jiang J, Su M, Liu Z, Guo W, Huang X, Xie R, Ge S, Hu J, Jiang Z. Predictors of relapse in chronic hepatitis B after discontinuation of anti-viral therapy. Aliment Pharmacol Ther. 2011;34:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Liu F, Wang L, Li XY, Liu YD, Wang JB, Zhang ZH, Wang YZ. Poor durability of lamivudine effectiveness despite stringent cessation criteria: a prospective clinical study in hepatitis B e antigen-negative chronic hepatitis B patients. J Gastroenterol Hepatol. 2011;26:456-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Orito E, Ichida T, Sakugawa H, Sata M, Horiike N, Hino K, Okita K, Okanoue T, Iino S, Tanaka E. Geographic distribution of hepatitis B virus (HBV) genotype in patients with chronic HBV infection in Japan. Hepatology. 2001;34:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 315] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 20. | Sun KX, Li J, Zhu FC, Liu JX, Li RC, Zhai XJ, Li YP, Chang ZJ, Nie JJ, Zhuang H. A predictive value of quantitative HBsAg for serum HBV DNA level among HBeAg-positive pregnant women. Vaccine. 2012;30:5335-5340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Gheorghiţa VI, Caruntu FA, Curescu M, Olaru I, Radu MN, Colţan G, Streinu-Cercel A. Use of quantitative serum HBsAg for optimization of therapy in chronic hepatitis B patients treated with pegylated interferon alfa-2a: a Romanian cohort study. J Gastrointestin Liver Dis. 2013;22:27-32. [PubMed] |
| 22. | Ma H, Yang RF, Wei L. Quantitative serum HBsAg and HBeAg are strong predictors of sustained HBeAg seroconversion to pegylated interferon alfa-2b in HBeAg-positive patients. J Gastroenterol Hepatol. 2010;25:1498-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Li WJ, Li BA, Zhao JM, Han JQ, Liu Y, Jiang L, Mao YL, Lu FM, Xu DP. [Quantitative analyses of intrahepatic HBV cccDNA and serum HBsAg in 54 patients with chronic hepatitis B]. Zhonghua Gan Zang Bing Zazhi. 2011;19:815-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Zoulim F, Carosi G, Greenbloom S, Mazur W, Nguyen T, Jeffers L, Brunetto M, Yu S, Llamoso C. Quantification of HBsAg in nucleos(t)ide-naïve patients treated for chronic hepatitis B with entecavir with or without tenofovir in the BE-LOW study. J Hepatol. 2015;62:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Seto WK, Lam YF, Fung J, Wong DK, Huang FY, Hung IF, Lai CL, Yuen MF. Changes of HBsAg and HBV DNA levels in Chinese chronic hepatitis B patients after 5 years of entecavir treatment. J Gastroenterol Hepatol. 2014;29:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Chan HL, Wong GL, Chim AM, Chan HY, Chu SH, Wong VW. Prediction of off-treatment response to lamivudine by serum hepatitis B surface antigen quantification in hepatitis B e antigen-negative patients. Antivir Ther. 2011;16:1249-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |