Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8249
Peer-review started: January 27, 2015
First decision: March 10, 2015
Revised: March 16, 2015
Accepted: May 4, 2015
Article in press: May 4, 2015
Published online: July 21, 2015
Processing time: 177 Days and 1.6 Hours
Although liver resection is considered the most effective treatment for hepatocellular carcinoma (HCC), treatment outcomes are unsatisfactory because of the high rate of HCC recurrence. Since we reported hepatitis B e-antigen positivity and high serum hepatitis B virus (HBV) DNA concentrations are strong risk factors for HCC recurrence after curative resection of HBV-related HCC in the early 2000s, many investigators have demonstrated the effects of viral status on HCC recurrence and post-treatment outcomes. These findings suggest controlling viral status is important to prevent HCC recurrence and improve survival after curative treatment for HBV-related HCC. Antiviral therapy after curative treatment aims to improve prognosis by preventing HCC recurrence and maintaining liver function. Therapy with interferon and nucleos(t)ide analogs may be useful for preventing HCC recurrence and improving overall survival in patients who have undergone curative resection for HBV-related HCC. In addition, reactivation of viral replication can occur after liver resection for HBV-related HCC. Antiviral therapy can be recommended for patients to prevent HBV reactivation. Nevertheless, further studies are required to establish treatment guidelines for patients with HBV-related HCC.
Core tip: Although liver resection is considered the most effective treatment for hepatocellular carcinoma (HCC), treatment outcomes are unsatisfactory because of the high rate of HCC recurrence. Many investigators have demonstrated the effects of viral status on HCC recurrence and post-treatment outcomes. Therapy with interferon and nucleos(t)ide analogs may be useful for preventing HCC recurrence and improving overall survival in patients who have undergone curative resection for hepatitis B virus (HBV)-related HCC. In addition, reactivation of viral replication can occur after liver resection for HBV-related HCC. Antiviral therapy can be recommended for patients to prevent HBV reactivation.
- Citation: Kubo S, Takemura S, Tanaka S, Shinkawa H, Nishioka T, Nozawa A, Kinoshita M, Hamano G, Ito T, Urata Y. Management of hepatitis B virus infection during treatment for hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol 2015; 21(27): 8249-8255
- URL: https://www.wjgnet.com/1007-9327/full/v21/i27/8249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i27.8249
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third most common cause of cancer mortality[1,2]. Chronic infection with hepatitis B virus (HBV) is a major risk factor for HCC development. Risk factors for HCC among HBV carriers include male sex and advanced age, longer infection, alcohol consumption, and hepatic fibrosis[3]. The incidence of HCC is reportedly 0.2 per 100 person-years among inactive carriers, between 0.8 and 1.0 among people with chronic hepatitis B infections without cirrhosis, and between 3.2 and 4.3 among patients with compensated cirrhosis[4]. Although liver resection and radiofrequency ablation therapy are curative treatments for HCC, treatment outcomes are often unsatisfactory because of the high rate of HCC recurrence, which includes metastases from primary carcinomas and multicentric carcinogenesis after treatment (i.e., multicentric recurrence). Risk factors for HCC recurrence include tumor factors such as tumor number and vascular invasion, which are related to metastasis from the primary carcinoma. Multicentric carcinogenesis is closely associated with persistent active hepatitis and hepatic fibrosis. Therefore, strategies for both HCC and chronic hepatitis B infection are required to improve the post-treatment outcome of HCC in patients with chronic hepatitis B infections[5]. Liver transplantation is the most radical treatment for HCC as well as the treatment of underlying disease including chronic hepatitis B infection. However, donor shortage is a major problem associated with liver transplantation.
Since we reported in the early 2000s that hepatitis B e-antigen (HBeAg) positivity and high serum concentrations of HBV DNA are strong risk factors for HCC recurrence after curative resection of HBV-related HCC[6-8], many investigators have reported the effects of viral status in HBV-infected individuals on HCC recurrence and post-treatment outcomes[9-13]. These studies suggest controlling viral status is important to prevent HCC recurrence and improve survival after curative treatment for HBV-related HCC. Since we first reported the reactivation of viral replication occurred after liver resection in patients with HBV-related HCC in 2001[14], some investigators have reported the same findings. It is important to prevent such reactivation after liver resection[15-17].
Two types of drugs, conventional interferon (IFN)-α or pegylated IFN (PEG-IFN)-alpha, and nucleos(t)ide analogs (NAs), have recently become available for the treatment of chronic hepatitis B infection. Studies have shown that IFN therapy reduces progression to cirrhosis and HCC development[18,19]. Meanwhile antiviral therapy with NAs including lamivudine (LAM), adefovir dipivoxil (ADV), and entecavir (ETV), suppresses viral replication and appears to be useful for preventing progression to cirrhosis and HCC development in patients with chronic hepatitis B infection[20-26].
This paper reviews the impacts of perioperative antiviral therapy on viral replication reactivation after treatment as well as on HCC recurrence and survival following curative treatment for HBV-related HCC.
Reactivation of HBV replication in patients who receive cytotoxic or immunosuppressive therapy is well recognized. Since we first reported the reactivation of viral replication occurring after liver resection in patients with HBV-related HCC[14], some investigators have also reported reactivation after chemoembolization, radiofrequency ablation therapy, and liver resection[15-17]. Reactivation occurred in 20%-30% of patients who underwent liver resection. The risk factors for HBV reactivation include high alanine aminotransferase activity, high viral load, the presence of wild-type DNA, and the detection of hepatitis B core antigen in hepatocytes, which are all features of the immune clearance phase in the natural course of HBV infection[14]. Huang et al[27] recently reported antiviral therapy using telbivudine can significantly decrease the perioperative reactivation of viral replication after liver resection for HBV-related HCC. We also did not have experienced acute exacerbation since the induction of NAs. Changes in serum HBV DNA levels should be monitored during the treatment for HBV-related HCC. Antiviral therapy can be recommended for patients with risk factor(s) for HBV reactivation.
IFN has been used to treat chronic hepatitis B infection for many years. IFNs are cytokines that possess a variety of biologic properties, including antiviral, immunomodulatory, antiproliferative, antiangiogenic, and tumoricidal effects[28]. Meta-analyses of controlled trials of IFN therapy in HBeAg-positive patients show that patients treated with IFN achieve greater HBeAg losses, suppression of HBV DNA levels, and alanine aminotransferase normalization than untreated control patients[29]. In addition, IFN therapy induces higher rates of hepatitis B surface antigen (HBsAg) seroclearance, resulting in lower rates of cirrhosis development, reduced HCC incidence, and better overall survival[30]. IFN conjugated with polyethylene glycol, i.e., PEG-IFN, has recently become widely used because it is convenient and patients with chronic hepatitis B infections treated with it exhibit clinical outcomes superior to those of patients treated with unconjugated IFN[31]. Several studies show that IFN effectively suppresses HBV replication, reduces HCC incidence, and improves outcomes in patients with chronic hepatitis B infections[32-34]. Although the potential effectiveness of IFN-α as an adjuvant therapy for preventing HCC recurrence has been demonstrated in clinical trials and meta-analyses[35-41], other studies report conflicting results[42,43] (Table 1). Furthermore, while the use of adjuvant IFN therapy in HCC has been studied extensively, it has not yet been accepted as a standard treatment after curative therapy, because previous studies have only involved small numbers of patients and varying types and doses of IFN. Therefore, further studies are required to establish appropriate postoperative therapy with IFN in HCC patients.
| Effects of antiviral therapy | ||||||
| Author | Year | Study design | Treatment | Adjuvant therapy | Tumor-free survival | Overall survival |
| Interferon | ||||||
| Sun et al[39] | 2006 | RCT | Resection | Inteferon-α | Postponed | Improved |
| Lo et al[37] | 2007 | RCT | Resection | Inteferon-α2b | Not significant | Improved in stage III/IVA patients |
| Chen et al[42] | 2012 | RCT | Resection | Inteferon-α2b | Not significant | Not significant |
| Nucleos(t)ide analogues | ||||||
| Kubo et al[45] | 2007 | Prospective cohort | Resection | LAM (with ADV rescue) | Improved | ND |
| Kuzuya et al[48] | 2007 | Retrospective cohort | Resection or RFA | LAM (with ADV rescue) | Not significant | Improved (tendency) |
| Yoshida et al[49] | 2008 | Retrospective cohort | RFA | LAM (with ADV rescue) | Not significant | Not significant |
| Chuma et al[11] | 2009 | Retrospective cohort | Resection or RFA | LAM (with ADV rescue) or ETV | Improved | ND |
| Koda et al[50] | 2009 | Cohort | Resection or RFA | LAM (with ADV rescue) or ETV | Not significant | Improved |
| Li et al[51] | 2010 | Prospective cohort | Resection | LAM (with ADV rescue) | Not significant | Improved |
| Chan et al[52] | 2011 | Retrospective cohort | Resection | LAM or ETV | Improved | Improved |
| Urata et al[44] | 2012 | Retrospective cohort | Resection | LAM (with ADV rescue) or ETV | Improved | Improved |
| Wu et al[53] | 2012 | Cohort | Resection | LAM, ETV, telbivudine | Improved | Improved |
| Su et al[54] | 2013 | Retrospective cohort | Rection | LAM or ETV or Pegylated interferon | Improved | Improved |
| Ke et al[55] | 2013 | Retrospective cohort | Resection | LAM | Not significant | Improved |
| Yin et al[56] | 2013 | Cohort including RCT | Resection | LAM (with ADV or ETV rescue) | Improved | Improved |
| Nishikawa et al[57] | 2014 | Retrospective cohort | Resection or RFA or PEI | LAM (with ADV rescue) or ETV | Not significant | Improved |
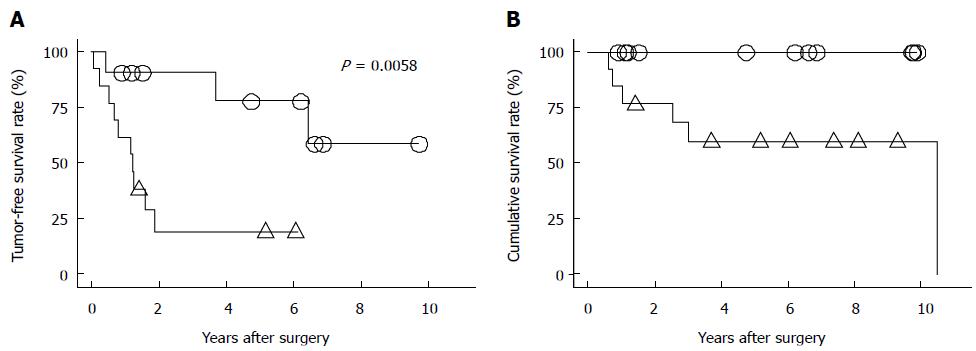
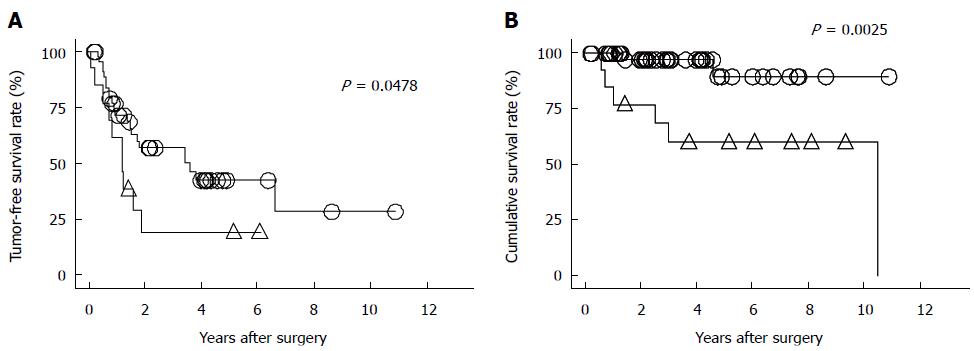
We reported that a high serum concentration of HBV DNA is a strong risk factor for HCC recurrence and poor survival after curative HCC resection[44] (Figure 1). Furthermore, we reported that the incidence of HCC recurrence was significantly higher in patients who experienced acute postoperative exacerbations of their hepatitis, showed constantly high serum concentrations of HBV DNA, and showed sustained HBsAg expression postoperatively[45]. Several subsequent studies also demonstrated high serum concentrations of HBV DNA are significantly associated with shorter survival times, with the cause of death being HCC recurrence[6,7,9-13,45]. It is well established that a high serum concentration of HBV-DNA is strongly associated with HCC development[46]. The risk of HCC in such patients is more than 10 times higher than that in patients with low serum concentrations of HBV DNA. Antiviral therapy with NAs including LAM, ADV, and ETV, has recently been reported to be useful for preventing progression to cirrhosis and HCC development in patients with chronic hepatitis B infections[20-26]. Since we reported that LAM may prevent HCC recurrence after curative resection for HCC[47] (Figure 2), several published reports describe the effects of NAs after HCC treatment[11,44,48-57] (Table 1). A recent meta-analysis shows that antiviral therapy with NAs reduces HCC-related mortality and HCC recurrence postoperatively, and improves overall survival in patients with HBV-related HCC[58-60]. A recent cohort study of patients with chronic hepatitis B infections treated with ETV demonstrates the importance of a sustained virologic response[61]. However, antiviral therapy using NAs cannot suppress HCC recurrence caused by metastases from the original tumor, because NAs do not have any anticancer activity. In addition, NAs cannot prevent multicentric recurrence of HCC, because the HBV DNA will already have been integrated into the host’s genome, which is the first step in hepatocarcinogenesis in patients with chronic hepatitis B infections and cirrhosis. Therefore, HCC may still develop despite the effective suppression of viral replication by antiviral agents. A retrospective study of 2795 Japanese patients with chronic hepatitis B infections shows that absence of treatment, male sex, family history of HBV carriage, age over 40 years, fibrosis exceeding grade 2, on a scale of 0-4, and albumin < 40 g/L and platelet count < 150000/mm3 are independent risk factors for HCC[22]. Close follow-up is necessary for elderly patients who have cirrhosis, even if antiviral therapy has been effective. We previously showed that antiviral therapy with NAs induces the remission of active hepatitis, maintains liver function, and increases the likelihood of successful treatment for HCC recurrence, even if recurrence developed after curative resection[44]. Similar results were subsequently reported in other studies, namely that antiviral therapy with NAs improved patient outcomes following curative treatment[48,50,51,55,56]. Drug resistance is increasingly emerging in association with long-term antiviral therapy. Hence, the effects of drug resistance on HCC recurrence following treatment for HBV-related HCC must be evaluated.
Antiviral therapy after curative treatment aims to improve prognosis by suppressing viral replication. Recent accumulating evidence indicates high serum HBV DNA levels, either preoperatively or postoperatively, is associated with a higher risk of HCC recurrence and that this affects the effectiveness of the antiviral therapy administered after curative treatment for HBV-related HCC. Although IFN has antiproliferative, antiangiogenic, and tumoricidal effects, IFN therapy may be accompanied by a substantial risk of hepatic decompensation in patients with advanced liver disease. While serum concentrations of HBV DNA become undetectable in most patients who undergo antiviral therapy with NAs, these compounds have no anticancer activity. Therefore, combining IFN and NAs may be an alternative strategy to prevent HCC recurrence after curative treatment for HBV-related HCC. Although IFN therapy and NAs are useful for preventing HCC recurrence and maintaining liver function after treatment for HBV-related HCC, there is insufficient evidence to reach a conclusion and consensus about the optimal perioperative antiviral therapy. Nevertheless, further studies are necessary to establish treatment guidelines for these patients.
P- Reviewer: Sonzogni A, Tziomalos K S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13557] [Article Influence: 677.9] [Reference Citation Analysis (1)] |
| 2. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4264] [Article Influence: 236.9] [Reference Citation Analysis (2)] |
| 3. | Chen CJ, Yu MW, Liaw YF. Epidemiological characteristics and risk factors of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S294-S308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 338] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1792] [Article Influence: 85.3] [Reference Citation Analysis (2)] |
| 5. | Kubo S, Takemura S, Sakata C, Urata Y, Uenishi T. Adjuvant therapy after curative resection for hepatocellular carcinoma associated with hepatitis virus. Liver Cancer. 2013;2:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Kubo S, Hirohashi K, Tanaka H, Tsukamoto T, Shuto T, Yamamoto T, Ikebe T, Wakasa K, Nishiguchi S, Kinoshita H. Effect of viral status on recurrence after liver resection for patients with hepatitis B virus-related hepatocellular carcinoma. Cancer. 2000;88:1016-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Kubo S, Hirohashi K, Tanaka H, Shuto T, Takemura S, Yamamoto T, Uenishi T, Kinoshita H, Nishiguchi S. Usefulness of viral concentration measurement by transcription-mediated amplification and hybridization protection as a prognostic factor for recurrence after resection of hepatitis B virus-related hepatocellular carcinoma. Hepatol Res. 2003;25:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Kubo S, Hirohashi K, Yamazaki O, Matsuyama M, Tanaka H, Horii K, Shuto T, Yamamoto T, Kawai S, Wakasa K. Effect of the presence of hepatitis B e antigen on prognosis after liver resection for hepatocellular carcinoma in patients with chronic hepatitis B. World J Surg. 2002;26:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Ohkubo K, Kato Y, Ichikawa T, Kajiya Y, Takeda Y, Higashi S, Hamasaki K, Nakao K, Nakata K, Eguchi K. Viral load is a significant prognostic factor for hepatitis B virus-associated hepatocellular carcinoma. Cancer. 2002;94:2663-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Hung IF, Poon RT, Lai CL, Fung J, Fan ST, Yuen MF. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol. 2008;103:1663-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Chuma M, Hige S, Kamiyama T, Meguro T, Nagasaka A, Nakanishi K, Yamamoto Y, Nakanishi M, Kohara T, Sho T. The influence of hepatitis B DNA level and antiviral therapy on recurrence after initial curative treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2009;44:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, Huo TI, Sheen IJ, Lee SD, Lui WY. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 355] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 13. | Qu LS, Jin F, Huang XW, Shen XZ. High hepatitis B viral load predicts recurrence of small hepatocellular carcinoma after curative resection. J Gastrointest Surg. 2010;14:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Kubo S, Nishiguchi S, Hamba H, Hirohashi K, Tanaka H, Shuto T, Kinoshita H, Kuroki T. Reactivation of viral replication after liver resection in patients infected with hepatitis B virus. Ann Surg. 2001;233:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Huang L, Li J, Lau WY, Yan J, Zhou F, Liu C, Zhang X, Shen J, Wu M, Yan Y. Perioperative reactivation of hepatitis B virus replication in patients undergoing partial hepatectomy for hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Lao XM, Wang D, Shi M, Liu G, Li S, Guo R, Yuan Y, Chen M, Li J, Zhang Y. Changes in hepatitis B virus DNA levels and liver function after transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatol Res. 2011;41:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Dan JQ, Zhang YJ, Huang JT, Chen MS, Gao HJ, Peng ZW, Xu L, Lau WY. Hepatitis B virus reactivation after radiofrequency ablation or hepatic resection for HBV-related small hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol. 2013;39:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Yang YF, Zhao W, Zhong YD, Xia HM, Shen L, Zhang N. Interferon therapy in chronic hepatitis B reduces progression to cirrhosis and hepatocellular carcinoma: a meta-analysis. J Viral Hepat. 2009;16:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Miyake Y, Kobashi H, Yamamoto K. Meta-analysis: the effect of interferon on development of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Gastroenterol. 2009;44:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 574] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 21. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 22. | Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, Okita K, Hayashi N, Okanoue T, Iino S. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res. 2005;32:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Yuen MF, Seto WK, Chow DH, Tsui K, Wong DK, Ngai VW, Wong BC, Fung J, Yuen JC, Lai CL. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther. 2007;12:1295-1303. [PubMed] |
| 24. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 681] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 25. | Schiff ER, Lee SS, Chao YC, Kew Yoon S, Bessone F, Wu SS, Kryczka W, Lurie Y, Gadano A, Kitis G. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2011;9:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 772] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 27. | Huang L, Li J, Yan J, Sun J, Zhang X, Wu M, Yan Y. Antiviral therapy decreases viral reactivation in patients with hepatitis B virus-related hepatocellular carcinoma undergoing hepatectomy: a randomized controlled trial. J Viral Hepat. 2013;20:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Kardinal CG, Moertel CG, Wieand HS, Schutt AJ, O’Connell MJ, Wright K, Wiesenfeld M, Tschetter LK, Krook JE. Combined doxorubicin and alpha-interferon therapy of advanced hepatocellular carcinoma. Cancer. 1993;71:2187-2190. [PubMed] |
| 29. | Wong DK, Cheung AM, O’Rourke K, Naylor CD, Detsky AS, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med. 1993;119:312-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 705] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 30. | Lin SM, Yu ML, Lee CM, Chien RN, Sheen IS, Chu CM, Liaw YF. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol. 2007;46:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 243] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 31. | Cooksley WG, Piratvisuth T, Lee SD, Mahachai V, Chao YC, Tanwandee T, Chutaputti A, Chang WY, Zahm FE, Pluck N. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat. 2003;10:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 351] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 32. | Niederau C, Heintges T, Lange S, Goldmann G, Niederau CM, Mohr L, Häussinger D. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med. 1996;334:1422-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 574] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 33. | Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Fukuda M, Koida I, Arase Y, Chayama K, Murashima N. Interferon decreases hepatocellular carcinogenesis in patients with cirrhosis caused by the hepatitis B virus: a pilot study. Cancer. 1998;82:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology. 1999;29:971-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 324] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 35. | Oon CJ. Long-term survival following treatment of hepatocellular carcinoma in Singapore: evaluation of Wellferon in the prophylaxis of high-risk pre-cancerous conditions. Cancer Chemother Pharmacol. 1992;31 Suppl:S137-S142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Lygidakis NJ, Pothoulakis J, Konstantinidou AE, Spanos H. Hepatocellular carcinoma: surgical resection versus surgical resection combined with pre- and post-operative locoregional immunotherapy-chemotherapy. A prospective randomized study. Anticancer Res. 1995;15:543-550. [PubMed] |
| 37. | Lo CM, Liu CL, Chan SC, Lam CM, Poon RT, Ng IO, Fan ST, Wong J. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:831-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Shen YC, Hsu C, Chen LT, Cheng CC, Hu FC, Cheng AL. Adjuvant interferon therapy after curative therapy for hepatocellular carcinoma (HCC): a meta-regression approach. J Hepatol. 2010;52:889-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Sun HC, Tang ZY, Wang L, Qin LX, Ma ZC, Ye QH, Zhang BH, Qian YB, Wu ZQ, Fan J. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol. 2006;132:458-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 40. | Breitenstein S, Dimitroulis D, Petrowsky H, Puhan MA, Müllhaupt B, Clavien PA. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg. 2009;96:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Lau WY, Lai EC, Lau SH. The current role of neoadjuvant/adjuvant/chemoprevention therapy in partial hepatectomy for hepatocellular carcinoma: a systematic review. Hepatobiliary Pancreat Dis Int. 2009;8:124-133. [PubMed] |
| 42. | Chen LT, Chen MF, Li LA, Lee PH, Jeng LB, Lin DY, Wu CC, Mok KT, Chen CL, Lee WC. Long-term results of a randomized, observation-controlled, phase III trial of adjuvant interferon Alfa-2b in hepatocellular carcinoma after curative resection. Ann Surg. 2012;255:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Xu JB, Qi FZ, Xu G, Chen GF, Huang MD, Zhang JH. Adjuvant interferon therapy after surgical treatment for hepatitis B/C virus-related hepatocellular carcinoma: A meta-analysis. Hepatol Res. 2014;44:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Urata Y, Kubo S, Takemura S, Uenishi T, Kodai S, Shinkawa H, Sakae M, Kaneda K, Ohata K, Nozawa A. Effects of antiviral therapy on long-term outcome after liver resection for hepatitis B virus-related hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2012;19:685-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Kubo S, Hirohashi K, Tanaka H, Tsukamoto T, Shuto T, Higaki I, Takemura S, Yamamoto T, Nishiguchi S, Kinoshita H. Virologic and biochemical changes and prognosis after liver resection for hepatitis B virus-related hepatocellular carcinoma. Dig Surg. 2001;18:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2364] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 47. | Kubo S, Tanaka H, Takemura S, Yamamoto S, Hai S, Ichikawa T, Kodai S, Shinkawa H, Sakaguchi H, Tamori A. Effects of lamivudine on outcome after liver resection for hepatocellular carcinoma in patients with active replication of hepatitis B virus. Hepatol Res. 2007;37:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Kuzuya T, Katano Y, Kumada T, Toyoda H, Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Honda T. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1929-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 49. | Yoshida H, Yoshida H, Goto E, Sato T, Ohki T, Masuzaki R, Tateishi R, Goto T, Shiina S, Kawabe T. Safety and efficacy of lamivudine after radiofrequency ablation in patients with hepatitis B virus-related hepatocellular carcinoma. Hepatol Int. 2008;2:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Koda M, Nagahara T, Matono T, Sugihara T, Mandai M, Ueki M, Ohyama K, Hosho K, Okano J, Kishimoto Y. Necleotide analogs for patients with HBV-related hepatocellular carcinoma increase the survival rate through improved liver function. Inter Med. 2009;48:11-17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Li N, Lai EC, Shi J, Guo WX, Xue J, Huang B, Lau WY, Wu MC, Cheng SQ. A comparative study of antiviral therapy after resection of hepatocellular carcinoma in the immune-active phase of hepatitis B virus infection. Ann Surg Oncol. 2010;17:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Chan AC, Chok KS, Yuen WK, Chan SC, Poon RT, Lo CM, Fan ST. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. 2011;146:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 53. | Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleotide analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906-1914. [RCA] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 695] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 54. | Su CW, Chiou YW, Tsai YH, Teng RD, Chau GY, Lei HJ, Hung HH, Huo TI, Wu JC. The Influence of Hepatitis B Viral Load and Pre-S Deletion Mutations on Post-Operative Recurrence of Hepatocellular Carcinoma and the Tertiary Preventive Effects by Anti-Viral Therapy. PLoS One. 2013;8:e66457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Ke Y, Ma L, You XM, Huang SX, Liang YR, Xiang BD, Li LQ, Zhong JH. Antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after radical hepatectomy. Cancer Biol Med. 2013;10:158-164. [PubMed] |
| 56. | Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, Guo W, Zhang H, Wang H, Cheng S. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31:3647-3655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 57. | Nishikawa H, Nishijima N, Arimoto A, Inuzuka T, Kita R, Kimura T, Osaki Y. Effect of nucleoside analog use in patients with hepatitis B virus-related hepatocellular carcinoma. Hepatol Res. 2014;44:608-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung SY, Chong CN, Wong J, Lee KF, Lai PB, Chan HL. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;33:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 59. | Qu LS, Liu JX, Kuai XL, Xu ZF, Jin F, Zhou GX. Significance of viral status on recurrence of hepatitis B-related hepatocellular carcinoma after curative therapy: A meta-analysis. Hepatol Res. 2014;44:750-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Sun P, Dong X, Cheng X, Hu Q, Zheng Q. Nucleot(s)ide analogues for hepatitis B virus-related hepatocellular carcinoma after curative treatment: a systematic review and meta-analysis. PLoS One. 2014;9:e102761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Wong GL, Chan HL, Chan HY, Tse PC, Tse YK, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW. Accuracy of risk scores for patients with chronic hepatitis B receiving entecavir treatment. Gastroenterology. 2013;144:933-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |