Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8492
Peer-review started: March 5, 2015
First decision: April 29, 2015
Revised: May 10, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: July 28, 2015
Processing time: 148 Days and 11.8 Hours
Autophagy is a highly-regulated, conserved cellular process for the degradation of intracellular components in lysosomes to maintain the energetic balance of the cell. It is a pro-survival mechanism that plays an important role during development, differentiation, apoptosis, ageing and innate and adaptive immune response. Besides, autophagy has been described to be involved in the development of various human diseases, e.g., chronic liver diseases and the development of hepatocellular carcinoma. The hepatitis C virus (HCV) is a major cause of chronic liver diseases. It has recently been described that HCV, like other RNA viruses, hijacks the autophagic machinery to improve its replication. However, the mechanisms underlying its activation are conflicting. HCV replication and assembly occurs at the so-called membranous web that consists of lipid droplets and rearranged endoplasmic reticulum-derived membranes including single-, double- and multi-membrane vesicles. The double-membrane vesicles have been identified to contain NS3, NS5A, viral RNA and the autophagosomal marker microtubule-associated protein 1 light chain 3, corroborating the involvement of the autophagic pathway in the HCV life-cycle. In this review, we will highlight the crosstalk of the autophagosomal compartment with different steps of the HCV life-cycle and address its implications on favoring the survival of infected hepatocytes.
Core tip: The hepatitis C virus (HCV) is the major cause of chronic liver disease worldwide. According to the world health organization, approximately 130-170 million people are chronically infected with HCV. It has recently been described that HCV hijacks the autophagosomal pathway. Autophagy is a conserved cellular process that catabolizes intracellular components to maintain cellular homeostasis. Besides, autophagy is involved in the development of human diseases. In this review, we will depict the data known so far, corresponding the interplay between the autophagosomal pathway and the different steps of the HCV life-cycle.
- Citation: Ploen D, Hildt E. Hepatitis C virus comes for dinner: How the hepatitis C virus interferes with autophagy. World J Gastroenterol 2015; 21(28): 8492-8507
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8492.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8492
Hepatitis C virus (HCV) is one of the major causes of chronic liver diseases worldwide including chronic hepatitis, fibrosis, cirrhosis and hepatocellular carcinoma (HCC). At present, more than 185 million people are persistently infected with HCV reflecting 3% of the world population[1]. Until now, there is no preventive vaccine available and the current standard therapy is a 24-48 wk course of pegylated interferon (IFN)-α and ribavirin (RBV) that is only effective in 40%-50% of the patients infected with genotype 1. Since 2013, two first-generation direct-acting antivirals (DAAs), telaprevir (TVR) and boceprevir (BOC), both NS3-4A protease inhibitors, are approved for therapy in combination with IFN-α and ribavirin in patients infected with the HCV genotype 1[2]. In 2015, new DAAs and host-targeted agents (HTAs) will be available to further improve the HCV treatment in IFN-free regimens. These second generation of antivirals, including NS3/4A protease inhibitors (Simeprevir, Faldaprevir, Asunaprevir), NS5A inhibitors (Daclatasavir) and NS5B inhibitors (Sofusbuvir), are currently under clinical evaluation and further target patients infected with the HCV genotypes 2-7 to induce an increased sustained virological response (SVR)[3-5]. However, despite the improvement of the HCV therapy, the number of patients chronically infected with HCV developing a liver cirrhosis or HCC will increase within the next years[1,6]. One important component of new HCV regimens remains the guanosine nucleoside analog ribavirin (RBV). RBV enters the cell by binding to nucleoside transporters like the equilibrative nucleoside transporter-1 (ENT1). Intracellularly, RBV is phosphorylated by cellular kinases to form the RBV-monophosphate (RMP), diphosphate (RDP), or triphosphate[7]. Its mode of action is versatile including the mutagenesis of viral RNA by incorporation of RBV-triphosphate, inhibition of IRES-RNA translation at the level of polyribosome formation, inhibition of the viral polymerase NS5B, inhibition of inosine monophosphate dehydrogenase (IMPDH), induction of TH1 response resulting in increased clearance of the infected cells and interaction with the IFN signaling pathway[8]. However, the antiviral activity of RBV alone is impaired in chronically HCV-infected patients. In line with this, it has recently been described that the HCV-dependent induction of autophagy impairs the uptake of RBV in persistently infected Huh7.5 cells by reduced expression of ENT1. ENT1 is expressed on the plasma membrane of many cell types[9] via a clathrin-dependent mechanism. The HCV-induced autophagic response provokes the consequential degradation of the clathrin heavy chain and thereby prevents the clathrin-mediated recycling of ENT1 to the plasma membrane forcing ENT1 for lysosomal degradation[10]. Based on this, the development of antiviral drugs that target factors involved in the autophagosomal pathway will be attractive.
Autophagy has been described to be involved in several steps of the HCV life-cycle including entry[11], replication[12-16], assembly and release[17-19]. In addition, it has been reported that HCV-induced autophagy impairs innate antiviral activity[20,21] and plays a role in the development of HCV-related liver diseases[22-24].
Based on published data, in this review we will summarize the crosstalk between the autophagic pathway and the different steps of the HCV life-cycle and discuss the impact of innate immunity for HCV-induced autophagy.
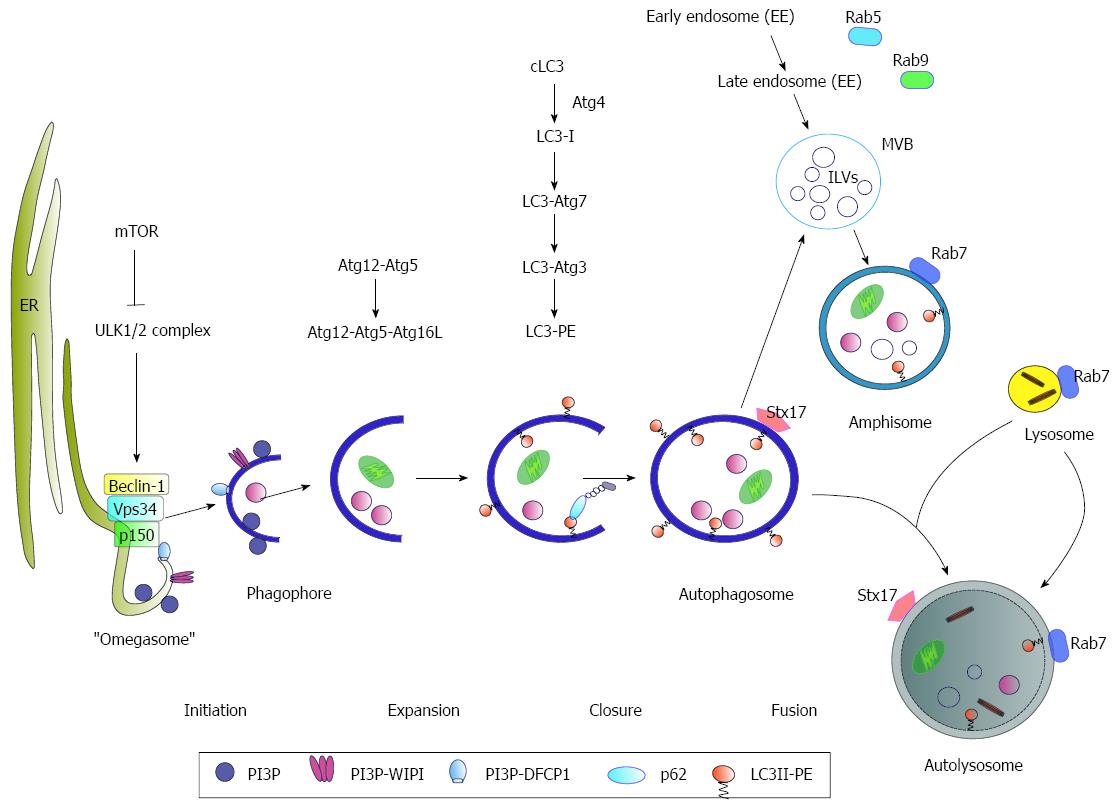
Autophagy (“self-eating”) is a highly regulated and conserved cellular process that catabolizes intracellular components to maintain cellular homeostasis. It is initiated by the formation of membrane crescents known as phagophores or isolation membranes that increase to enclosed double-membrane vesicles, called autophagosomes. The autophagosome finally fuses with lysosomes to form autolysosomes where their cargo is digested by lysosomal proteases[25] (Figure 1). Autophagy is tightly regulated by more than 32 so-called autophagy-related genes (Atg) as a response to nutrient starvation, damaged organelles, protein-aggregates, ER-/oxidative-stress or infection with pathogens[26]. One important regulator represents the mammalian target of rapamycin (mTOR) that represses autophagy by phosphorylation of Unc-like kinase 1 and 2 (ULK1/2-complex). During starvation, mTOR kinase activity is inhibited by AMP-activated protein kinase (AMPK) resulting in the induction of autophagy[27]. As an initial step, Beclin1 forms a complex with the class III phosphatidylinositol-3-kinase (PI3K3) Vps34, p150 and Atg14 (PI3K-complex). The activated ULK1/2- and PI3K-complexes further catalyze the formation of an autophagosome-specific phosphatidylinositol-3-phosphate (PI3P)-enriched environment[28]. The PI3P further recruits the effector DFCP1- (double FYVE-containing protein 1) and WIPI- (WD-repeat domain phosphoinositide-interacting) proteins, leading to phagophore nucleation by formation of an ER-associated Ω-structure (“omegasome”). Autophagosomal membranes can further originate from Golgi-[29], mitochondrial-[30], or plasma membrane (PM)-[31] derived membranes. After the nucleation process, the formation of the autophagosome occurs. For this, the two ubiquitin-like conjugation systems Atg5-Atg12-Atg16L and Atg4-Atg3-LC3-II are required. Here, Atg12 is conjugated to Atg5 by ubiquitination-like reactions that require Atg7 (E1-like) and Atg10 (E2-like). The Atg12-Atg5 conjugate associates with Atg16L resulting in the formation of the Atg12-Atg5-Atg16L complex (E3-like). Subsequently, the microtubule-associated protein 1 light chain 3 (LC3) is cleaved at its carboxy terminus by Atg4. The cleaved cytosolic LC3-I is finally converted to the lipidated form LC3-II by conjugation to phosphatidylethanolamine (PE) through Atg7 and Atg3 and localized to the autophagosomal membrane[32]. The autophagosomes can either directly fuse with lysosomes to form the autolysosome or fuse with late endosomes/MVBs (multivesicular bodies) forming an amphisome prior fusion with the lysosome[33]. The fusion of the autophagosome with the lysosome has been described to be stimulated by Rab7-GTPase activity[34]. In a recent study, it was reported that Rab7 is associated with lipid droplets (LDs) where it plays an important role during “lipophagy”, as activated Rab7 promotes the interaction of LDs with MVBs as well with lysosomes[35]. Another factor involved in autophagosome-lysosome fusion is the autophagosomal SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein) syntaxin 17 (Stx17). Stx17 can be found on the outer membrane of enclosed completed autophagosomes where it promotes its fusion with lysosomes by interaction with SNAP29 and the autophagosomal SNARE VAMP8[36-38].
Taken together, autophagy is a highly regulated, conserved and complex intracellular mechanism that requires the interplay of various factors to maintain the cell homeostasis and to finally ensure the survival of the cell. Autophagosomes can either directly fuse with lysosomes to form the autolysosome or fuse with late endosomes/MVBs forming an amphisome prior fusion with the lysosome.
Autophagy can be triggered by ER stress that results in the unfolded protein response (UPR). UPR activates three signaling pathways: the inositol-required protein 1 (IRE1), the activating transcription factor-6 (ATF6) and the protein kinase (PKR)-like ER kinase (PERK) pathway[39]. Upon activation of ER stress, IRE1 RNase catalyzes the removal of a small intron from the X-box binding protein 1 (XBP1) mRNA, resulting in spliced XBP1 (sXBP1) that encodes for an active transcription factor. XBP1 binds to the ER stress response element (ERSE) and the UPR element (UPRE), resulting in expression of more than 380 target genes, including the ER chaperone 78 kDa glucose-regulated protein (GRP78) and ER-associated degradation (ERAD) factors[40,41]. In response to ER stress, ATF6 is released from GRP78 and translocates to the Golgi where it undergoes protease cleavage to obtain an active transcription factor (cATF6). The cATF6 shuttles to the nucleus where it binds to ERSEs and UPREs, resulting in gene expression of mainly ER chaperones to remove misfolded proteins[42]. Activation of PERK results in phosphorylation of eukaryotic translation factor 2α (eIF2α) following the inhibition of translation, induction of ATF4 and expression of the CCAAT/enhancer-binding protein-homologous protein (CHOP)[43,44]. Induction of UPR after elevated intracellular reactive oxidant species (ROS) further results in a PERK-dependent activation of the transcription factor NF-E2 related factor 2 (Nrf2)[45]. PERK induces the phosphorylation of Nrf2 and dissociation from Keap1. Besides the PERK-Nrf2 pathway, indirect activation of Nrf2 via IRE1α-JNK-Nrf2 occurs[46]. Nrf2 belongs to the Cap´n´collar-bacic leucine zipper (CNC-bZIP) transcription factor that plays a crucial role in the defense against oxidative stress. One protection mechanism against oxidative stress is the expression of cytoprotective genes. Many of these genes harbor a short cis acting sequence in their promoter, the antioxidant response element (ARE, 5´-TGANNNNGC-3´), and encode for proteins involved in the detoxification of the cells, e.g., NQO1, GCS, GPx or catalytic subunits of the proteasome PSMB5 and PSMB6[47-49]. In its inactive form, Nrf2 is localized in the cytoplasm of the cell, bound to the actin-binding protein Keap1 which mediates its ubiquitin-dependent degradation. Upon activation, Nrf2 dissociates from Keap1 and translocates to the nucleus where it binds to ARE sequences and together with other proteins (e.g., sMaf proteins) activates the transcription of detoxifying genes[47,50]. Moreover, a crosstalk between Nrf2 and the autophagic pathway has been described[46,51]. It has recently been published that phosphorylation of the autophagy adaptor protein p62 activates the Keap1-Nrf2 pathway during selective autophagy as phosphorylated p62 (p-p62) binds to the Keap1 binding site of Nrf2 and therefore competitively inhibits the Keap1-Nrf2 interaction, followed by release of Nrf2 and increased expression of ARE-dependent cytoprotective genes[52]. p62, also known as sequestome-1, is a stress-inducible protein that regulates several transduction pathways involved in cell survival and cell death. In line with this, inhibition of autophagy results in the upregulation of p62 and activation of Nrf2[51].
The studies indicate that autophagy can be activated by endoplasmic reticulum (ER) stress-induced UPR resulting in activation of three signaling pathways.
HCV is an RNA virus that belongs to the Hepacivirus within the Flaviviridae family. Due to its high genetic variability, HCV can be divided into 7 genotypes (1-7) and several subtypes that differ 20%-30% in their sequence and display a different geographical distribution and treatment response[53].
The HCV genome is a single-stranded, positive orientated RNA genome with a size of 9600 bases length. The viral RNA encodes for a large polyprotein precursor of approximately 3100 amino acids that is co- and/or posttranslationally cleaved by viral or cellular proteases into the mature structural (core, E1, E2) and p7 protein and the nonstructural (NS) proteins (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B). The structural proteins form the viral particle, whereas the NS proteins are involved in viral replication and the assembly process[54,55].
The HCV life-cycle is closely linked to very low density (VLDL) synthesis, leading to the pleiomorphic, VLDL-like structure of the so-called lipoviroparticles (LVPs). LVPs display a heterogenity in their density ranging from 1.25 g/mL to 1.03 g/mL (human sera) or 1.10 g/mL to 1.05 g/mL (HCVcc)[56-61]. Their infectivity inversely correlates to their density, with low-densitiy particles being more infectious than high-density particles[62-64]. Thus, the presence of lipids and apolipoproteins such as apoE, apoB, apoC1, C2, C3, and apoA1 is important for the entry, assembly and release of viral particles.
The entry process occurs in a coordinated way including several host factors located on the cell surface. The LVPs initially bind the LDL-receptor (LDLR) and glycosaminoglycans (GAG) via apoE[65-68] followed by subsequent interaction with the scavenger receptor class B type I (SR-BI)[68] and the tetraspanin CD81[69,70]. As reported previously, the viral particles primary bind the heparan sulfate proteoglycans (HSPGs) syndecan-1 and syndecan-4[71,72]. In line with this, apoE has been described to interact with HSPGs to mediate the lipoprotein uptake. It has recently been reported that LVP-associated apoE and not the viral envelope glycoproteins mediate interaction with the HSPGs. For this, N- and 6-O-sulfation on the HS is essential and the minimal length of an HS decasaccharide is required[73,74]. The interaction of the viral particle with its major coreceptors SR-BI and CD81 occurs via E2[69,68]. Here, the highly conserved region of E2, spanning amino acids 502-520, has been proposed to act as a fusion peptide. The peptide consists of glycine and non-polar amino acids and non-charged residues, exhibiting a globular structure with no regular secondary structures, atypical for a fusion peptide[75]. However, the amino acids Y507, V514, and V515 have been identified to be involved in interaction with CD81 and SR-BI and neutralizing antibodies, thus promoting membrane fusion[76,77].
After the relocation of the LVPs to the tight junction proteins claudin-1 (CLDN-1)[78] and occludin (OCLN)[79-81], the virus becomes internalized by clathrin-mediated endocytosis[82,83] and is finally released into the cytosol in a pH-dependent manner[83,84]. It has recently been described that two receptor tyrosine kinases (RTK): epidermal growth factor receptor (EGFR) and ephidrin type A receptor 2 (EPHA2)[85,86] - and the Niemann-Pick C1-like 1 (NPC1L1) cholesterol uptake receptor[87] as well as the transferrin receptor[88] are additional cofactors involved in the entry process.
Taken together, the entry of the viral particles occurs in a highly organized way including the interaction of the viral particle with specific host factors as LDL-receptor, SR-BI, CD81, CLDN-1, OCLN, EGFR and EPHA2.
The uncoated positive-strand RNA genome is transported to the rough endoplasmatic reticulum (rER) where it serves as template for the synthesis of the HCV polyprotein precursor that is co- and post-translationally processed by cellular and viral proteases into the mature structural (core, E1, E2) and non-structural proteins (p7, NS2, NS3, NS4A-B, NS5A-B)[89]. HCV replication occurs in replication complexes (RCs) at the so-called membranous web (MW) that consists of lipid droplets (LDs) and rearranged ER-derived membranes including single-, double- and multi-membrane vesicles[90,91]. The double-membrane vesicles (DMVs) have been identified to contain NS3, NS5A, viral RNA and the autophagosome marker microtubule-associated protein 1 light chain 3 (LC3-II), the lipidated form of LC3-I, suggesting an involvement of the autophagic pathway in the HCV life-cycle[91]. In addition, the DMVs are highly enriched in cholesterol[92]. The formation of the MW is induced by the viral proteins NS4B[93] and NS5A as well as by cellular host factors such as the phosphatidylinositol 4-kinase III α (PI4K-IIIα)[90,94]. Interactions of NS5A with PI4K-IIIα induces the accumulation of phosphatidylinositol 4-phospate (PI4P) in the MW. The PI4P-enriched environment is further stabilized by the interaction of NS5A with ARFGAP1, a GTPase-activating protein for ARF1, an adaptor protein involved in COPI trafficking[95]. Moreover, NS4B synergistically modulates core-mediated activity of sterol regulatory element-binding proteins (SREBP) and fatty acid synthetase (FAS), thus mediating lipogenesis and lipid accumulation in hepatoma cells[96]. Viral replication is catalyzed by the viral RNA-dependent RNA polymerase (RdRp) NS5B. NS5B presents the classical right-hand structure, consisting of finger, palm and thumb domains[97]. In addition, NS5B contains a β-loop insertion in the thumb domain and a C-terminal membrane-anchoring linker in its active site that are involved in the initiation of RNA synthesis[98]. The highly active site of NS5B further offers possible targets for antiviral strategies with higher barriers to the development of resistance[2]. In addition, host factors such as cyclophilin A (CypA) and microRNA (miR)-122 have been identified to participate in the viral replication[99]. MiR-122 is a small non-coding RNA that is highly expressed in hepatocytes and is involved in proliferation and differentiation of hepatocytes, lipid metabolism as well as in the development of liver disease. Besides, miR-122 stimulates HCV replication by binding to the 5´-UTR of the HCV genome[100,101]. CypA is a peptidyl-prolyl isomerase (PPIase) that catalyzes the cis-trans isomerization of peptide bonds at proline (Pro) residues and therefore assists in protein-folding[102,103]. The immunosuppressant CypA inhibitor, cyclosporine A (CsA), has been identified to inhibit HCV replication and translation in cell culture[104]. In line with this, it was recently reported that NS5A and CypA act in concert and are required for DMV formation. This suggests that CypA and NS5A inhibitors block HCV replication by inhibiting formation of the membranous web[105]. Due to their importance during the viral replication, the involved viral proteins (NS3/4A, NS5A, NS5B, p7) and cellular factors (miR-122, CypA, PI4K-IIIα) represent potential targets for antiviral therapy.
Summing up, HCV replication occurs in replication complexes at the so-called membranous web that consists of lipid droplets and rearranged ER-derived membranes including single-, double- and multi-membrane vesicles. This process is mainly regulated by the crosstalk between HCV NS-proteins and core and cellular host factors.
The assembly of the virions occurs on the surface of core-associated cytosolic lipid droplets (cLDs) in close proximity to the RC at the membranous web[106] via budding at the ER membrane and seems to depend on VLDL-assembly[107]. cLDs are organelles for the storage of neutral lipids and consist of a hydrophobic core of triacylglycerol (TAG) and cholesterol ester (CE) surrounded by a phospholipid monolayer that is surrounded by a coat of proteins, the so-called PAT-proteins including perilipin, adipose differentiation-related protein (ADRP) and tail interacting protein of 47 kDa (TIP47)[108]. During HCV infection, the core protein induces the formation of the LDs[109] and recruits NS proteins and the RC to LD-associated membranes[106]. The recruitment of core and NS5A to the LDs is mediated by the diacylglycerolacyltransferase-1 (DGAT-1)[110], an enzyme involved in triglyceride synthesis and LD maturation[111] and the mitogen-activated protein kinase (MAPK)-regulated protein cytosolic phospholipase A2 (cPLA2)[112]. In addition, NS5A interacts with LD-associated Rab18 and thereby promotes the interaction between the sites of viral replication and the LDs[113]. Proteomic analyses of LD-proteins in core-expressing cells revealed a higher content of the lipid droplet associated protein TIP47, whereas the amount of ADRP was significantly decreased[114]. Besides its role in the biogenesis of LDs[115], TIP47 has been described to act as an intracellular sorting factor that is involved in the retrograde trafficking of the mannose-6-phosphate receptor (M6PR) from late endosomes to the trans-Golgi network (TGN)[116]. Structural analysis of TIP47 revealed a high structural homology to apoE[117]. ApoE plays an important role in the VLDL pathway and has been described to directly interact with the viral protein NS5A[118,119]. This interaction is crucial for the virus assembly and the release of infectious viral particles[120,61]. It was recently observed that TIP47 binds the viral protein NS5A and targets the de novo synthesized viral RNA that is bound to NS5A from the replicon complex to the LDs, the assembly platform[121,122].
The initial step of virion assembly represents the interaction of NS5A with the cLD-bound core protein[106,123,124]. For this process, the phosphorylation of specific serine residues of NS5A-domain III (DIII) by casein kinase IIα (CKIIα) is essential[125]. In addition, the viral proteins p7 and NS2 play a crucial role in the organization of the assembly complex. NS2 interacts with the viroporin p7. The p7-NS2-complex further interacts with the viral proteins NS3-4A and promotes the disruption of the core protein from the cLDs to the nascent viral particle[126,127]. The viral budding has been described to require late components of the ESCRT (endosomal sorting complex required for transport) pathway[128,129].
Maturation of viral particles is closely linked to VLDL synthesis as they are highly enriched in apoE, apoB and the microsomal transfer protein (MTP), proteins that are involved in VLDL-assembly[107]. The virions are packaged as LVPs that have similar densities to VLDLs and envelopment and maturation are thought to proceed at the luminal LD (luLD), the VLDL precursors[130].
The mature viral particles are finally released through the secretory pathway. During this process, the surface proteins E1 and E2 are modified by glycosylation and their disulfide bonds are reorganized[131-133].
For this, the interaction of TIP47 with activated GTP-bound Rab9 is essential. It has previously been described that the TIP47/GTP-Rab9 complex is important for the release of the viral particles and TIP47 becomes part of the viral particle. The intracellular trafficking of the HCV particles loaded with TIP47 that lacks the Rab9 binding site ends in the autolysosomal compartment with degradation of these viral particles[134]. Besides, heterogeneous nuclear ribonucleoprotein K (HNRNPK) has been identified as a host restriction factor that regulates the viral assembly. In HCV-infected cells, HNRNPK interacts with the viral plus-strand RNA and the core protein in close proximity of the LDs. The proposed mechanism indicates that HNRNPK binds the viral RNA to regulate its availability for packaging. Bound RNA cannot be incorporated into newly synthesized viral particles and thereby enters a new replication cycle[135].
These data so far suggest that the release of the viral particles is linked to the VLDL pathway. However, the exact mechanism remains enigmatic and needs to be further investigated.
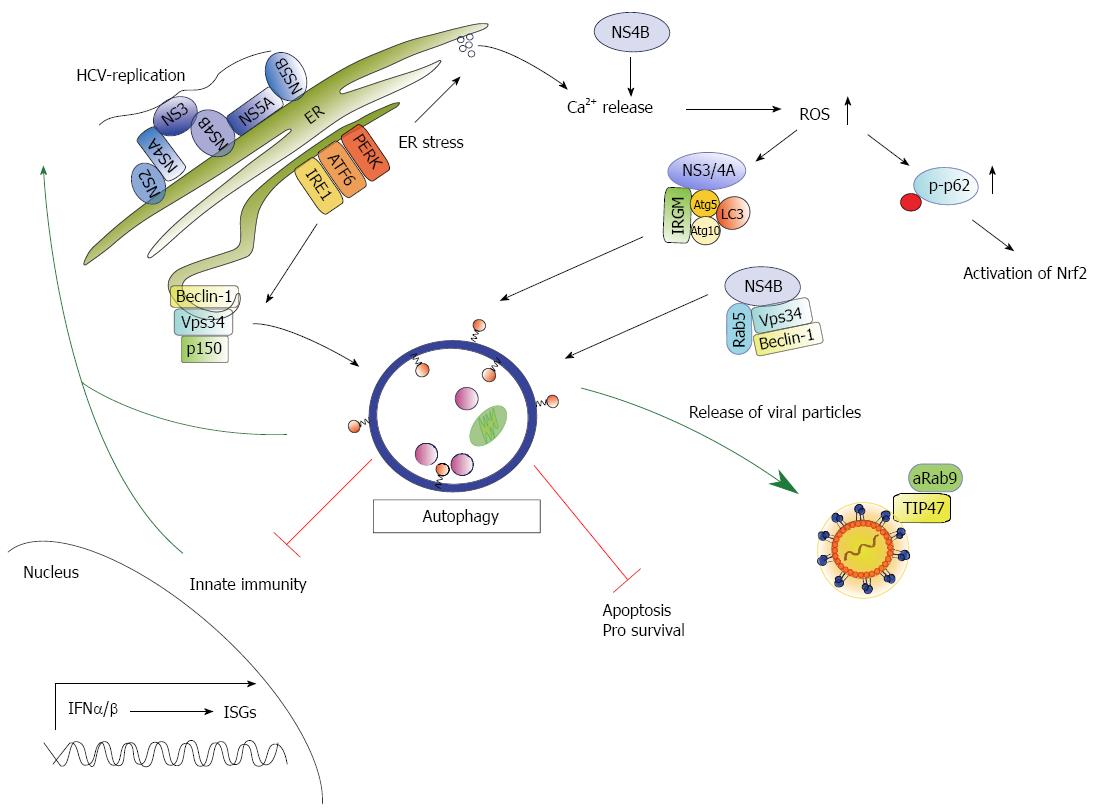
The data concerning HCV and its role during UPR/autophagy are conflicting, and the mechanisms underlying its activation are not completely understood. As HCV replication occurs at the membranous web, it is believed that HCV induces ER stress following the induction of UPR in vitro[14,21,136,137] and in vivo[138]. For instance, the viral glycoproteins E1-E2 form a complex retained in the ER membrane that can form aggregates resulting in ER stress[139,140] (Table 1 and Figure 2). Furthermore, HCV infection is associated with oxidative stress in liver cells that results in elevated ROS[141]. As a response to the increased ER-/oxidative stress, the direct PERK-Nrf2 or indirect IRE1α-JNK-Nrf2 pathways trigger the expression of detoxifying genes[46]. In addition, the elevated ROS levels trigger phosphorylation of the autophagy adaptor protein p62 on Ser351 by the mTOR kinase. Phosphorylation of p62 results in the release of Nrf2 from Keap1 followed by increased expression of cytoprotective genes[52] (Figure 2). In a recent study of our group, we observed that HCV interferes with the production of cytoprotective genes by an impaired Nrf2/ARE-signaling. Here, the core protein triggers the delocalization of sMaf proteins to the cytosol where sMaf proteins bind to NS3 and prevent the entry of Nrf2 into the nucleus resulting in elevated ROS levels. Therefore, the increased ROS levels in HCV replicating cells cannot be efficiently compensated due to the impaired Nrf2 activity resulting in the induction of autophagy[142] (Table 1). Moreover, it has been reported that liver regeneration is impaired in mice lacking Nrf2 due to increased ROS levels that negatively affect insulin signaling[143]. UPR can be further activated by HCV NS4B through the activation of the ATF6- or IRE1-pathway as NS4B interferes with Ca2+ homeostasis resulting in elevated ROS[141,144] (Table 1). In addition, NS4B forms a complex with Rab5 and the PI3 kinase Vps34, triggering the induction of autophagy[16]. Active Rab5 activates autophagy by inhibition of mTOR kinase activity[145] and interaction with Beclin1 and Vps34[146]. Hence, interaction between Rab5 and NS4B have been described to be crucial for HCV replication[16,147] (Table 1). Grégoire et al[148] further identified the human immunity-associated GTPase family M (IRGM) protein to be involved in the autophagy modulation of different RNA-viruses including HCV. IRGM interacts with HCV NS3/4A and different autophagosomal proteins, e.g., Atg5, Atg10, LC3[18,148,149] and thereby triggers autophagy (Table 1 and Figure 2). These studies so far indicate that HCV can induce autophagy by elevated ROS levels via UPR or by direct interference with the autophagic pathway.
| Viral proteins | Cellular interacting partner/pathway | Function | Ref. |
| Core | - | - | - |
| E1-E2 | UPR-pathway | ER-retention of heterodimeric E1-E2-complexesInduction of UPR | Cocquerel et al[139], 1998 |
| E1-E2 | UPR-pathway | Formation of E1-E2 aggregates at ERInduction of UPR | Choukhi et al[140], 1998 |
| E2 | - | - | - |
| P7 | - | - | - |
| NS2 | - | - | - |
| NS3 | sMafs | Entry of Nrf2 into nucleus prohibitedElevated ROS-levelsInduction of autophagy | Carvajal-Yepes et al[142], 2011 |
| NS3/NS4A | IRGM | Activation of autophagy | Grégoire et al[18], 2011 |
| NS3/4A | Interaction of Atg5-Atg12 conjugate with RIGI/Mda5 and IPS-1/MAVSCleavage of these helicasesInhibition of IFN-signaling | Shrivastava et al[20], 2012 | |
| NS4B | UPR-pathway | Interference with Ca2+-homeostasis resulting in elevated ROSInduction of UPR via activation of ATF6- or Xbp1-pathwayActivation of NfκB via Ca2+-homeostasis and elevated ROS | Li et al[144], 2009 |
| NS4B | Rab5 + Vps34 | Inhibition of mTOR kinase activity and interaction with Vps34 and Beclin1Activation of autophagy | Su et al[16], 2011 Stone et al[147], 2007 Simonsen et al[146], 2009 Li et al[145], 2010 |
| NS4B | Atg5 | Possible involvement in formation of membranous web | Guévin et al[152], 2010 |
| NS5A | - | Autolysosome formation | Shrivastava et al[20], 2012 |
| NS5A | TIP47/Rab9PKR | Release of viral particlesParticles loaded with TIP47 lacking Rab9 are targeted towards the autolysosome | Ploen et al[121], 2013 |
| NS5B | Atg5 | Possible role in establishment of infection | Guévin et al[152], 2010 |
Autophagy has been described to be involved in different steps of the HCV life-cycle. HCV replication occurs in “replication factories” at the so-called membranous web (MW)[93] that consists of LDs and rearranged ER-derived membranes including single-, double- and multi-membrane vesicles. The double membrane vesicles (DMVs) display the main component of the MW and are thought to be induced by NS5A during the early stage of infection[90,92,150]. The single membrane vesicles (SMVs) are induced by NS4B and have been reported to contain NS3 and NS5A. Multi-membrane vesicles (MMVs) can only be detected in the late stage of infection and are thought to occur as a consequence to the HCV-induced autophagic response[90]. Indeed, the DMVs have been identified to contain NS3, NS5A, viral RNA and the autophagosomal marker LC3-II suggesting a link to the autophagosomal compartment[91]. Using immunofluorescence-analysis, Romero-Brey et al[90] could identify markers of early endosomes (EE), late endosomes (LE), COP vesicles, mitochondria, LDs and Rab proteins that are associated to the MW. Thus, a crosstalk between factors involved in HCV replication with individual autophagosomal factors may contribute to the formation of the MW. During the early steps of the HCV life-cycle, Atg5 has been described as a proviral factor required for translation and/or delivery of incoming viral RNA. Atg5 induces formation of DMVs in embryonic stem cells[151] and was found to be associated with the membranous web in Huh7 cells and further colocalizes with NS4B (Table 1). Besides, Atg5 colocalizes with the viral polymerase NS5B during the onset of infection. This interaction is abrogated during the course of infection[152]. In another study, Beclin1, Atg4B and Atg12 were identified as additional proviral factors required for establishment of viral replication[11,153].
Furthermore, the EE marker Rab5 has been identified to interact with NS4B and the autophagosomal regulators Beclin1 and Vps34, resulting in the activation of autophagy[16] (Table 1 and Figure 2). NS5A was described to induce the formation of DMVs and MMVs[90,150] and has been recently identified to induce autophagy as it triggers fusion of the autophagosome with the lysosome[15]. Moreover, NS5A interacts with the adaptor protein ARFGAP1, involved in COPI trafficking, to stabilize the PI4P enriched environment in the MW[95]. In addition, Sir et al[13] could detect NS5A, NS5B and viral RNA on autophagosomal structures.
Taken together, induction of autophagy is crucial for the onset of the infection process. Interaction between HCV NS proteins and molecules of the autophagic pathway is required for the reorganization of intracellular membranes resulting in the formation of the MW.
Ait-Goughoulte et al[12] indicated that serial passages of an HCV genotype 1a (clone H77) isolated in immortalized human hepatocytes (IHH) resulted in the accumulation of LC3B-positive autophagic vesicles. This could be confirmed by other groups using different cells types and full-length as well as subgenomic replicons of different virus strains[14,17,91,154]. Ke et al[21,137] further demonstrated that HCV induces complete autophagy via the unfolded protein response (UPR) to promote viral replication. In contrast to this, using subgenomic replicons, Sir et al[14] claimed that maturation of autophagic vesicles seems to be incomplete in HCV replicating cells, since degradation of the long lived protein p62, a polyubiquitin binding protein that is known to be degraded during autophagy, is not enhanced. Subgenomic replicons enable the analysis of the crosstalk between HCV genome replication and autophagy in the absence of processes that are triggered by virus morphogenesis or release. Based on this, they further demonstrated that down-regulation of Atg7 and LC3 that are crucial for autophagosome formation reduced HCV replication. Moreover, they were able to co-preciptate the HCV replication complex with autophagosomes indicating that the membranous structure of virus-induced autophagosomes may serve as site of RNA replication in the early state of infection[13]. However, inhibition of PI3K3, involved in the initial step of autophagy, did not affect HCV replication. The data regarding the involvement of the autophagic pathway during HCV replication are conflicting and not completely understood. The inconsistent results might be explained by the use of different cell culture systems. In vitro studies of HCV are based on human hepatoma cell lines (Huh7). Huh7 clones behave differently and vary in their ability to induce an innate immune response. In line with this, it has been reported that HCV-induced autophagic response represses the innate antiviral immunity[11,153-155]. Based on this, one could argue that the contradictory results can be in part deduced from the different cellular background. In addition, the conflicting results can be referred to different experimental settings using either full length or subgenomic HCV replicons of different virus strains. Nevertheless the published data indicate that autophagy favors HCV replication.
Particle assembly and release occurs in the MW and is closely linked to the VLDL synthesis. The mature viral particles are finally released via the secretory pathway. However, the detailed mechanism still remains to be elucidated. Initial experiments of our group suggested an involvement of the autophagic pathway in the release of the LVPs. Here, the TIP47/Rab9 complex plays an important role (Table 1 and Figure 2) as particles loaded with TIP47 lacking Rab9 are targeted towards the autophagosome[134]. Moreover, the inhibition of autophagolysosome formation resulted in a decrease of released infectious viral particles (not published data). Additionally, Tanida et al[17] recently observed that the silencing of Beclin-1 and Atg7 reduces the release of infectious viral particles, but did not affect the intracellular amount of viral RNA. Besides, they could only detect a poor colocalization of LC3-II with LDs, NS5A and core. Moreover, Grégoire et al[18,148] observed that immunity-associated GTPase family M (IRGM) interacts with the autophagy-associated proteins Atg5, Atg10, MAP1CLC3C and SH3GBL1 and is involved in the activation of HCV-induced autophagy and release of viral particles (Figure 2).
These studies indicate that autophagy participates in the release of the viral particles. However, the detailed mechanisms are not understood. Further studies are required to elucidate the detailed role of autophagy for the release of HCV.
Besides its role in maintaining cell homeostasis, autophagy is involved in the defense against pathogens[156]. Autophagosomes hereby deliver intracellular pathogen-associated molecular patterns (PAMPs) to endosomal pattern recognition receptors (PRRs) or MHC-specific compartments in order to provoke an innate or adaptive immune response[157,158]. The innate immune response represents the first barrier after viral infection and can be activated by specific immune sensors (toll-like receptors (TLRs), RIG-I-like receptors (RLRs), and the nucleotide oligomerization domain (NOD)-like receptors) which detect PAMPs or intracellular viral nucleic acids. It has been described that autophagy can be directly activated by PAMP-dependent stimulation of TLRs resulting in the induction of an interferon (IFN)-response[159,160]. Furthermore, the autolysosomal degradation of viral particles or proteins leads to MHC class II-dependent antigen presentation and subsequent activation of the adaptive immune response[161]. Many viruses have developed strategies to escape the innate immune response. Like other viruses, HCV infection triggers an innate immune response through RIG-I/Mda5-mediated IFN signaling in order to establish an antiviral state[162,163]. However, to escape the induction of an IFN-response, HCV proteins interfere with different steps of the IFN signaling pathway. The HCV NS3/4A protease e.g., inhibits IFN signaling via blocking the RIG-I and TLR3 pathway[20,149]. In addition, HCV interferes with the activation of the double-stranded viral RNA-sensing kinase protein kinase R (PKR). HCV triggers the phosphorylation of PKR resulting in the phosphorylation of eIF2α and the consequential inhibition of translation including translation of ISGs. PKR is targeted by HCV core, E2 and NS5A that modulate the kinase activity by different mechanisms. In hepatocarcinoma (HCC) positive cells, PKR/core interaction results in the cell cycle arrest in the G2/M phase due to PKR phosphorylation on Thr446. Overexpression of the core protein further leads to increased PKR activity that does not correlate with enhanced eIF2α phosphorylation[164]. Moreover, the core protein activates the expression of OAS-1[165] and PKR[166] in the HCV genotype 1b. Further inactivation of PKR by NS5A has been described for the HCV genotype 1a and 1b[167-169]. In vitro studies indicate a PKR/E2 interaction and inhibition of PKR. As E2 accumulates in the ER and PKR can be found in the cystosol of the cell, in vivo studies suggest a PKR interaction with cytosolic unglycosylated E2[170] resulting in the inactivation of PKR via inhibition of PKR-like ER-resident kinase (PERK)[171]. The paradoxon is that the ability of HCV to partially activate PKR may be an advantage for the virus during an IFN response since it preferentially suppresses the translation of ISGs[172]. Moreover, the activation of PKR results in the activation of the autophagic response[157,173,174]. Recent studies indicate that HCV-induced autophagy represses the innate immune response[10,11,21,137,175]. Silencing of Beclin-1 and Atg7 in immortalized human hepatocytes (IHHs) infected with HCV restored the IFN signaling pathway by expression of OAS-1, IFN-α, IFN-β, IFI-27 and induced apoptosis[20]. In line with this, HCV-induced autophagy augments the PAMP-mediated innate immune response (Figure 2). The silencing of autophagy or autolysosome formation by treatment with chloroquine (CQ) and bafilomycin (BFLA) resulted in the upregulation of the innate immune response[21,137]. Moreover, recent studies demonstrate that persistent HCV infection results in an impaired immune response after treatment with IFNα and Ribavirin, whereas treatment with IFNλ provokes a strong sustained virological response[175]. As a result, persistent HCV infection leads to chronic ER stress and an autophagic response followed by impaired type I, but not type III IFN signaling and impaired JAK-STAT signaling. Moreover, the uptake of the antiviral drug RBV is impaired in persistently HCV-infected Huh7.5 cells due to a decreased surface expression of the equilibrative nucleoside transporter-1(ENT1) as described above[10]. Besides its role in controlling HCV-dependent innate immunity, autophagy is involved in the survival of the HCV-infected host cell to promote viral replication (Figure 2). Taguwa et al[155] observed that cells infected with HCV genotype 1b prevented vacuole formation by incomplete autolysosome formation to maintain persistent infection. Besides, it was reported that HCV induces autophagy by the upregulation of Beclin-1 and activation of mTOR signaling, thereby promoting hepatocyte cell growth[15].
Based on this, the induction of autophagy is part of the viral escape strategy to counteract viral infection.
Autophagy is a highly regulated and conserved cellular mechanism to maintain cellular homeostasis and to promote cell survival. As described in this review, autophagy interferes with different steps of the HCV life cycle to establish a persistent viral infection. However, the detailed mechanisms are not completely understood and remain to be elucidated. Moreover, HCV-induced autophagy represses apoptosis to promote cell survival. In addition, recent studies indicate that the HCV-induced autophagic response interferes with antiviral immunity as it reduces the innate immune response in HCV-infected cells. Hence, the disruption of autophagy restores IFN signaling and could restore the defective RBV-dependent antiviral response in vitro. Based on this, the improvement of existing antiviral therapies and development of new antiviral strategies targeting the autophagosomal response will be promising. Moreover, the development of vaccines to eradicate HCV infection and the linked HCV-associated pathogenesis depict an auspicious future perspective.
P- Reviewer: Liu HM, Roohvand F S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1077] [Cited by in RCA: 1145] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 2. | Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 3. | Kim do Y, Ahn SH, Han KH. Emerging therapies for hepatitis C. Gut Liver. 2014;8:471-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Kronenberger B, Zeuzem S. New developments in HCV therapy. J Viral Hepat. 2012;19 Suppl 1:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Peiffer KH, Akhras S, Himmelsbach K, Hassemer M, Finkernagel M, Carra G, Nuebling M, Chudy M, Niekamp H, Glebe D. Intracellular accumulation of subviral HBsAg particles and diminished Nrf2 activation in HBV genotype G expressing cells lead to an increased ROI level. J Hepatol. 2015;62:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Thomas DL. Global control of hepatitis C: where challenge meets opportunity. Nat Med. 2013;19:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 7. | Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 734] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 8. | Koh C, Liang TJ. What is the future of ribavirin therapy for hepatitis C? Antiviral Res. 2014;104:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Curr Drug Metab. 2004;5:63-84. [PubMed] |
| 10. | Panigrahi R, Chandra PK, Ferraris P, Kurt R, Song K, Garry RF, Reiss K, Coe IR, Furihata T, Balart LA. Persistent hepatitis C virus infection impairs ribavirin antiviral activity through clathrin-mediated trafficking of equilibrative nucleoside transporter 1. J Virol. 2015;89:626-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Dreux M, Gastaminza P, Wieland SF, Chisari FV. The autophagy machinery is required to initiate hepatitis C virus replication. Proc Natl Acad Sci USA. 2009;106:14046-14051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 388] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 12. | Ait-Goughoulte M, Kanda T, Meyer K, Ryerse JS, Ray RB, Ray R. Hepatitis C virus genotype 1a growth and induction of autophagy. J Virol. 2008;82:2241-2249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Sir D, Kuo CF, Tian Y, Liu HM, Huang EJ, Jung JU, Machida K, Ou JH. Replication of hepatitis C virus RNA on autophagosomal membranes. J Biol Chem. 2012;287:18036-18043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Sir D, Chen WL, Choi J, Wakita T, Yen TS, Ou JH. Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology. 2008;48:1054-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 292] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 15. | Shrivastava S, Bhanja Chowdhury J, Steele R, Ray R, Ray RB. Hepatitis C virus upregulates Beclin1 for induction of autophagy and activates mTOR signaling. J Virol. 2012;86:8705-8712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Su WC, Chao TC, Huang YL, Weng SC, Jeng KS, Lai MM. Rab5 and class III phosphoinositide 3-kinase Vps34 are involved in hepatitis C virus NS4B-induced autophagy. J Virol. 2011;85:10561-10571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Tanida I, Fukasawa M, Ueno T, Kominami E, Wakita T, Hanada K. Knockdown of autophagy-related gene decreases the production of infectious hepatitis C virus particles. Autophagy. 2009;5:937-945. [PubMed] |
| 18. | Grégoire IP, Richetta C, Meyniel-Schicklin L, Borel S, Pradezynski F, Diaz O, Deloire A, Azocar O, Baguet J, Le Breton M. IRGM is a common target of RNA viruses that subvert the autophagy network. PLoS Pathog. 2011;7:e1002422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Stoeckl L, Funk A, Kopitzki A, Brandenburg B, Oess S, Will H, Sirma H, Hildt E. Identification of a structural motif crucial for infectivity of hepatitis B viruses. Proc Natl Acad Sci USA. 2006;103:6730-6734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Shrivastava S, Raychoudhuri A, Steele R, Ray R, Ray RB. Knockdown of autophagy enhances the innate immune response in hepatitis C virus-infected hepatocytes. Hepatology. 2011;53:406-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Ke PY, Chen SS. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J Clin Invest. 2011;121:37-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 22. | Ke PY, Chen SS. Autophagy in hepatitis C virus-host interactions: potential roles and therapeutic targets for liver-associated diseases. World J Gastroenterol. 2014;20:5773-5793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Das GC, Hollinger FB. Molecular pathways for glucose homeostasis, insulin signaling and autophagy in hepatitis C virus induced insulin resistance in a cellular model. Virology. 2012;434:5-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Bao L, Chandra PK, Moroz K, Zhang X, Thung SN, Wu T, Dash S. Impaired autophagy response in human hepatocellular carcinoma. Exp Mol Pathol. 2014;96:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5421] [Cited by in RCA: 5289] [Article Influence: 311.1] [Reference Citation Analysis (0)] |
| 26. | Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2886] [Cited by in RCA: 2757] [Article Influence: 183.8] [Reference Citation Analysis (0)] |
| 27. | Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 1127] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 28. | Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14:759-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1041] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 29. | Yen WL, Shintani T, Nair U, Cao Y, Richardson BC, Li Z, Hughson FM, Baba M, Klionsky DJ. The conserved oligomeric Golgi complex is involved in double-membrane vesicle formation during autophagy. J Cell Biol. 2010;188:101-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 30. | Reggiori F, Shintani T, Nair U, Klionsky DJ. Atg9 cycles between mitochondria and the pre-autophagosomal structure in yeasts. Autophagy. 2005;1:101-109. [PubMed] |
| 31. | Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol. 2010;12:747-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 723] [Cited by in RCA: 694] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 32. | Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19:2092-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 868] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 33. | Fader CM, Colombo MI. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ. 2009;16:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Silva LM, Jung JU. Modulation of the autophagy pathway by human tumor viruses. Semin Cancer Biol. 2013;23:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Schroeder B, Schulze RJ, Weller SG, Sletten AC, Casey CA, McNiven MA. The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology. 2015;61:1896-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 36. | Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151:1256-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 989] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 37. | Itakura E, Mizushima N. Syntaxin 17: the autophagosomal SNARE. Autophagy. 2013;9:917-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Hegedűs K, Takáts S, Kovács AL, Juhász G. Evolutionarily conserved role and physiological relevance of a STX17/Syx17 (syntaxin 17)-containing SNARE complex in autophagosome fusion with endosomes and lysosomes. Autophagy. 2013;9:1642-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 980] [Cited by in RCA: 950] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 40. | Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2117] [Cited by in RCA: 2229] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 41. | Yoshida H, Oku M, Suzuki M, Mori K. pXBP1(U) encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1(S) in mammalian ER stress response. J Cell Biol. 2006;172:565-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 317] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 42. | Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99-111. [PubMed] |
| 43. | Jiang H, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the Subunit of Eukaryotic Initiation Factor 2 Is Required for Activation of NF- B in Response to Diverse Cellular Stresses. Mol Cell Biol. 2003;23:5651-5663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 352] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 44. | Nakajima S, Kitamura M. Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response. Free Radic Biol Med. 2013;65:162-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 45. | Cullinan SB, Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38:317-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 435] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 46. | Digaleh H, Kiaei M, Khodagholi F. Nrf2 and Nrf1 signaling and ER stress crosstalk: implication for proteasomal degradation and autophagy. Cell Mol Life Sci. 2013;70:4681-4694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2965] [Cited by in RCA: 2854] [Article Influence: 158.6] [Reference Citation Analysis (0)] |
| 48. | Kwak M, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants Enhance Mammalian Proteasome Expression through the Keap1-Nrf2 Signaling Pathway. Mol Cell Biol. 2003;23:8786-8794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 409] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 49. | Kwak MK, Kensler TW. Induction of 26S proteasome subunit PSMB5 by the bifunctional inducer 3-methylcholanthrene through the Nrf2-ARE, but not the AhR/Arnt-XRE, pathway. Biochem Biophys Res Commun. 2006;345:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 51. | Riley BE, Kaiser SE, Kopito RR. Autophagy inhibition engages Nrf2-p62 Ub-associated signaling. Autophagy. 2011;7:338-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 923] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 53. | Simmonds P. Genetic diversity and evolution of hepatitis C virus--15 years on. J Gen Virol. 2004;85:3173-3188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 632] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 54. | Lindenbach BD, Rice CM. The ins and outs of hepatitis C virus entry and assembly. Nat Rev Microbiol. 2013;11:688-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 55. | Pietschmann T, Lohmann V, Rutter G, Kurpanek K, Bartenschlager R. Characterization of cell lines carrying self-replicating hepatitis C virus RNAs. J Virol. 2001;75:1252-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 288] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 56. | André P, Perlemuter G, Budkowska A, Bréchot C, Lotteau V. Hepatitis C virus particles and lipoprotein metabolism. Semin Liver Dis. 2005;25:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 57. | Andre P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Brechot C, Paranhos-Baccala G, Lotteau V. Characterization of Low- and Very-Low-Density Hepatitis C Virus RNA-Containing Particles. J Virol. 2002;76:6919-6928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 517] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 58. | Lindenbach BD, Meuleman P, Ploss A, Vanwolleghem T, Syder AJ, McKeating JA, Lanford RE, Feinstone SM, Major ME, Leroux-Roels G. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci USA. 2006;103:3805-3809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 343] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 59. | Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1849] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 60. | Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294-9299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1475] [Cited by in RCA: 1466] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 61. | Gastaminza P, Dryden KA, Boyd B, Wood MR, Law M, Yeager M, Chisari FV. Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture. J Virol. 2010;84:10999-11009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 62. | Chang KS, Jiang J, Cai Z, Luo G. Human apolipoprotein e is required for infectivity and production of hepatitis C virus in cell culture. J Virol. 2007;81:13783-13793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 63. | Bradley D, McCaustland K, Krawczynski K, Spelbring J, Humphrey C, Cook EH. Hepatitis C virus: buoyant density of the factor VIII-derived isolate in sucrose. J Med Virol. 1991;34:206-208. [PubMed] |
| 64. | Hijikata M, Shimizu YK, Kato H, Iwamoto A, Shih JW, Alter HJ, Purcell RH, Yoshikura H. Equilibrium centrifugation studies of hepatitis C virus: evidence for circulating immune complexes. J Virol. 1993;67:1953-1958. [PubMed] |
| 65. | Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci USA. 1999;96:12766-12771. [PubMed] |
| 66. | Germi R, Crance JM, Garin D, Guimet J, Lortat-Jacob H, Ruigrok RW, Zarski JP, Drouet E. Cellular glycosaminoglycans and low density lipoprotein receptor are involved in hepatitis C virus adsorption. J Med Virol. 2002;68:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Barth H, Schafer C, Adah MI, Zhang F, Linhardt RJ, Toyoda H, Kinoshita-Toyoda A, Toida T, Van Kuppevelt TH, Depla E. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J Biol Chem. 2003;278:41003-41012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 349] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 68. | Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017-5025. [PubMed] |
| 69. | Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G. Binding of hepatitis C virus to CD81. Science. 1998;282:938-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 1552] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 70. | Bertaux C, Dragic T, Bertaux C, Dragic T. Different domains of CD81 mediate distinct stages of hepatitis C virus pseudoparticle entry. J Virol. 2006;80:4940-4988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Shi Q, Jiang J, Luo G. Syndecan-1 serves as the major receptor for attachment of hepatitis C virus to the surfaces of hepatocytes. J Virol. 2013;87:6866-6875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 72. | Lefèvre M, Felmlee DJ, Parnot M, Baumert TF, Schuster C. Syndecan 4 is involved in mediating HCV entry through interaction with lipoviral particle-associated apolipoprotein E. PLoS One. 2014;9:e95550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 73. | Xu Y, Martinez P, Séron K, Luo G, Allain F, Dubuisson J, Belouzard S. Characterization of hepatitis C virus interaction with heparan sulfate proteoglycans. J Virol. 2015;89:3846-3858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 74. | Jiang J, Cun W, Wu X, Shi Q, Tang H, Luo G. Hepatitis C virus attachment mediated by apolipoprotein E binding to cell surface heparan sulfate. J Virol. 2012;86:7256-7267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 75. | Khan AG, Whidby J, Miller MT, Scarborough H, Zatorski AV, Cygan A, Price AA, Yost SA, Bohannon CD, Jacob J. Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2. Nature. 2014;509:381-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 230] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 76. | Lavie M, Sarrazin S, Montserret R, Descamps V, Baumert TF, Duverlie G, Séron K, Penin F, Dubuisson J. Identification of conserved residues in hepatitis C virus envelope glycoprotein E2 that modulate virus dependence on CD81 and SRB1 entry factors. J Virol. 2014;88:10584-10597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 77. | Douam F, Lavillette D, Cosset FL. The mechanism of HCV entry into host cells. Prog Mol Biol Transl Sci. 2015;129:63-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 78. | Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wölk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 942] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 79. | Liu S, Yang W, Shen L, Turner JR, Coyne CB, Wang T. Tight junction proteins claudin-1 and occludin control hepatitis C virus entry and are downregulated during infection to prevent superinfection. J Virol. 2009;83:2011-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 80. | Dorner M, Horwitz JA, Robbins JB, Barry WT, Feng Q, Mu K, Jones CT, Schoggins JW, Catanese MT, Burton DR. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 81. | Dorner M, Horwitz JA, Donovan BM, Labitt RN, Budell WC, Friling T, Vogt A, Catanese MT, Satoh T, Kawai T. Completion of the entire hepatitis C virus life cycle in genetically humanized mice. Nature. 2013;501:237-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 82. | Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, Wychowski C, Rouillé Y. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80:6964-6972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 432] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 83. | Meertens L, Bertaux C, Dragic T. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J Virol. 2006;80:11571-11578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 84. | Koutsoudakis G, Kaul A, Steinmann E, Kallis S, Lohmann V, Pietschmann T, Bartenschlager R. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J Virol. 2006;80:5308-5320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 332] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 85. | Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 568] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 86. | Zona L, Lupberger J, Sidahmed-Adrar N, Thumann C, Harris HJ, Barnes A, Florentin J, Tawar RG, Xiao F, Turek M. HRas signal transduction promotes hepatitis C virus cell entry by triggering assembly of the host tetraspanin receptor complex. Cell Host Microbe. 2013;13:302-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 87. | Sainz B, Barretto N, Martin DN, Hiraga N, Imamura M, Hussain S, Marsh KA, Yu X, Chayama K, Alrefai WA. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med. 2012;18:281-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 352] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 88. | Martin DN, Uprichard SL. Identification of transferrin receptor 1 as a hepatitis C virus entry factor. Proc Natl Acad Sci USA. 2013;110:10777-10782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 89. | Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1005] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 90. | Romero-Brey I, Merz A, Chiramel A, Lee JY, Chlanda P, Haselman U, Santarella-Mellwig R, Habermann A, Hoppe S, Kallis S. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS Pathog. 2012;8:e1003056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 383] [Cited by in RCA: 402] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 91. | Ferraris P, Blanchard E, Roingeard P. Ultrastructural and biochemical analyses of hepatitis C virus-associated host cell membranes. J Gen Virol. 2010;91:2230-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 92. | Paul D, Hoppe S, Saher G, Krijnse-Locker J, Bartenschlager R. Morphological and biochemical characterization of the membranous hepatitis C virus replication compartment. J Virol. 2013;87:10612-10627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 93. | Egger D, Wölk B, Gosert R, Bianchi L, Blum HE, Moradpour D, Bienz K. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol. 2002;76:5974-5984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 633] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 94. | Reiss S, Rebhan I, Backes P, Romero-Brey I, Erfle H, Matula P, Kaderali L, Poenisch M, Blankenburg H, Hiet MS. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe. 2011;9:32-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 406] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 95. | Li H, Yang X, Yang G, Hong Z, Zhou L, Yin P, Xiao Y, Chen L, Chung RT, Zhang L. Hepatitis C virus NS5A hijacks ARFGAP1 to maintain a phosphatidylinositol 4-phosphate-enriched microenvironment. J Virol. 2014;88:5956-5966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Park CY, Jun HJ, Wakita T, Cheong JH, Hwang SB. Hepatitis C virus nonstructural 4B protein modulates sterol regulatory element-binding protein signaling via the AKT pathway. J Biol Chem. 2009;284:9237-9246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 97. | Lesburg CA, Cable MB, Ferrari E, Hong Z, Mannarino AF, Weber PC. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nat Struct Biol. 1999;6:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 571] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 98. | Appleby TC, Perry JK, Murakami E, Barauskas O, Feng J, Cho A, Fox D, Wetmore DR, McGrath ME, Ray AS. Viral replication. Structural basis for RNA replication by the hepatitis C virus polymerase. Science. 2015;347:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 99. | Lohmann V. Hepatitis C virus RNA replication. Curr Top Microbiol Immunol. 2013;369:167-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 100. | Roberts AP, Lewis AP, Jopling CL. miR-122 activates hepatitis C virus translation by a specialized mechanism requiring particular RNA components. Nucleic Acids Res. 2011;39:7716-7729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 101. | Hu J, Xu Y, Hao J, Wang S, Li C, Meng S. MiR-122 in hepatic function and liver diseases. Protein Cell. 2012;3:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 102. | Fischer G, Aumüller T. Regulation of peptide bond cis/trans isomerization by enzyme catalysis and its implication in physiological processes. Rev Physiol Biochem Pharmacol. 2003;148:105-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 103. | Yang F, Robotham JM, Nelson HB, Irsigler A, Kenworthy R, Tang H. Cyclophilin A is an essential cofactor for hepatitis C virus infection and the principal mediator of cyclosporine resistance in vitro. J Virol. 2008;82:5269-5278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 104. | Watashi K, Hijikata M, Hosaka M, Yamaji M, Shimotohno K. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003;38:1282-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 409] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 105. | Chatterji U, Bobardt M, Tai A, Wood M, Gallay PA. Cyclophilin and NS5A inhibitors, but not other anti-hepatitis C virus (HCV) agents, preclude HCV-mediated formation of double-membrane-vesicle viral factories. Antimicrob Agents Chemother. 2015;59:2496-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 106. | Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 980] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 107. | Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M, Ye J. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci USA. 2007;104:5848-5853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 429] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 108. | Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 521] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 109. | Boulant S, Douglas MW, Moody L, Budkowska A, Targett-Adams P, McLauchlan J. Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule- and dynein-dependent manner. Traffic. 2008;9:1268-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 110. | Camus G, Herker E, Modi AA, Haas JT, Ramage HR, Farese RV, Ott M. Diacylglycerol acyltransferase-1 localizes hepatitis C virus NS5A protein to lipid droplets and enhances NS5A interaction with the viral capsid core. J Biol Chem. 2013;288:9915-9923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 111. | Shavinskaya A, Boulant S, Penin F, McLauchlan J, Bartenschlager R. The lipid droplet binding domain of hepatitis C virus core protein is a major determinant for efficient virus assembly. J Biol Chem. 2007;282:37158-37169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 112. | Menzel N, Fischl W, Hueging K, Bankwitz D, Frentzen A, Haid S, Gentzsch J, Kaderali L, Bartenschlager R, Pietschmann T. MAP-kinase regulated cytosolic phospholipase A2 activity is essential for production of infectious hepatitis C virus particles. PLoS Pathog. 2012;8:e1002829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 113. | Salloum S, Wang H, Ferguson C, Parton RG, Tai AW. Rab18 binds to hepatitis C virus NS5A and promotes interaction between sites of viral replication and lipid droplets. PLoS Pathog. 2013;9:e1003513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 114. | Sato S, Fukasawa M, Yamakawa Y, Natsume T, Suzuki T, Shoji I, Aizaki H, Miyamura T, Nishijima M. Proteomic profiling of lipid droplet proteins in hepatoma cell lines expressing hepatitis C virus core protein. J Biochem. 2006;139:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 115. | Bulankina AV, Deggerich A, Wenzel D, Mutenda K, Wittmann JG, Rudolph MG, Burger KN, Höning S. TIP47 functions in the biogenesis of lipid droplets. J Cell Biol. 2009;185:641-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 116. | Díaz E, Pfeffer SR. TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell. 1998;93:433-443. [PubMed] |
| 117. | Hickenbottom SJ, Kimmel AR, Londos C, Hurley JH. Structure of a lipid droplet protein; the PAT family member TIP47. Structure. 2004;12:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 118. | Benga WJ, Krieger SE, Dimitrova M, Zeisel MB, Parnot M, Lupberger J, Hildt E, Luo G, McLauchlan J, Baumert TF. Apolipoprotein E interacts with hepatitis C virus nonstructural protein 5A and determines assembly of infectious particles. Hepatology. 2010;51:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 119. | Cun W, Jiang J, Luo G. The C-terminal alpha-helix domain of apolipoprotein E is required for interaction with nonstructural protein 5A and assembly of hepatitis C virus. J Virol. 2010;84:11532-11541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 120. | Jiang J, Luo G. Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles. J Virol. 2009;83:12680-12691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 121. | Ploen D, Hafirassou ML, Himmelsbach K, Sauter D, Biniossek ML, Weiss TS, Baumert TF, Schuster C, Hildt E. TIP47 plays a crucial role in the life cycle of hepatitis C virus. J Hepatol. 2013;58:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 122. | Vogt DA, Camus G, Herker E, Webster BR, Tsou CL, Greene WC, Yen TS, Ott M. Lipid droplet-binding protein TIP47 regulates hepatitis C Virus RNA replication through interaction with the viral NS5A protein. PLoS Pathog. 2013;9:e1003302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 123. | Appel N, Zayas M, Miller S, Krijnse-Locker J, Schaller T, Friebe P, Kallis S, Engel U, Bartenschlager R. Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog. 2008;4:e1000035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 375] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 124. | Masaki T, Suzuki R, Murakami K, Aizaki H, Ishii K, Murayama A, Date T, Matsuura Y, Miyamura T, Wakita T. Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. J Virol. 2008;82:7964-7976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 295] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 125. | Tellinghuisen TL, Foss KL, Treadaway J. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 2008;4:e1000032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 126. | Jirasko V, Montserret R, Lee JY, Gouttenoire J, Moradpour D, Penin F, Bartenschlager R. Structural and functional studies of nonstructural protein 2 of the hepatitis C virus reveal its key role as organizer of virion assembly. PLoS Pathog. 2010;6:e1001233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 127. | Popescu CI, Callens N, Trinel D, Roingeard P, Moradpour D, Descamps V, Duverlie G, Penin F, Héliot L, Rouillé Y. NS2 protein of hepatitis C virus interacts with structural and non-structural proteins towards virus assembly. PLoS Pathog. 2011;7:e1001278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 128. | Ariumi Y, Kuroki M, Maki M, Ikeda M, Dansako H, Wakita T, Kato N. The ESCRT system is required for hepatitis C virus production. PLoS One. 2011;6:e14517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 129. | Corless L, Crump CM, Griffin SD, Harris M. Vps4 and the ESCRT-III complex are required for the release of infectious hepatitis C virus particles. J Gen Virol. 2010;91:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 130. | Bartenschlager R, Penin F, Lohmann V, André P. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 2011;19:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 131. | Coller KE, Heaton NS, Berger KL, Cooper JD, Saunders JL, Randall G. Molecular determinants and dynamics of hepatitis C virus secretion. PLoS Pathog. 2012;8:e1002466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 132. | Vieyres G, Thomas X, Descamps V, Duverlie G, Patel AH, Dubuisson J. Characterization of the envelope glycoproteins associated with infectious hepatitis C virus. J Virol. 2010;84:10159-10168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 133. | Counihan NA, Rawlinson SM, Lindenbach BD. Trafficking of hepatitis C virus core protein during virus particle assembly. PLoS Pathog. 2011;7:e1002302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 134. | Ploen D, Hafirassou ML, Himmelsbach K, Schille SA, Biniossek ML, Baumert TF, Schuster C, Hildt E. TIP47 is associated with the hepatitis C virus and its interaction with Rab9 is required for release of viral particles. Eur J Cell Biol. 2013;92:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 135. | Poenisch M, Metz P, Blankenburg H, Ruggieri A, Lee JY, Rupp D, Rebhan I, Diederich K, Kaderali L, Domingues FS. Identification of HNRNPK as regulator of hepatitis C virus particle production. PLoS Pathog. 2015;11:e1004573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 136. | Tardif KD, Mori K, Siddiqui A. Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J Virol. 2002;76:7453-7459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 137. | Ke PY, Chen SS. Autophagy: a novel guardian of HCV against innate immune response. Autophagy. 2011;7:533-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 138. | Asselah T, Bièche I, Mansouri A, Laurendeau I, Cazals-Hatem D, Feldmann G, Bedossa P, Paradis V, Martinot-Peignoux M, Lebrec D. In vivo hepatic endoplasmic reticulum stress in patients with chronic hepatitis C. J Pathol. 2010;221:264-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 139. | Cocquerel L, Meunier JC, Pillez A, Wychowski C, Dubuisson J. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J Virol. 1998;72:2183-2191. [PubMed] |
| 140. | Choukhi A, Ung S, Wychowski C, Dubuisson J. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J Virol. 1998;72:3851-3858. [PubMed] |
| 141. | Bartosch B, Thimme R, Blum HE, Zoulim F. Hepatitis C virus-induced hepatocarcinogenesis. J Hepatol. 2009;51:810-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 142. | Carvajal-Yepes M, Himmelsbach K, Schaedler S, Ploen D, Krause J, Ludwig L, Weiss T, Klingel K, Hildt E. Hepatitis C virus impairs the induction of cytoprotective Nrf2 target genes by delocalization of small Maf proteins. J Biol Chem. 2011;286:8941-8951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 143. | Beyer TA, Xu W, Teupser D, auf dem Keller U, Bugnon P, Hildt E, Thiery J, Kan YW, Werner S. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27:212-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 144. | Li S, Ye L, Yu X, Xu B, Li K, Zhu X, Liu H, Wu X, Kong L. Hepatitis C virus NS4B induces unfolded protein response and endoplasmic reticulum overload response-dependent NF-kappaB activation. Virology. 2009;391:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 145. | Li L, Kim E, Yuan H, Inoki K, Goraksha-Hicks P, Schiesher RL, Neufeld TP, Guan KL. Regulation of mTORC1 by the Rab and Arf GTPases. J Biol Chem. 2010;285:19705-19709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 146. | Simonsen A, Tooze SA. Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. J Cell Biol. 2009;186:773-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 382] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 147. | Stone M, Jia S, Heo WD, Meyer T, Konan KV. Participation of rab5, an early endosome protein, in hepatitis C virus RNA replication machinery. J Virol. 2007;81:4551-4563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 148. | Grégoire IP, Rabourdin-Combe C, Faure M. Autophagy and RNA virus interactomes reveal IRGM as a common target. Autophagy. 2012;8:1136-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 149. | Vescovo T, Refolo G, Romagnoli A, Ciccosanti F, Corazzari M, Alonzi T, Fimia GM. Autophagy in HCV infection: keeping fat and inflammation at bay. Biomed Res Int. 2014;2014:265353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 150. | Romero-Brey I, Bartenschlager R. Membranous replication factories induced by plus-strand RNA viruses. Viruses. 2014;6:2826-2857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 212] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 151. | Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657-668. [PubMed] |
| 152. | Guévin C, Manna D, Bélanger C, Konan KV, Mak P, Labonté P. Autophagy protein ATG5 interacts transiently with the hepatitis C virus RNA polymerase (NS5B) early during infection. Virology. 2010;405:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 153. | Dreux M, Chisari FV. Autophagy proteins promote hepatitis C virus replication. Autophagy. 2009;5:1224-1225. [PubMed] |
| 154. | Dreux M, Chisari FV. Impact of the autophagy machinery on hepatitis C virus infection. Viruses. 2011;3:1342-1357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 155. | Taguwa S, Kambara H, Fujita N, Noda T, Yoshimori T, Koike K, Moriishi K, Matsuura Y. Dysfunction of autophagy participates in vacuole formation and cell death in cells replicating hepatitis C virus. J Virol. 2011;85:13185-13194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 156. | Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 723] [Cited by in RCA: 716] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 157. | Orvedahl A, Levine B. Eating the enemy within: autophagy in infectious diseases. Cell Death Differ. 2009;16:57-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 158. | Chiramel AI, Brady NR, Bartenschlager R. Divergent roles of autophagy in virus infection. Cells. 2013;2:83-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 159. | Delgado MA, Deretic V. Toll-like receptors in control of immunological autophagy. Cell Death Differ. 2009;16:976-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 160. | Into T, Inomata M, Takayama E, Takigawa T. Autophagy in regulation of Toll-like receptor signaling. Cell Signal. 2012;24:1150-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 161. | Münz C. Antigen processing by macroautophagy for MHC presentation. Front Immunol. 2011;2:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 162. | Katze MG, Fornek JL, Palermo RE, Walters KA, Korth MJ. Innate immune modulation by RNA viruses: emerging insights from functional genomics. Nat Rev Immunol. 2008;8:644-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 163. | Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1338] [Cited by in RCA: 1484] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 164. | Alisi A, Mele R, Spaziani A, Tavolaro S, Palescandolo E, Balsano C. Thr 446 phosphorylation of PKR by HCV core protein deregulates G2/M phase in HCC cells. J Cell Physiol. 2005;205:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 165. | Dansako H, Naganuma A, Nakamura T, Ikeda F, Nozaki A, Kato N. Differential activation of interferon-inducible genes by hepatitis C virus core protein mediated by the interferon stimulated response element. Virus Res. 2003;97:17-30. [PubMed] |
| 166. | Delhem N, Sabile A, Gajardo R, Podevin P, Abadie A, Blaton MA, Kremsdorf D, Beretta L, Brechot C. Activation of the interferon-inducible protein kinase PKR by hepatocellular carcinoma derived-hepatitis C virus core protein. Oncogene. 2001;20:5836-5845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 167. | Gale MJ, Korth MJ, Tang NM, Tan SL, Hopkins DA, Dever TE, Polyak SJ, Gretch DR, Katze MG. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology. 1997;230:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 608] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 168. | Gale M, Blakely CM, Kwieciszewski B, Tan SL, Dossett M, Tang NM, Korth MJ, Polyak SJ, Gretch DR, Katze MG. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol Cell Biol. 1998;18:5208-5218. [PubMed] |
| 169. | François C, Duverlie G, Rebouillat D, Khorsi H, Castelain S, Blum HE, Gatignol A, Wychowski C, Moradpour D, Meurs EF. Expression of hepatitis C virus proteins interferes with the antiviral action of interferon independently of PKR-mediated control of protein synthesis. J Virol. 2000;74:5587-5596. [PubMed] |
| 170. | Pavio N, Taylor DR, Lai MM. Detection of a Novel Unglycosylated Form of Hepatitis C Virus E2 Envelope Protein That Is Located in the Cytosol and Interacts with PKR. J Virol. 2002;76:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 171. | Pavio N, Romano PR, Graczyk TM, Feinstone SM, Taylor DR. Protein Synthesis and Endoplasmic Reticulum Stress Can Be Modulated by the Hepatitis C Virus Envelope Protein E2 through the Eukaryotic Initiation Factor 2 Kinase PERK. J Virol. 2003;77:3578-3585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 172. | Garaigorta U, Chisari FV. Hepatitis C virus blocks interferon effector function by inducing protein kinase R phosphorylation. Cell Host Microbe. 2009;6:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 173. | Lee HK, Iwasaki A. Autophagy and antiviral immunity. Curr Opin Immunol. 2008;20:23-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 174. | Dabo S, Meurs EF. dsRNA-dependent protein kinase PKR and its role in stress, signaling and HCV infection. Viruses. 2012;4:2598-2635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 175. | Chandra PK, Bao L, Song K, Aboulnasr FM, Baker DP, Shores N, Wimley WC, Liu S, Hagedorn CH, Fuchs SY. HCV infection selectively impairs type I but not type III IFN signaling. Am J Pathol. 2014;184:214-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |