Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.7933
Peer-review started: January 10, 2015
First decision: February 10, 2015
Revised: March 11, 2015
Accepted: April 16, 2015
Article in press: April 17, 2015
Published online: July 14, 2015
Processing time: 185 Days and 7.4 Hours
Esophageal cancer is one of the most unknown and deadliest cancers worldwide, mainly because of its extremely aggressive nature and poor survival rate. Esophageal cancer is the 6th leading cause of death from cancer and the 8th most common cancer in the world. The 5-year survival is around 15%-25%. There are clear differences between the risk factors of both histological types that affect their incidence and distribution worldwide. There are areas of high incidence of squamous cell carcinoma (some areas in China) that meet the requirements for cost-effectiveness of endoscopy for early diagnosis in the general population of those areas. In Europe and United States the predominant histologic subtype is adenocarcinoma. The role of early diagnosis of adenocarcinoma in Barrett’s esophagus remains controversial. The differences in the therapeutic management of early esophageal carcinoma (high-grade dysplasia, T1a, T1b, N0) between different parts of the world may be explained by the number of cancers diagnosed at an early stage. In areas where the incidence is high (China and Japan among others) early diagnoses is more frequent and has led to the development of endoscopic techniques for definitive treatment that achieve very effective results with a minimum number of complications and preserving the functionality of the esophagus.
Core tip: Esophageal cancer is a disease with a non-negligible impact, being the 6th leading cause of death from cancer, and with a very high morbidity and mortality due to diagnosed in advanced stages. A better understanding of the epidemiology, the natural history, and the risk factors could lead to an earlier diagnosis and treatment by endoscopic methods or by other less aggressive techniques. As a result, we could improve treatment outcomes, even though less aggressive modalities. This article provides a global perspective by comparing the management of esophageal cancer in Western and Eastern countries with particular emphasis on current prevention strategies.
- Citation: Domper Arnal MJ, Ferrández Arenas &, Lanas Arbeloa &. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015; 21(26): 7933-7943
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/7933.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.7933
Esophageal cancer is the 6th leading cause of death from cancer and the 8th most common cancer in the world. The 5-year survival is around 15%-25% and the best results are related to early diagnosis, which is commonly known as "early stages"[1].
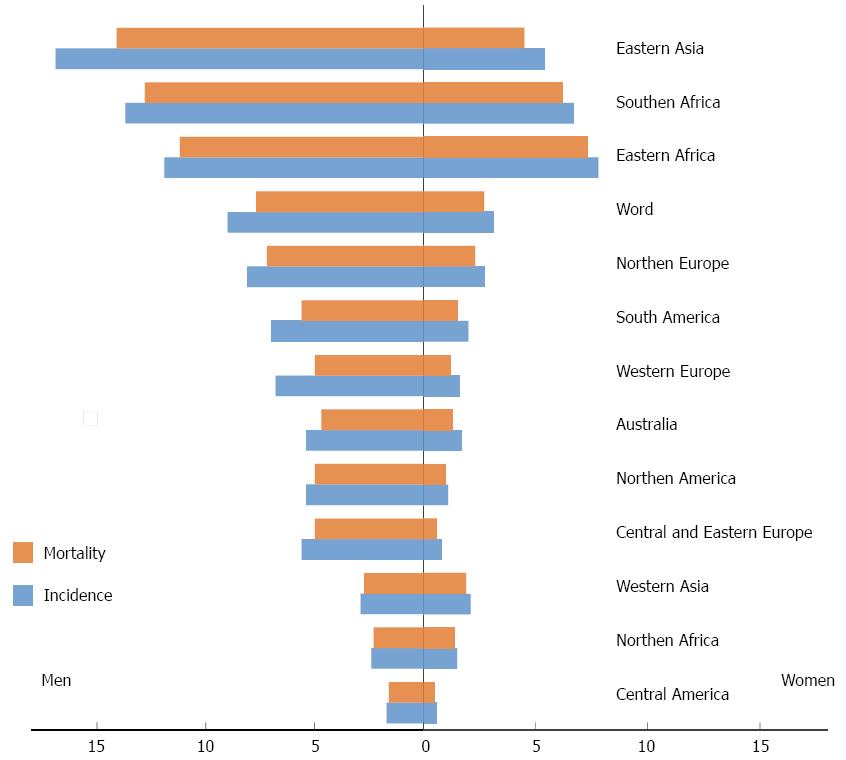
Esophageal squamous cell carcinoma is the predominant histological type worldwide. However, at present, in countries like United States, Australia, United Kingdom and Western Europe (Finland, France, Norway), there is a preponderance of adenocarcinoma subtype, having squamous carcinoma moved to second place[1]. The so-called “Asian Esophageal Cancer Belt” encompasses areas such as Turkey, Iran, Kazakhstan and northern and central China, with an estimated esophageal squamous carcinoma of more than 100 cases/100000 person-years. Another area with high incidence of squamous cell carcinoma is southeastern Africa, with similar rates to those observed in Eastern countries. In the United States, from 1975-2004, the age-adjusted incidence in white males has increased from 5.76 to 8.34 cases/100000 person-years at the expense of the adenocarcinoma histological subtype. Nevertheless squamous carcinoma remains the most common subtype in American black males, but still adenocarcinoma, is one of the few cancers that contributes to an increased mortality from cancer among American men[2]. The trend towards dominance of adenocarcinoma subtype is not limited only to North America. In European countries like the United Kingdom, France or Norway the age-adjusted incidence increased by 39.6% for men and 37.5% for women in the last five years[1]. There is also a significant difference between gender distributions; the incidence of this disease is about 2-4 fold higher among males compared to females[3]. The incidence rates of squamous neoplasia in men in the territory of “Asian Esophageal Cancer Belt” are around 23 cases/100000 person-years and 16 cases/100000 person-years for females. In South Africa similar rates for males have been estimated[4]. Mortality rates follow, overall, a major parallelism with incidence rates in each country[5] (Figure 1). Regarding race, age-adjusted mortality for black individuals have a tendency to decrease, but still it is two-fold higher compared to Caucasians (7.79 vs 3.96, P < 0.05)[2].
Risk factors of esophageal cancer are slightly different between the two major subtypes.
Gender and race: Squamous cell carcinoma is the most frequent histological type in black individuals and white women, while adenocarcinoma is predominant in white men (P < 0.001)[2]. The incidence of esophageal squamous cell carcinoma is generally higher in men than women in most countries, and black men, compared to whites in the United States[4].
Smoking: Smoking is one of the major risk factor for developing esophageal squamous carcinoma. Smokers have a 5-fold risk of developing this disease compared to non-smokers[4]. However, there are parts of the world where smoking is not such an important risk factor and racial differences could account for these geographical differences. In a prospective study of risk factors for esophageal cancer in the province of Linxia, China, smoking was not an important risk factor compared to other parts of the world, while diet-related factors seem to play a major role in esophageal carcinogenesis. A study from Taiwan compared current and former smokers to people who never smoked and found that the OR was 4.2 and 3.4 respectively for smokers and former smokers compared to people without this habit[4].
Alcohol: Alcohol is a clear risk factor for squamous carcinoma. The relative risk (RR) increases with the amount of alcohol ingested varying between 1.8 and 7.4 depending on the weekly volume[4]. The intake of certain types of drink creates worldwide “hot spots” of squamous cell carcinoma of the esophagus in areas of Northern France consuming Brandie, corn beer in Southeast Africa, distilled sugary drinks in Puerto Rico, or certain whiskies in Carolina, United States. In Northern China, alcohol is not consumed regularly and therefore the risk associated with this habit is not relevant[4].
Diet and nutrients: Tea, mate and coffee have been extensively studied as potential risk factors associated with esophageal carcinoma and its geographical distribution, particularly in regions of South America. There is little evidence for carcinogenicity relationship through its components except for mate, which has been linked for both amount consumed and temperature[4]. Foods rich in nitrogenous components are historically related to the high incidence of squamous cell carcinoma in certain regions of China. A meta-analysis published in 2003, shows an OR of 2 for individuals who eat foods rich in such compounds compared to those who do not[4].
The “chewers of areca nut” (often mixed with tobacco), are common in regions such as Southeast Asia and India and have been linked to the development of squamous carcinoma. In Taiwan, where the tobacco is not included in the chewable mixture, the OR for chewers is 2.3[4]. Similarly, people living in developing countries that have significant deficits of minerals and vitamins, mainly due to low intake of foods like fruits and vegetables also have an OR of 2[4].
Genetics: There are conditions with a genetic basis, such as Tylosis, an autosomal dominant disease, that are clearly related to the development of esophageal squamous carcinoma. Familial aggregation in population of high incidence of esophageal carcinoma, such as northern regions of China has also been reported[4]. Four genome-wide association studies (GWAS), three of them conducted in Chinese population and one in Japanese population have shown genetic susceptibility factors in the development of squamous carcinoma, especially in heavy alcohol and tobacco users. Two nucleotide polymorphisms (SNPs, single nucleotide polymorphisms) deserve special attention because encode enzymes metabolizing alcohol: alcohol dehydrogenase 1B (rs1229984, OR = 1.79) and aldehyde dehydrogenase 2 family (rs671, OR = 1.67). Other GWAS found association at two loci, one located in the enzyme phospholipase C and another in a particular region of chromosome 20 (C20orf54)[3]. Regarding association with squamous carcinoma a GWAS dataset that included 453852 SNPs from 1898 squamous carcinoma patients and 2100 control subjects of Chinese population was reviewed. The authors identified candidate causal SNPs, and pathways (ICSNPathway) analysis identified seven candidate SNPs, five genes, and seven pathways, which together revealed seven hypothetical biological mechanisms. The three strongest hypothetical biological mechanisms were rs4135113, rs1800450 and rs3769823[6].
Gender and race: The incidence of esophageal adenocarcinoma is 8-fold more common in men than in women and 5-fold more common in whites than in blacks in the United States[4].
Gastroesophageal reflux disease and Barrett’s esophagus: The prevalence of gastroesophageal reflux disease (GERD) in the Western population is about 10%-20%, and about 30 to 60 million people in the United States. This entity is capable of producing esophageal adenocarcinoma directly or, more commonly, through an intermediate pre-neoplastic lesion, the Barrett's esophagus (BE). The increased incidence of BE in the last 30 years, is correlated with an increased incidence of adenocarcinoma in the same period. Barrett’s esophagus is a pre-malignant lesion that develops in 6%-14% of patients with GERD and of which, around 0.5%-1% will develop adenocarcinoma[4]. In a study performed in Spain, the incidence of adenocarcinoma during follow-up of patients with BE was 0.48% per year (95%CI: 0.006%-2.62%), for an incidence of 1 per 210 patient-years[7]. The largest study is a nationwide, population-based, cohort study conducted in Denmark, involving all patients with BE during the period from 1992 through 2009, using data from the Danish Pathology Registry and the Danish Cancer Registry. The study included 11028 patients with BE for a median of 5.2 years. The incidence rate for adenocarcinoma was 1.2 cases per 1000 person-years (95%CI: 0.9-1.5). As compared with the risk in the general population, the RR of adenocarcinoma among patients with BE was 11.3 (95%CI: 8.8-14.4). However, the annual risk of esophageal adenocarcinoma was 0.12% (95%CI: 0.09-0.15). Current surveillance guidelines assume a risk for adenocarcinoma of 0.5%-1%, far from the results obtained in this study. Detection of low-grade dysplasia was associated with an incidence rate for adenocarcinoma of 5.1 cases per 1000 person-years compared to 1.0 case per 1000 person-years among patients without dysplasia. These data question the rationale for ongoing surveillance in patients who have Barrett’s esophagus without dysplasia[8].
Obesity: Obesity is a major and consistent risk factor for the development of esophageal adenocarcinoma. It has become a serious public-related disease in developed countries. By 2015, an estimated 75% of the American people will be overweight (BMI > 25) and 41% obese (BMI > 30). The OR of developing adenocarcinoma is 1.52 (95%CI: 1.33-1.74, P < 0.0001) for those with BMI in the 25-30 rank compared with those who have normal-weight. A high BMI (> 25) was associated with an increased risk of oesophageal adenocarcinoma (males, OR = 2.2; 95%CI: 1.7-2.7; females, OR = 2.0; 95%CI: 1.4-2.9)[9]. Higher levels of BMI were associated with increased risk of oesophageal adenocarcinoma (overweight males, OR = 1.8; 95%CI: 1.5-2.2; obese males, OR = 2.4; 95%CI: 1.9-3.2)[10]. Two main mechanisms have been proposed for the development of esophageal adenocarcinoma in obese patients. First, a physical mechanism involving an increase in the incidence of GERD, and second a hormonal-dependent mechanism mainly mediated by inflammatory markers that are secreted by adipocytes[4].
Tobacco, alcohol and nutritional deficit: Alcohol is not related to the presence of adenocarcinoma, but smoking tobacco is a known risk factor, with an OR of 2.7 (95%CI: 1.64-4.45) relative to non-smokers[11].
In a Swedish population study, an inverse relationship was found between intake of total dietary fiber and the presence of adenocarcinoma of the gastro-esophageal junction. Similarly, in a United States case-control study it was found that a diet rich in vitamins, fruits and vegetables protect against the development of this disease[4].
Drugs: Observational studies with a large number of patients showed that the use of non-steroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors (PPIs) and statins in patients with BE, reduced the progression to adenocarcinoma[4]. The most studied agents have been acid suppressants. A systematic review with meta-analysis of studies evaluating the association between PPIs and histamine receptor antagonists (H2RAs) and risk of esophageal adenocarcinoma or high-grade dysplasia (HGD) in patients with BE has been recently published. The authors identified seven observational studies (2813 patients with BE, 317 cases of esophageal adenocarcinoma or HGD, 84.4% PPI users). On meta-analysis, PPI use was associated with a 71% reduction in risk of esophageal adenocarcinoma and/or HGD in patients with BE (adjusted OR = 0.29; 95%CI: 0.12-0.79). There was a trend towards a dose-response relationship with PPI use for > 2-3 years protective against esophageal adenocarcinoma or HGD [three studies; PPI use > 2-3 years vs < 2-3 years: OR = 0.45, (95%CI: 0.19-1.06) vs OR= 1.09 (95%CI: 0.47-2.56)]. Considerable heterogeneity was observed. Two studies reported the association between H2RA use and risk of esophageal adenocarcinoma and/or HGD (1352 patients with BE, 156 cases of esophageal adenocarcinoma, 25.4% on H2RAs), and both studies did not show a significant effect[12]. The largest study was published short after and challenged these results. In such nationwide case-control study carried out in Denmark, no cancer-protective effects from PPI’s were seen. In fact, among 9883 patients with a new diagnosis of BE the authors identified 140 cases with incident esophageal adenocarcinomas and/or high-grade dysplasia, with a median follow-up time of 10.2 years. The relative risk of esophageal adenocarcinoma or high-grade dysplasia was 2.2 (95%CI: 0.7-6.7) and 3.4 (95%CI: 1.1-10.5) in long-term low- and high-adherence PPI users respectively. Such results could partly be due to confounding by indication or a true negative effect from PPIs. Based on these results and until the results from future studies can elucidate what the association might be, continuous PPI therapy might not be necessary in all patients and could be directed at symptom control[13].
Genetic aspects: Very recently, it has been demonstrated using GWAS, that risk of BE and esophageal adenocarcinoma is influenced by many germline genetic variants of small effect and that shared polygenic effects contribute to the risk of these two diseases. In fact, the authors found that the genetic correlation between BE and esophageal adenocarcinoma was high (rg = 1.0; SE = 0.37) and estimated a statistically significant polygenic overlap between BE and esophageal adenocarcinoma [one-sided P = 1 × 10(-6)]. These data strongly suggest that shared genes underlie the development of BE and esophageal adenocarcinoma[14].
GWAS type studies have also been conducted to elucidate susceptibility loci. The first genome-wide association study of esophageal adenocarcinoma, together with BE has been recently published. The most significant results were for cancer and pre-cancer combined suggesting that much of the genetic basis for esophageal adenocarcinoma lies in the development of BE, rather than its to esophageal adenocarcinoma. The authors found three novel genome-wide significant loci for esophageal adenocarcinoma and BE combined, and extended existing findings at the FOXF1 and HLA loci. One of the novel regions is chromosome 3p13, near FOXP1, a gene encoding a transcription factor, which regulates esophageal development. Interestingly, two of the other regions (BARX1/9q22.32 and FOXF1/16q24.1) contain risk associated SNPs which disrupt binding of FOXP1. Further dissection of these loci is likely to lead to insights into the etiology of this rapidly fatal cancer[15] (Table 1).
| Risk factor | Squamous cell carcinoma | Adenocarcinoma |
| Geography | Southeastern Africa, Asia, Iran, South America | Western Europe, North America (United States), Australia |
| Race | Black > White | White > Black |
| Gender | Male > Female | Male > Female |
| Alcohol | ++++ | - |
| Tobacco | ++++ | ++ |
| Obesity | - | +++ |
| GERD | - | ++++ |
| Diet: Low fruits and vegetables | ++ | + |
| Socioeconomic conditions | ++ | - |
| Genetic aspects | ++ | + |
Esophageal cancer is a health problem worldwide with high mortality due to its natural history and the common diagnosis in advanced stages. Therefore, its detection at an early stage would improve outcomes of mortality significantly. Squamous dysplasia is the precursor lesion of esophageal squamous cell carcinoma; Barrett’s esophagus is the pre-neoplastic lesion preceding adenocarcinoma[16].
Screening of BE-associated adenocarcinoma by endoscopy is a worldwide clinical practice although it has not been proven cost-effective. According to current guidelines, random endoscopic biopsies should be taken in all 4 quadrants and each 2 cm of columnar epithelium, and ideally performed with high-resolution endoscopes and NBI (narrow banding imaging)[17].
The results of large cohort studies suggest that the annual cancer risk for patients with non-dysplastic Barrett’s esophagus is low in European populations (0.12%-0.40% per year)[8]. Dysplasia within BE lesions signals a marked increase in cancer risk: the annual risk is approximately 1% for patients with low-grade dysplasia and more than 5% for patients with high-grade dysplasia. However, 80% to 90% of cases of esophageal adenocarcinoma are diagnosed in patients without known BE. Endoscopic screening results in detection of BE in 6% to 12% of patients with prolonged GERD symptoms, most frequently white men older than 50 years of age[18].
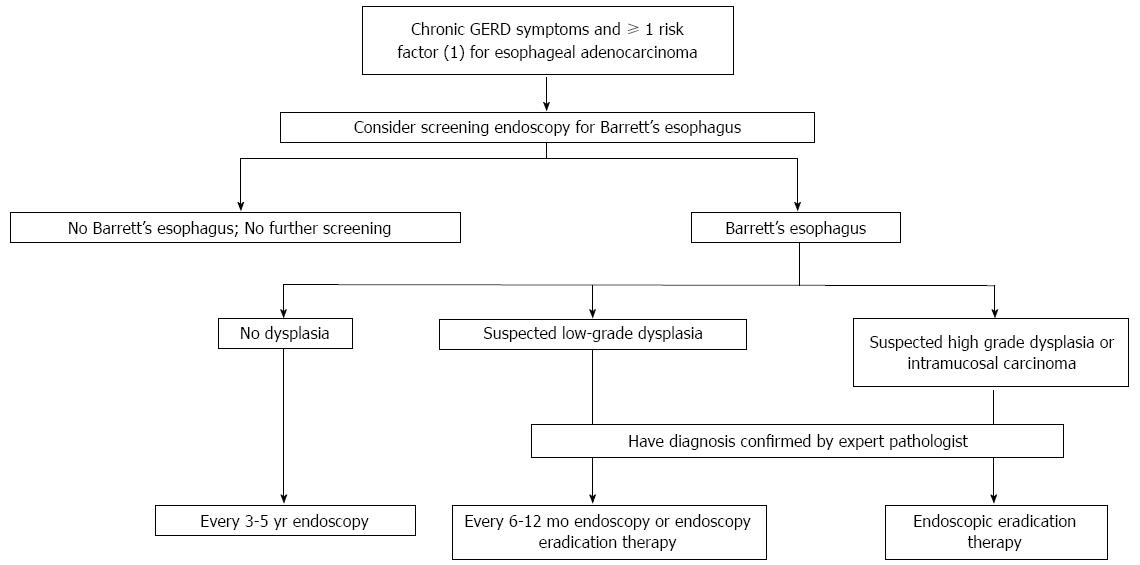
A recent review by Spechler and Souza suggests that people with chronic GERD symptoms and at least 1 risk factor for esophageal carcinoma are suitable for active endoscopic screening for Barrett’s esophagus and early adenocarcinoma with adequate surveillance depending on the lesion found on the index endoscopy and pathology (Figure 2). The main caveat of such strategy is that the target population focuses on GERD patients although around 40% of esophageal adenocarcinomas have no prior history of GERD[19].
In 2006, a systematic review, expert workshop and economic modelling was performed focused on Surveillance of BE. Such study identified 3 cost-utility analyses of surveillance of BE that used Markov modeling and confined their analysis to 50- or 55-year-old white men with GERD symptoms. In one study, the authors concluded that the only cost-effective strategy was once in a lifetime screening of 50-year-old white men with GERD, followed by surveillance of those with dysplasia only. In the other 2 studies (performed by the same group) surveillance of BE every 5 years compared with no surveillance was cost-effective; however the model was very sensitive to the incidence of adenocarcinoma and quality of life (utility value) in the post-esophagectomy state. Moreover, the incremental cost-effectiveness ratio for 5-yearly surveillance was no longer within the range usually considered cost-effective[20].
These models are American, so there are almost certainly differences in practice from Europe and possible underlying differences in the epidemiology and natural history of the disease. In European public services there is a major difficulty in knowing what proportion of patients with GERD have an endoscopy and at what stage of the disease, whereas in the United States, those who present to health services are more likely to be investigated at an earlier stage. The costs of the procedures involved are also likely to be very different.
They key of surveillance may underlie on what patients may benefit from it. Is dysplasia a good marker? Should genetic markers be used? A recent cost-utility analysis from Australia compared (1) No surveillance; (2) 2-yearly endoscopic surveillance of patients with non-dysplastic BE and 6-monthly surveillance of patients with low-grade dysplasia; and (3) a hypothetical strategy of biomarker-modified surveillance. In a total of 2040 patient-years of follow-up and by using best available estimates of the malignant potential of BE, endoscopic surveillance of patients with non-dysplastic BE is unlikely to be cost-effective for the majority of patients and depends heavily on progression rates between dysplasia grades. However, strategies that modify surveillance according to cancer risk might be cost-effective, if high-risk individuals can be identified and prioritized for surveillance[21]. However, unless newly emerging technologies improve the quality-adjusted survival benefit conferred by endoscopic surveillance, current strategies are unlikely to be cost-effective in Europe. Obsolete assumptions and incomplete analyses reduce the quality of published evaluations. For these reasons new evaluations are required that encompass the growing evidence base for new technologies, such as new endoscopic therapies for high-grade dysplasia and intramucosal cancer[22].
Another fact that should be added in the evaluation is that, despite the absence of direct evidence from randomized trials, most but not all observational studies have shown that patients in whom adenocarcinoma is detected during endoscopic surveillance for BE are more likely to have early-stage cancer, receive curative therapy, and survive longer than symptomatic patients in whom adenocarcinoma is detected during the clinical workout[18].
Esophageal squamous cell carcinoma is the predominant histologic subtype in Asia and the incidence and mortality are higher in China than in Japan. In Japan, the incidence of this disease is declining from the late 90 s to the present. By contrast, in China, esophageal cancer is the 4th most frequently diagnosed cancer and the 4th leading cause of death from cancer. Incidence rates are higher in rural areas of China compared to urban areas, especially in regions such as Henan, Hebei, Linxia and Shanxi[23]. As mentioned before, squamous dysplasia is a precursor lesion of squamous carcinoma. It is hardly detectable in asymptomatic individuals and there is no standardized screening program to detect this condition[23].
In Japan there are controversies about whether dysplasia should be actively detected by gastroenterologists. There are no reliable data on the actual prevalence of dysplasia in Japanese asymptomatic patients, but a recent study of 1345 asymptomatic individuals, who underwent endoscopy during a health check, found a prevalence of dysplasia of 3% in this population. There are no prospective studies and the relationship between dysplasia and squamous carcinoma development in this population is still unknown[23].
In China, endoscopic screening in high-risk areas (defined as an incidence higher than 30 cases per 100000 inhabitants per year) has been shown to detect precursor lesions in asymptomatic patients with dysplasia, with high rates of what is known as “esophageal early cancer”. The main dysplastic lesion associated with esophageal squamous cell carcinoma in prospective population studies in the Chinese region of Linxia is the high-grade dysplasia, which is associated with an RR of 28.3 (95%CI: 15.3-52.3) for developing the disease compared to patients who have a normal esophageal mucosa[23,24].
Endoscopy: Endoscopy is the gold standard for the diagnosis of pre-cancerous squamous lesions. Squamous dysplasia may go undetected when using standard endoscopy and therefore chromoendoscopy techniques have been suggested to improve the performance of the test. The most simple and effective for the detection of squamous dysplasia is Lugol staining. The sensitivity and specificity of white-light endoscopy for the detection of high-grade dysplasia and cancer is 62% and 79% respectively, compared with a much higher sensitivity of 96%, at the expense of a slight loss of specificity of 63%, when using Lugol chromoendoscopy[16].
Most of the studies, if not all, have been performed in Asia where the incidence of squamous carcinoma is high. The prevalence of low, medium, high grade and invasive carcinoma, using Lugol chromoendoscopy, are 28%, 21.9%, 6.3% and 0% to 9.5% respectively in expert hands, and many of these lesions can be treated with endoscopic resection[16]. In this regard, a prospective population study was conducted in 2014 in Henan, one of the areas of Northern China with high incidence of esophageal carcinoma, in the context of a screening program with biopsies taken and guided by chromoendoscopy. A total of 36154 people between 40 and 69 years were examined. The study detected 7.1% of people with low-grade dysplasia, 2.3% with intermediate grade dysplasia and 1.6% with cancerous lesions, being 87.32% of them early carcinomas (high-grade dysplasia, carcinoma mucosa-submucosa) cases[25].
The results of several cost-benefit studies about endoscopic screening of esophageal squamous carcinoma have shown that such strategy is only cost-effective in areas of high incidence of squamous cell carcinoma, such as in Northern and rural areas of China. However, some variations may occur even in high-risk areas. The geographical and the economic status of the region have a great impact in the onset of esophageal carcinoma regarding the age of onset, the number needed to screen, the precursor lesions that have to be identified and the intervals for a proper surveillance in people with such lesions[26,27].
A recent study, based on economic parameters and management, made a comparison between 12 different existing screening methods in high-risk/high incidence of squamous cell carcinoma in China. The two key strategies to be followed to ensure cost-effective programs taking into account the acceptance of the population and the distribution of wealth in different regions were: (1) screening once throughout life and starting at the age of 50, following up after 5 years of detecting low-grade dysplasia and 3 years after intermediate-grade dysplasia, for areas with limited access to healthcare, impoverished and with a difficult track the target population economy[26]; and (2) screening three times over life, starting at the age of 40, and monitoring low-grade dysplasia and intermediate-grade dysplasia as above, for areas with appropriate access to health care, and economies that are more advanced and good monitoring program by the target population[26].
One of the questions is whether these results can be applied to Western countries. There are no European studies suggesting that endoscopic screening for squamous esophageal carcinoma is either necessary or cost-effective. The low incidence of squamous esophageal carcinoma in the European population and the predominance of public health systems might be some of the main reasons why screening of this condition is not an option even in individuals with risk factors.
Other screening techniques: There are areas in the world with high incidence of squamous carcinoma, beyond those already mentioned, where screening program using the gold standard technique with Lugol chromoendoscopy have not been shown to be cost-effective. An Iranian review published in 2013 suggested that new screening strategies, cheaper and more effective, should be tracked. They propose combining the individual risk factors of patients with cytology techniques without endoscopy and/or tissue or serum markers of risk detected enzimoimmunoassay techniques or micro-RNA[28].
There is a relatively large number of extraction techniques without endoscopic for esophageal cytology which include inflatable balls and sponges, recently developed, but these techniques have a sensitivity of only 24%-47% for dysplasia-cancer and 18%-44% for cancer, despite having good specificity of 81%-92% and 99%-100% for dysplasia-cancer and cancer respectively. The low number of suitable samples and low sensitivity makes them unsuitable for effective screening[16].
Very few studies looking at blood biomarkers on people of countries with high incidence of squamous carcinoma have been performed, but most suggest that these should be used in the future in combination with other screening techniques to optimize the results[16].
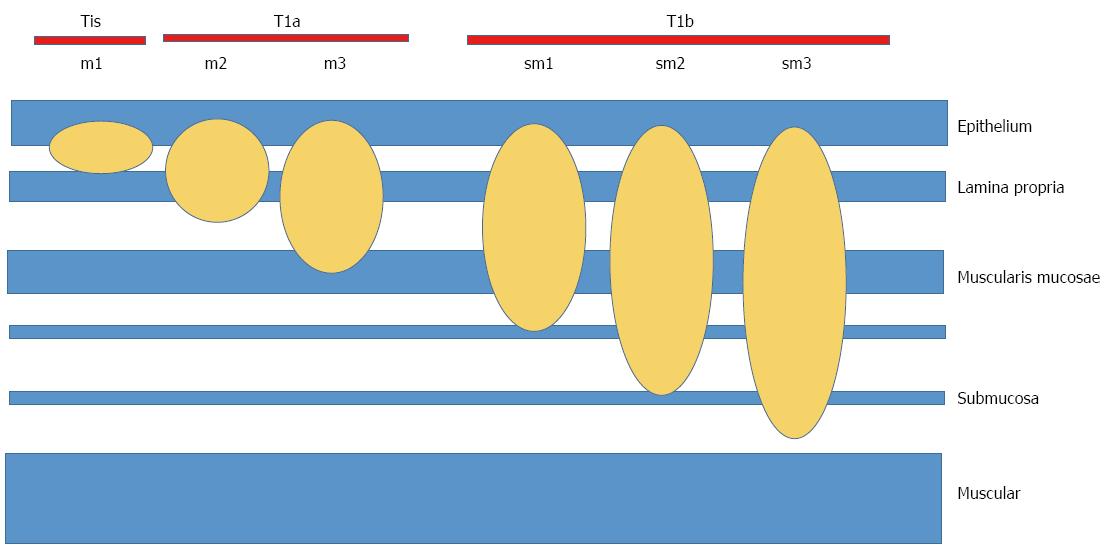
Early esophageal carcinoma (EEC) is defined as those early stages in which the neoplastic involvement does not exceed the submucosa, and there are no nodes involved (DAG, T1a, T1b, N0)[29,30] (Table 2, Figure 3).
| T: Primary tumor: |
| Tx: It can not be evaluated |
| T0: No evidence of primary tumor |
| Tis: High-grade dysplasia (intra-epithelial neoplasia noninvasive) |
| T1: Tumor invades own lamina, muscularis mucosae and submucosa: |
| T1a: Tumor invades own lamina or muscularis mucosae |
| T1b: Tumor invades the submucosa |
| T2: Tumor invades the muscularis |
| T3: Tumor invades the adventitia |
| T4: Tumor invades adjacent structures: |
| T4a: Tumor invades resectable pleura, pericardium, or diaphragm |
| T4b: Unresectable tumor that invades other adjacent structures: aorta, vertebral body, trachea, etc. |
| N: Regional lymph nodes: |
| Nx: They can not be evaluated |
| N1: Metastasis in 1-2 regional lymph nodes |
| N2: Metastasis in 3-6 regional lymph nodes |
| N3: Metastasis in 7 or more regional lymph nodes |
| M: Distant metastasis: |
| M0: None |
| M1: There are distant metastases |
There are big differences among treatment for early esophageal cancer between Western and Asian countries. In fact, the Asian attitude is more aggressive in managing these patients.
Most Western studies convey the idea that the rate of lymph node metastasis in T1b tumors is too high to be considered a safe endoscopic therapy as a definitive treatment for this neoplastic disease. It is estimated that the risk of nodal metastases in tumors confined to the mucosa (T1a), mainly adenocarcinomas in clinical practice, is 1%-2%, therefore, an endoscopic local treatment may be considered sufficient as definitive treatment. In tumors invading the submucosa (T1b), the risk of nodal metastases exceeds 10%, therefore a definitive endoscopic treatment is not feasible in principle[31]. In this type of tumor stages (high-grade dysplasia, T1a) the most common therapeutic approach is the combination of endoscopic resection techniques by means of mucosal resection (EMR) to remove the neoplastic tissue associated with ablative techniques such as radiofrequency to remove the remaining metaplastic/dysplastic residual tissue. Its therapeutic efficacy is up to 98%, and its potential complications include bleeding, perforation and residual stenosis[31,32].
For stage T1a, esophagectomy is seen today as a second treatment option, with a success rate similar to endoscopic cancer but with a much larger treatment morbidity. However, esophagectomy should be considered in patients in whom the risk of recurrence is considered high (7%-30%), such as multifocal lesions and long BE segments associated with neoplasia where it is not possible to associate ablative techniques[33].
A review of 46 studies involving 7645 patients with esophageal cancer T1N0 concluded that in T1b sm1 adenocarcinomas, well or moderately differentiated without lymphovascular invasion or lymph node metastasis, endoscopic treatment is the preferred option because the rate of lymph node involvement is lower than suspected (6% in sm1). However, in m3 T1a squamous carcinomas, lymph node involvement is higher than previously presumed and esophagectomy with lymphadenectomy should be considered[31,32].
A number of articles from Asia, mainly Japan and China, have a more aggressive approach from the point of view of endoscopic management of early esophageal cancer. T1a and T1b lesions, regardless of histological type, with confirmed no lymph node metastases, are managed by endoscopy resection, since it is considered that this technique has the same efficacy as esophagectomy. A recently published population-based study comparing the survival of both techniques for T0/T1 stages, with a total of 430 patients who received endoscopic treatment over 1586 patients who received surgical treatment showed no differences in mortality after 2 (endoscopy: 10.5% vs 12.7% surgery, P = 0.27) or 5 years (endoscopy: 36.7% vs 42.8% surgery, P = 0.16) of follow-up[34]. The fundamental treatment of neoplasia at this stage is suggested to be the combination of definitive endoscopic treatments such as EMR or ESD (endoscopic submucosal dissection) with ablative treatments to eradicate the rest of metaplastic/dysplastic tissue if necessary[35].
The main objective of this approach is to preserve the esophagus as a functional organ and avoid the morbidity of surgery at that level. The EMR was the first endoscopic technique developed. However, it has its limitations. In a meta-analysis of five case-control studies that included 319 lesions treated with ESD and 476 lesions treated with EMR it was observed that ESD shower better “en bloc” and histologically resection rates, and lower recurrence, without increasing the incidence of procedure-related complications but at the cost of a longer process and higher costs[36]. In fact, in a similar meta-analysis of 21 studies, 1152 patients and 1240 lesions treated with ESD, with an average follow-up period between 12 and 53 mo, it was observed that the rates of resection as a whole were 99% (95%CI: 99%-100%), and R0 resection rate of 90%. In lesions less than 25 mm higher a percentage of R0 resections (92% vs 85%, P < 0.001) was achieved. The complication rate was very low, the most significant being stenosis, with an incidence of 5% (95%CI: 3%-8%). The authors conclude that it is a safe and effective technique[37].
Squamous cell carcinoma is still the most common histologic type in the world. The areas with the highest incidence are found in Africa and the Middle East. The risk factors most frequently involved, are the abuse of tobacco and alcohol, as well as mutations in metabolizing pathways of these substances, and nutritional deficits. In areas of high incidence, defined as 30 or more cases per 100000 person-years, justified the mass screening of squamous carcinoma, a fact that improves detection rates of early squamous cell carcinoma and its management without surgery, with a high proportion of patients treated with endoscopic resection strategy.
There has been a shift from squamous carcinoma to adenocarcinoma as the most frequent histological type of esophageal carcinoma in fundamental areas of Europe such as Norway, United Kingdom, in the United States and in Australia. Differentiating risk factors are fundamentally obesity, GERD and BE as well as the influence of toxics such as tobacco. BE is a precursor of adenocarcinoma, but the rate of cancer transformation in European and United States populations is low, which questions surveillance programs and the search for an early diagnosis of adenocarcinoma in BE, which is common clinical practice today. In any case, the rate of detection of early stage adenocarcinoma is lower in Western countries and treatment, therefore, is less conservative, with high proportion of patients treated with surgical techniques to achieve eradication of the disease.
P- Reviewer: Chuang SE, Grassilli E, Ji Y, Neilsen PM, Tavares A S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1961] [Article Influence: 163.4] [Reference Citation Analysis (5)] |
| 2. | Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598-5606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 736] [Cited by in RCA: 747] [Article Influence: 62.3] [Reference Citation Analysis (8)] |
| 3. | Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer. 2012;31:281-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (2)] |
| 4. | Wheeler JB, Reed CE. Epidemiology of esophageal cancer. Surg Clin North Am. 2012;92:1077-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 5. | International agency for research on cancer. Available from: http://globocan.iarc.fr/Default.aspx. |
| 6. | Yang X, Zhu H, Qin Q, Yang Y, Yang Y, Cheng H, Sun X. Genetic variants and risk of esophageal squamous cell carcinoma: a GWAS-based pathway analysis. Gene. 2015;556:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Alcedo J, Ferrández A, Arenas J, Sopeña F, Ortego J, Sainz R, Lanas A. Trends in Barrett’s esophagus diagnosis in Southern Europe: implications for surveillance. Dis Esophagus. 2009;22:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 8. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 980] [Article Influence: 70.0] [Reference Citation Analysis (1)] |
| 9. | Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3505] [Cited by in RCA: 3679] [Article Influence: 216.4] [Reference Citation Analysis (1)] |
| 10. | Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 273] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 11. | Oze I, Matsuo K, Ito H, Wakai K, Nagata C, Mizoue T, Tanaka K, Tsuji I, Tamakoshi A, Sasazuki S. Cigarette smoking and esophageal cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2012;42:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 13. | Hvid-Jensen F, Pedersen L, Funch-Jensen P, Drewes AM. Proton pump inhibitor use may not prevent high-grade dysplasia and oesophageal adenocarcinoma in Barrett’s oesophagus: a nationwide study of 9883 patients. Aliment Pharmacol Ther. 2014;39:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Ek WE, Levine DM, D’Amato M, Pedersen NL, Magnusson PK, Bresso F, Onstad LE, Schmidt PT, Törnblom H, Nordenstedt H. Germline genetic contributions to risk for esophageal adenocarcinoma, Barrett’s esophagus, and gastroesophageal reflux. J Natl Cancer Inst. 2013;105:1711-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Levine DM, Ek WE, Zhang R, Liu X, Onstad L, Sather C, Lao-Sirieix P, Gammon MD, Corley DA, Shaheen NJ. A genome-wide association study identifies new susceptibility loci for esophageal adenocarcinoma and Barrett’s esophagus. Nat Genet. 2013;45:1487-1493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Lao-Sirieix P, Fitzgerald RC. Screening for oesophageal cancer. Nat Rev Clin Oncol. 2012;9:278-287. [PubMed] |
| 17. | Fernández G, Baiges A, Visa L, Castelles A. Cáncer de esófago. España: CTO editorial. . |
| 18. | Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 998] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 19. | Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med. 2014;371:836-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 370] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 20. | Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N. Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling. Health Technol Assess. 2006;10:1-142, iii-iv. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Gordon LG, Mayne GC, Hirst NG, Bright T, Whiteman DC, Watson DI. Cost-effectiveness of endoscopic surveillance of non-dysplastic Barrett’s esophagus. Gastrointest Endosc. 2014;79:242-56.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Hirst NG, Gordon LG, Whiteman DC, Watson DI, Barendregt JJ. Is endoscopic surveillance for non-dysplastic Barrett’s esophagus cost-effective? Review of economic evaluations. J Gastroenterol Hepatol. 2011;26:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, Wei W, Inoue M, Tanaka H. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 429] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 24. | Wang GQ, Abnet CC, Shen Q, Lewin KJ, Sun XD, Roth MJ, Qiao YL, Mark SD, Dong ZW, Taylor PR. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Lu YF, Liu ZC, Li ZH, Ma WH, Wang FR, Zhang YB, Lu JB. Esophageal/gastric cancer screening in high-risk populations in Henan Province, China. Asian Pac J Cancer Prev. 2014;15:1419-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Yang J, Wei WQ, Niu J, Liu ZC, Yang CX, Qiao YL. Cost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of China. World J Gastroenterol. 2012;18:2493-2501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Yang J, Wei WQ, Niu J, He YT, Liu ZC, Song GH, Zhao de L, Qiao YL, Yang CX. Estimating the costs of esophageal cancer screening, early diagnosis and treatment in three high risk areas in China. Asian Pac J Cancer Prev. 2011;12:1245-1250. [PubMed] |
| 28. | Roshandel G, Nourouzi A, Pourshams A, Semnani S, Merat S, Khoshnia M. Endoscopic screening for esophageal squamous cell carcinoma. Arch Iran Med. 2013;16:351-357. [PubMed] |
| 29. | Ponce J, Castells T, Gomollón F, Esteve M, Martín de Argila C, Molero X, Vázquez Sequeiros E, editors . Treatment of gastroenterological diseases. 3rd ed. Madrid: Elsevier 2011; 155-156. |
| 30. | Westerterp M, Koppert LB, Buskens CJ, Tilanus HW, ten Kate FJ, Bergman JJ, Siersema PD, van Dekken H, van Lanschot JJ. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Lin JL. T1 esophageal cancer, request an endoscopic mucosal resection (EMR) for in-depth review. J Thorac Dis. 2013;5:353-356. [PubMed] |
| 32. | Gockel I, Sgourakis G, Lyros O, Polotzek U, Schimanski CC, Lang H, Hoppo T, Jobe BA. Risk of lymph node metastasis in submucosal esophageal cancer: a review of surgically resected patients. Expert Rev Gastroenterol Hepatol. 2011;5:371-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, Manner H, Guenter E, Huijsmans J, Vieth M. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 34. | Wani S, Drahos J, Cook MB, Rastogi A, Bansal A, Yen R, Sharma P, Das A. Comparison of endoscopic therapies and surgical resection in patients with early esophageal cancer: a population-based study. Gastrointest Endosc. 2014;79:224-232.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Hammad H, Kaltenbach T, Soetikno R. Endoscopic submucosal dissection for malignant esophageal lesions. Curr Gastroenterol Rep. 2014;16:386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Wang J, Ge J, Zhang XH, Liu JY, Yang CM, Zhao SL. Endoscopic submucosal dissection versus endoscopic mucosal resection for the treatment of early esophageal carcinoma: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:1803-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 37. | Sun F, Yuan P, Chen T, Hu J. Efficacy and complication of endoscopic submucosal dissection for superficial esophageal carcinoma: a systematic review and meta-analysis. J Cardiothorac Surg. 2014;9:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |