Published online Jun 21, 2015. doi: 10.3748/wjg.v21.i23.7172
Peer-review started: December 29, 2014
First decision: January 22, 2015
Revised: February 20, 2015
Accepted: April 9, 2015
Article in press: April 9, 2015
Published online: June 21, 2015
Processing time: 173 Days and 8.4 Hours
AIM: To investigate the abundance and potential diagnostic significance of neuroligin-1 and glutamate (Glu) in Hirschsprung’s disease (HSCR).
METHODS: Ninety children with HSCR and 50 children without HSCR matched for similar nutritional status, age and basal metabolic index were studied. The expression and localization of neuroligin-1 and Glu were assessed using double-labeling immunofluorescence staining of longitudinal muscles with adherent myenteric plexus from the surgically excised colon of children with HSCR. Western blot analysis, quantitative real-time PCR (qRT-PCR) and immunohistochemistry were performed to evaluate the abundance of neuroligin-1 and Glu in different HSCR-affected segments (ganglionic, transitional, and aganglionic segments). Enzyme-linked immunosorbent assay (ELISA) was used to detect and compare serum Glu levels in the long-segment HSCR, short-segment HSCR and non-HSCR samples.
RESULTS: Neuroligin-1 and Glu were co-expressed highest to lowest in the ganglionic, transitional and aganglionic segments based on Western blot (neuroligin-1: 0.177 ± 0.008 vs 0.101 ± 0.006, 0.177 ± 0.008 vs 0.035 ± 0.005, and 0.101 ± 0.006 vs 0.035 ± 0.005, P < 0.005; Glu: 0.198 ± 0.006 vs 0.115 ± 0.008, 0.198 ± 0.006 vs 0.040 ± 0.003, and 0.115 ± 0.008 vs 0.040 ± 0.003, P < 0.005) and qRT-PCR (neuroligin-1: 9.58 × 10-5± 9.94 × 10-6vs 2.49 × 10-5± 1.38 × 10-6, 9.58 × 10-5± 9.94 × 10-6vs 7.17 × 10-6 ± 1.12 × 10-6, and 2.49 × 10-5± 1.38 × 10-6vs 7.17 × 10-6± 1.12 × 10-6, P < 0.005). Serum Glu level was the highest to lowest in the non-HSCR, short-type HSCR and long-type HSCR samples based on ELISA (in nmol/μL, 0.93 ± 0.31 vs 0.57 ± 0.25, 0.93 ± 0.31 vs 0.23 ± 0.16, and 0.57 ± 0.25 vs 0.23 ± 0.16, P < 0.005).
CONCLUSION: Neuroligin-1 and Glu may represent new markers of ganglion cells, whose expression may correlate with the pathogenesis, diagnosis, differential diagnosis or classification of HSCR.
Core tip: Based on our results derived from a large set of clinical samples and various experimental methods, neuroligin-1 and glutamate (Glu) were first shown to be co-expressed in ganglion cells; thus neuroligin-1 and Glu may serve as new markers of this cell type, especially for excitatory synapses in the enteric nervous system. Moreover, the decreased abundance of neuroligin-1 and Glu in aganglionic segments may correlate with excessive intestinal contraction because of abnormal excitatory signaling that may ultimately result in Hirschsprung’s disease (HSCR). The serum Glu concentration may serve as a valuable adjunct measure for establishing a diagnosis and classification of HSCR.
- Citation: Wang J, Du H, Mou YR, Niu JY, Zhang WT, Yang HC, Li AW. Abundance and significance of neuroligin-1 and glutamate in Hirschsprung’s disease. World J Gastroenterol 2015; 21(23): 7172-7180
- URL: https://www.wjgnet.com/1007-9327/full/v21/i23/7172.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i23.7172
The pathogenesis of Hirschsprung’s disease (HSCR), a congenital disease with an incidence of 1 in 5000 human births, is typically regarded as the absence of enteric neurons in the distal gut, causing intestinal obstruction and proximal megacolon[1].
Although it was demonstrated that certain genes such as RET, SOX10, GDNE, EDNRB and ECE1 are related to the pathogenesis of HSCR[2-7], the alteration of these genes has been observed in only 30% of HSCR cases[3]. Therefore, the actual pathogenesis of HSCR and the mechanism underlying the loss of intestinal peristalsis in the distal diseased gut are unknown.
Neuroligins are cell adhesion molecules with a highly conserved structure that have been shown to function with neurexins in the formation and function of synapses in the central nervous system (CNS)[8]. Neuroligins are expressed in post-synapses, and neurexins are expressed in pre-synapses[9]. They coordinate to mediate CNS synaptogenesis by including secretion of proteins such as pentraxins to transduce signals between neurons[10,11]. Furthermore, the formation of different synapses (excitatory or inhibitory synapse) may be mediated by distinct neuroligin-neurexin combinations. For example, neuroligin-1 is localized to excitatory glutamatergic synapses, and neuroligin-2 is localized to inhibitory GABAergic synapses[11,12].
We know that some type of bi-directional communication occurs between the CNS and the enteric nervous system (ENS) and that gut function is affected by different psychological states and stresses communicated from the brain[13]. Given this information, it is of interest to determine whether the neuroligins expressed in the CNS are also expressed in the ENS. Our previous studies[14,15] showed that in the ENS of HSCR patients, the expression of neuroligins is down-regulated in aganglionic segments. Because neuroligin-1 was found to be localized to excitatory synapses, which are closely associated with the expression and release of glutamate (Glu), a type of excitatory neurotransmitter whose level may reflect the level of neuroligin-1[11,12], we sought to determine whether the pathogenesis of HSCR is related to abnormal excitatory signaling caused by alterations in the neuroligin-1 and Glu levels and the relationship between them. Moreover, we sought to identify a new method to more effectively diagnose, differentially diagnose and classify HSCR by measuring the serum Glu concentrations, which may be very valuable for clinical examinations.
In this study, the methods of immunofluorescence staining, Western blot, quantitative real-time PCR (qRT-PCR), and enzyme-linked immunosorbent assay (ELISA) were applied to resolve these issues and we believe that these results may be valuable for further research.
The study was reviewed and approved (No. 12025) by the Institution Review Board of Qilu Hospital, Shandong University. All colon tissues were collected from the surgically excised waste tissues, and neither tissue collection nor blood collection, which was performed via a routine preoperative blood test, caused any harm to the HSCR children.
From January 2010 to December 2013, more than 200 children with HSCR and 500 children with indirect inguinal hernia (IIH) were treated at the Department of Pediatric Surgery of Qilu Hospital, Shandong University. Experimental samples were collected from all of these patients, however, to reduce the influence of related effects during comparisons, only 90 patients with pathologically confirmed HSCR (HSCR group, 50 cases of short-segment HSCR and 40 cases of long-segment HSCR) and 50 patients with IIH (negative control or non-HSCR group ) who were matched for nutritional status (serum total protein, serum albumin, hemoglobin, blood urea nitrogen, body length and weight), age and basal metabolic index (BMI) were included in this study (Table 1). IIH patients were selected as the non-HSCR group because IIH was the most common disease displaying normal intestine function that required pediatric surgery, thereby facilitating participant recruitment and statistical analysis.
| Item | HSCR(n = 90) | non-HSCR(n = 50) | P value |
| Age (mo) | 7.2 ± 3.15 | 7.9 ± 2.07 | NS |
| Serum total protein (g/L) | 68.8 ± 5.17 | 70.2 ± 4.29 | NS |
| Serum albumin (g/L) | 48.2 ± 7.63 | 50.5 ± 6.19 | NS |
| Hemoglobin (g/L) | 129.1 ± 10.07 | 131.9 ± 9.89 | NS |
| Blood urea nitrogen (mmol/L) | 3.23 ± 1.01 | 2.91 ± 1.27 | NS |
| Length (cm) | 66.2 ± 4.94 | 67.9 ± 5.18 | NS |
| Weight (kg) | 7.8 ± 2.11 | 8.2 ± 2.75 | NS |
| Basal metabolic index | 8.6% ± 0.04% | 9.1% ± 0.05% | NS |
Detailed information regarding the antibodies and primers used is provided in Table 2. Other commercial reagents used were as follows: total RNA isolation kit (RNAiso Plus, TaKaRa, Japan); reverse transcription kit (PrimeScript® RT reagent Kit with gDNA Eraser, TaKaRa, Japan); SYBR® Premix Ex TaqTM II Tli RNaseH Plus (TaKaRa, Japan); protein extraction kit (Beyotime, China); BCA protein concentration determination kit (Beyotime, China); SDS-PAGE gel preparation kit (Beyotime, China); serum glutamate ELISA kit (E1258Hu, Uscn Life Science Inc., China); 3,3-diaminobenzidine (DAB; ZSGB-BIO, China); normal goat serum (Laboratoired’ Hormonologie, Marloie, Belgium); polymer helper (ZSGB-BIO, China); and TRIS (Merck- Belgolabo, Overijse, Belgium).
| Antigen | Primary antibody | Dilution | Applications |
| Neuroligin-1 | Goat-anti-human polyclonal | 1/100 | Detect Nlgn-1 with immunofluorescence on LMMP |
| Neuroligin-1 | Goat-anti-human polyclonal | 1/50 | Detect Nlgn-1 with Immunohistochemistry on paraffin-embedded sections |
| Neuroligin-1 | Goat-anti-human polyclonal | 1/200 | Detect Nlgn-1 with Western-blot |
| Glutamate | Mouse-anti-human monoclonal | 1/200 | Detect Glu with immunofluorescence on LMMP |
| Glutamate | Mouse-anti-human monoclonal | 1/200 | Detect Glu with Immunohistochemistry on LMMP |
| Glutamate | Mouse-anti-human monoclonal | 1/400 | Detect Nlgn-1 with Western-blot |
| β-actin | Rat-anti-human polyclonal | 1/2000 | Western-blot internal reference |
| Secondary antibody | Dilution | Applications | Source |
| Donkey anti-goat Texas Red Secondary | 1/500 | Label Nlgn-1 with immunofluorescence | ZSGB-BIO China |
| Goat anti-mouse FITC Secondary | 1/200 | Label Glu with immunofluorescence | ZSGB-BIO China |
| Horseradish Peroxidase-conjugated goat-anti-rat IgG | 1/500 | Detect β-actin with Western-blot | SANTA CRUZ United States |
| Horseradish Peroxidase-conjugated rabbit-anti-goat IgG | 1/1000 | Detect Nlgn-1 with Western-blot | SANTA CRUZ United States |
| Horseradish Peroxidase-conjugated goat-anti-mouse IgG | 1/1000 | Detect Glu with Western-blot | SANTA CRUZ United States |
| Primer | Primer sequence (5’→3’) | Annealing temperature (°C) | Product size (bp) |
| Neuroligin-1 | F: GCAAGACCAGAGCAGAGACT | 59 | 314 |
| R: CACCACCAAAGAATCCAATGTT | |||
| β-actin | F: AGCGAGCATCCCCCAAAGTT | 60 | 285 |
| R: GGGCACGAAGGCTCATCATT |
Tissue samples of 3-cm thickness consisting of aganglionic, transitional and ganglionic segments were harvested from the surgically excised colon of each child with HSCR[14,16]. The specimens were collected in quintuplicate. The two samples that were used to prepare longitudinal muscles with adherent myenteric plexus (LMMP) were placed in a dish coated with Sylgard elastomer (Dow Corning Co., Midland, MI, United States) and the mucosa, submucosa and circular muscle were removed under a stereomicroscope[14,16]. One sample was used to prepare the paraffin-embedded sections. Two additional fresh 100-mg pieces specimens were stored at -80 °C in disinfected tubes and prepared for Western blot analysis and qRT-PCR assay[16]. Additionally, fresh blood samples (1 mL) were collected during routine preoperative blood collection from all 140 patients (90 in the HSCR group and 50 in the IIH group). The samples were allowed to clot for two hours at room temperature (RT) or overnight at 4 °C and were then centrifuged for 20 min at approximately 1000 g. Serum samples were then stored in aliquots at -80 °C and were prepared for ELISA[16].
Double-labeled immunofluorescence staining was performed on the LMMP samples to identify whether neuroligin-1 is expressed in the excitatory post-synapses, where Glu is expressed, and to determine whether there are differences in expression between the aganglionic and ganglionic segments. The experimental methods were primarily similar to those which we used previously[14]. At RT, after rinsing in 10 mmol/L TRIS and 0.15 mol/L sodium chloride, two 1 cm × 1 cm LMMP patches (one each from the aganglionic and ganglionic segments) were incubated for 1 h in 3% normal goat serum and TBS-TX to reduce background staining and were then incubated overnight in the primary antibodies (against neuroligin-1 and Glu) diluted in TBS-TX. Then the samples were incubated in the dark for 2 h at RT in TBS containing the secondary antibodies (the Texas red-conjugated donkey anti-goat and FITC-conjugated donkey anti-goat). Finally, a laser scanning confocal microscope was used for the selective detection of green (FITC) and red (Texas red) fluorochromes, and the red and green fluorescence signals were digitally combined.
Immunohistochemical staining was used to determine the abundance of neuroligin-1 and Glu in both the LMMP and paraffinized sections from the aganglionic, transitional and ganglionic segments. After immersion in 3% hydrogen peroxide solution (H2O2) and incubation for 10 min to inactivate endogenous peroxidases, the samples were blocked with 3% goat normal serum diluted in 3% Triton-PBS for 1 h at RT. The slices were incubated in primary antibodies (against neuroligin-1 or Glu) for 24 h at 4 °C. PBS alone served as a negative control in which the primary antibody was omitted. Polymer helper and polyperoxidase-conjugated anti-goat IgG were sequentially added dropwise, and the samples were incubated at 37 °C for 20 min after the addition of the polymer helper and for 30 min after the addition polyperoxidase-conjugated anti-goat IgG. Finally, DAB was added as a chromogen to stain the samples.
Western blot, which was performed using a technique that was primarily similar to our previously described method[14,16], was employed to detect the levels of neuroligin-1 protein and Glu. Thirty micrograms of proteins were separated from 25 mg specimens from three different segments in the HSCR patients and were subjected to 10% SDS-PAGE, followed by transfer to PVDF membranes and blocking with 5% (w/v) nonfat milk for 1 h at RT. After washing three times with Tris-buffered saline-Tween solution (TBST), the membranes were incubated in antibodies against neuroligin-1, Glu and β-actin overnight at 4 °C. Subsequently, the membranes were incubated in horseradish peroxidase-conjugated rabbit anti-goat IgG and rabbit anti-rat IgG for 1 h at RT. Then, ECL and a chemiluminescence kit were applied for imagining on X-ray film (Millipore Corporation, Billerica, MA, United States). The expression levels were calculated as the relative gray values (neuroligin-1 IOD/β-actin IOD or Glu IOD/β-actin IOD) for analysis using Gel-Pro Analyzer 4.0 software.
As we described previously[16], 25 mg of specimens from different segments from HSCR patients were obtained for RNA extraction, and 1 μg of each specimen was then used for a cDNA synthesis reaction (20-μL reaction volume) using SYBR® Premix Ex TaqTM II (Perfect Real Time ). A qRT-PCR reaction was then performed according to instructions provided with the SYBR® Premix Ex TaqTM II (Tli RNaseH Plus) quantitative fluorescence kit. The reaction solution including 10 μL of SYBR® Premix Ex Taq II, 1 μL of the forward primer (10 μmol/L), 1 μL of the reverse primer (10 μmol/L) and 2 μL of cDNA was mixed and then subjected to qRT-PCR using the LightCycler® System Real Time fluorescence ratio PCR instrument. The Ct value of neuroligin-1 from each sample was measured, and the 2-△Ct value was calculated for further analysis.
Aliquots of 140 serum samples (90 from the HSCR group and 50 from the IIH group; 10 μL per sample) that were stored at -80 °C were used to detect the serum Glu levels as specified by the Glu ELISA kit instructions. Finally, after the measurement of the optical density (OD) value, the actual serum Glu concentration was calculated.
The averaged data in this study are summarized as the mean ± SD, and P values less than 0.05 were considered to be significant. For comparisons of two groups, unpaired t-test was performed. One-way ANOVA and the Tukey’s test were performed to compare three groups. All statistical analyses were performed using GraphPad Prism® 5 software for Windows (La Jolla, CA, United States). The statistical methods of this study were reviewed by Professor Xue Fuzhong, a biostatistician of School of Public Health of Shandong University.
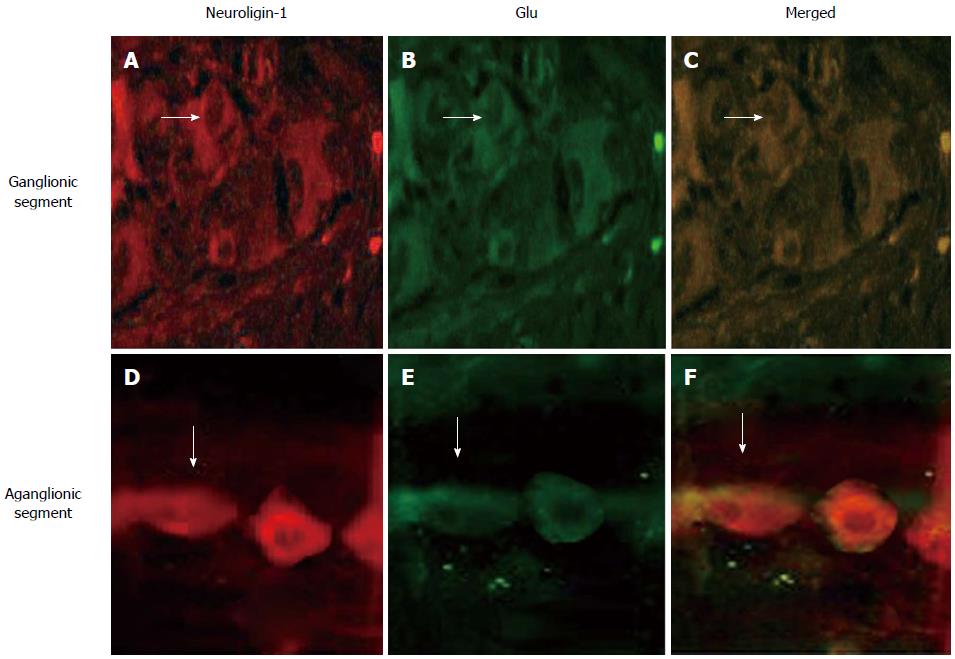
Figure 1 shows that in both the ganglionic and aganglionic segments, neuroligin-1 (A, D, red) was co-expressed (merged, C, F, yellow) with Glu (B, E, green), illustrating that neuroligin-1 was expressed in the excitatory post-synapses. However, the abundance and density of neuroligin-1 and Glu expression were lower in the aganglionic segments (D, E, F) than in the ganglionic segments (A, B, C) (white arrows indicates positively stained ganglion cells, which exhibit a fusiform or triangular shape).
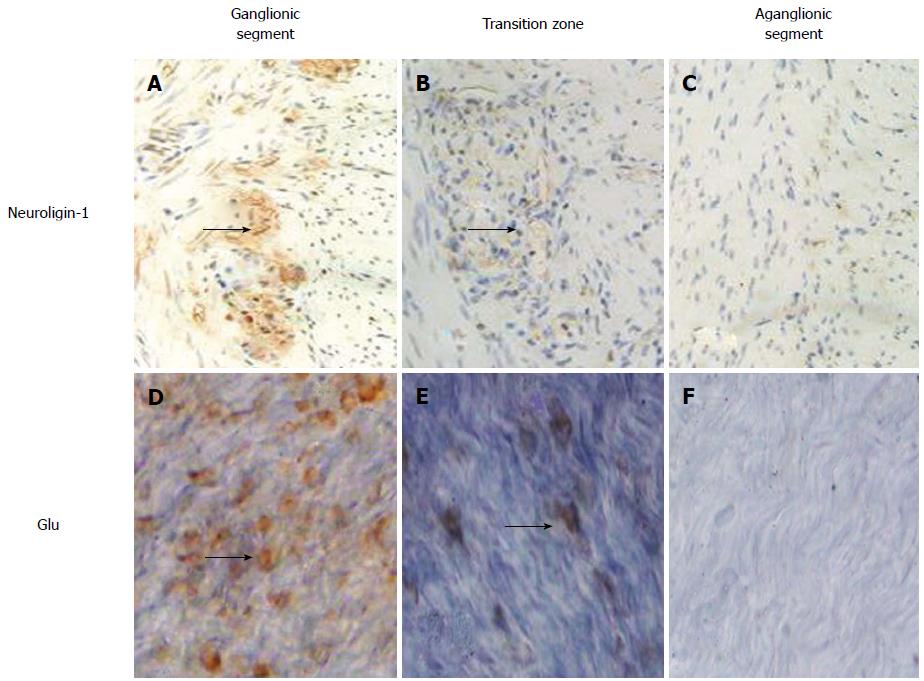
Figure 2 shows that the abundance and density of both neuroligin-1 in the paraffin-embedded sections (A, B, C) and Glu in LMMP (D, E, F) were highest to lowest in the ganglionic (A, D), transitional (B, E) and aganglionic segments (C, F). The black arrows indicate positively stained ganglion cells that were expressed between the longitudinal muscle and the circular muscle in both the paraffin-embedded sections (A, B, C) and the LMMP (D, E, F).
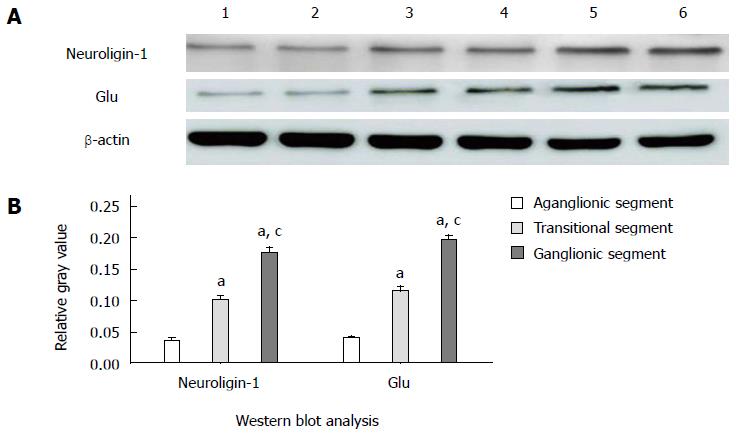
Figure 3 shows that neuroligin-1 and Glu were both significantly expressed in the ganglionic colonic segments (the relative gray values were 0.177 ± 0.008 and 0.198 ± 0.006, respectively; n = 90), moderately expressed in the transitional colonic segments (the relative gray values were 0.101 ± 0.006 and 0.115 ± 0.008, respectively; n = 90) and were clearly weekly expressed in the aganglionic colonic segments (the relative gray values were 0.035 ± 0.005 and 0.040 ± 0.003, respectively; n = 90). The differences in the gray values were significant (neuroligin-1: 0.177 ± 0.008 vs 0.101 ± 0.006, 0.177 ± 0.008 vs 0.035 ± 0.005, and 0.101 ± 0.006 vs 0.035 ± 0.005, P < 0.005; Glu: 0.198 ± 0.006 vs 0.115 ± 0.008, 0.198 ± 0.006 vs 0.040 ± 0.003, and 0.115 ± 0.008 vs 0.040 ± 0.003, P < 0.005).
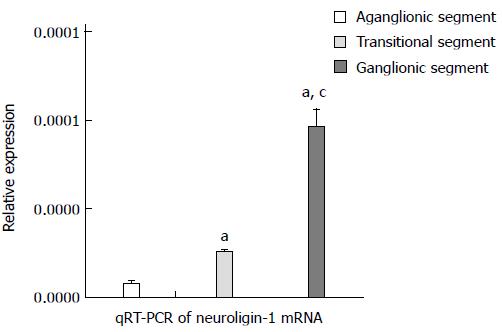
The qRT-PCR assay showed that neuroligin-1 mRNA expression was highest to lowest in the ganglionic (the relative expression level was 9.58 × 10-5± 9.94 × 10-6, n = 90), transitional (the relative expression level was 2.49 × 10-5± 1.38 × 10-6, n = 90) and aganglionic segments (the relative expression level was 7.17 × 10-6± 1.12 × 10-6, n = 90). These values were consistent with the results obtained via Western blot and immunohistochemical staining, and the differences in expression were significant (9.58 × 10-5± 9.94 × 10-6vs 2.49 × 10-5± 1.38 × 10-6, 9.58 × 10-5± 9.94 × 10-6vs 7.17 × 10-6± 1.12 × 10-6 and 2.49 × 10-5± 1.38 × 10-6vs 7.17 × 10-6± 1.12 × 10-6, P < 0.005) (Figure 4).
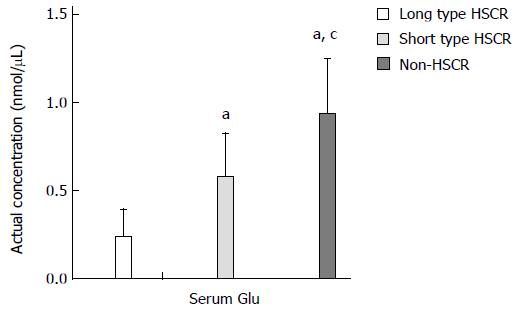
ELISA was used to detect and compare the serum Glu concentration. The results showed that the serum Glu concentration was lowest to highest in the long-segment HSCR (0.23 ± 0.16 nmol/μL), short-segment HSCR (0.57 ± 0.25 nmol/μL) and non-HSCR samples (0.93 ± 0.31 nmol/μL). The differences in the serum Glu concentration were significant (in nmol/μL, 0.93 ± 0.31 vs 0.57 ± 0.25, 0.93 ± 0.31 vs 0.23 ± 0.16, and 0.57 ± 0.25 vs 0.23 ± 0.16, P < 0.005) (Figure 5).
Neuroligins are a family of ubiquitously expressed post-synaptic cell adhesion molecules expressed in the brain that interact with neurexins. They are differentially localized to the post-synaptic boutons of excitatory and inhibitory synapses[17], which form the foundation of signal transduction between neurons via the transport of neurotransmitters[14]. Neuroligin-1 is enriched at the postsynaptic densities of excitatory glutamatergic synapses; neuroligin-2, however, is preferentially localized to inhibitory GABAergic synapses[17]. It has been shown that the balance of excitatory/inhibitory synapses in the brain plays a key role in neuronal plasticity mechanisms including learning and memory and some mental disorders, such as autism[18-22]. Furthermore, behavioral and cognitive deficiencies may be caused by a mismatch of neurexin and neuroligin expression in the CNS[23].
It has been accepted that coordinated interaction and cooperating mechanisms of the gastrointestinal tract results in normal gastrointestinal motility[24]. HSCR is regarded as a congenital disease resulting in neuronal intestinal malformations that display a typical pathology characterized by the absence of ganglion cells in the diseased digestive tract. During embryonic development, the failure of ganglion cells to innervate the lower gastrointestinal tract results in aganglionosis and dysfunction of the ENS[25]. Although certain genes such as RET, GDNF, NRTN, PHOX2B, EDNRB, EDN3, ECE1, SOX10, ZFHX1B, KIAA1279 and NRG1 have been shown to be altered in neural crest cell development in HSCR[26,27], whether enteric ganglion cells are influenced (such as their distribution and function) by the pathogenic genetic variations noted above remains to be fully elucidated[28].
Furthermore, primarily because of the uncertain pathogenesis of HSCR, the diagnosis of HSCR in clinical practice is occasionally difficult, and the diagnostic methods used primarily involve imaging examination, rendering the diagnosis, differential diagnosis and classification of this disease very difficult in infants[29,30]. Thus, it would be helpful to identify a novel easy diagnostic method for both the differential diagnosis and classification (long-type or short-type) of HSCR.
Related research has demonstrated that both gut digestion and motility and immunological processes are confined by the bi-directional communication of the brain-gut axis[13]. Therefore, we aimed to determine the pathogenesis of HSCR in terms of synaptic function and neuroligin expression based on this communication and the understanding of neuroligin-neurexin funtion. Our previous studies[14,15] revealed that neuroligins are expressed in post-synaptic neurons of HSCR patients but are down-regulated in the aganglionic colonic segment. Subsequently, based on our previous findings, we studied the expression of neuroligin-1 (a neuroligin subtype) and serum Glu and explored the relationship of their abundance to HSCR and the potential significance of any such relationship.
The findings of our study demonstrated the following: (1) in the ENS, neuroligin-1 is co-expressed with Glu in the same excitatory post-synapses between longitudinal muscle and circular muscle; (2) the abundance of neuroligin-1 and Glu was highest to lowest in the ganglionic, transitional and aganglionic colonic segments; and (3) the abundance of serum Glu was lowest to highest in the long-segment HSCR, short-segment HSCT and non-HSCR samples.
Our present results suggested that the abnormality of neuroligin-1 expression is closely related to HSCR and that neuroligin-1 may serve as a novel marker of ganglion cells, especially in excitatory synapses. The decreased abundance of neuroligin-1 in aganglionic segments may correlate with the excessive intestinal contraction resulting from abnormal excitatory signaling, potentially leading to HSCR. Furthermore, the difference in serum Glu concentrations may provide a valuable adjunct measure for diagnosing HSCR or for determining the length of the transition zone, which may be applied as an easy method to determine the classification of HSCR (long or short type). Of course, our present conclusions provide only basic information, and further investigation is needed. In the future, we will investigate the relationship between neuroligin-1 and neuroligin-2 and between Glu and GABA to examine the pathogenesis of HSCR from the perspective of abnormal synaptic development and will examine the value of a novel easy diagnostic method for HSCR based on the serum concentrations of Glu and GABA and their relationship.
Hirschsprung’s disease (HSCR) is a congenital neuronal intestinal malformation characterized by the absence of ganglion cells in the lower digestive tract. This disorder causes great harm to children and clinically manifests as intestinal obstruction, colon perforation or enterocolitis. Although there have been numerous studies of HSCR, the actual pathogenesis remains unclear and the preoperative diagnosis and classification (long or short-type) of HSCR have been restricted to imaging examinations; thus, this disorder warrants further investigation.
The aims of this study were to investigate the pathogenesis of HSCR from the perspective of synapses in the enteric nervous system (ENS) primarily by detecting the expression of neuroligin-1 and glutamate (Glu) and to evaluate a new method for the diagnosis, differential diagnosis and classification of HSCR.
This study demonstrated for the first time that the abnormal expression of neuroligin-1 and Glu was closely related to the pathogenesis of HSCR and that neuroligin-1 may serve as a novel marker of ganglion cells in the ENS, especially in excitatory snypases. Additionally, an abnormal concentration of serum Glu may be primarily considered as a novel method for the diagnosis, differential diagnosis and classification of HSCR.
The study was based on the bi-directional communication between the CNS and the ENS and previous studies of synapses and neuroligins in the ENS. Based on this fundamental research, a problem in clinical practice was investigated and was aimed to be solved.
Neuroligins are postsynaptic proteins implicated in the formation, development and function of synapses by acting together with neurexins, which are pre-synaptic proteins in the CNS. Post-synapses are primarily divided into excitatory post-synapses expressing neuroligin-1 and inhibitory post-synapses expressing neuroligin-2. Furthermore, the excitatory neurotransmitter Glu is primarily released by excitatory post-synapses, and the inhibitory neurotransmitter γ-aminobutyric acid (GABA) is primarily released by inhibitory post-synapses. Therefore, the expression of neuroligin-1 or neuroligin-2 may be reflected by the localization of Glu and GABA to some degree.
The authors present an interesting study to further investigate physiologic changes that occur in HSCR. The data reported may be of some interest in their field
P- Reviewer: Cologne KG S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Roberts RR, Bornstein JC, Bergner AJ, Young HM. Disturbances of colonic motility in mouse models of Hirschsprung’s disease. Am J Physiol Gastrointest Liver Physiol. 2008;294:G996-G1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 406] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 3. | Emison ES, McCallion AS, Kashuk CS, Bush RT, Grice E, Lin S, Portnoy ME, Cutler DJ, Green ED, Chakravarti A. A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature. 2005;434:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 350] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Kaymakçi A, Narter F, Yazar AS, Yilmaz MS. Congenital central hypoventilation syndrome with hirschsprung’s disease due to PHOX2B gene mutation in a Turkish infant. Turk J Pediatr. 2012;54:519-522. [PubMed] |
| 5. | Heanue TA, Pachnis V. Prospective identification and isolation of enteric nervous system progenitors using Sox2. Stem Cells. 2011;29:128-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Burkardt DD, Graham JM, Short SS, Frykman PK. Advances in Hirschsprung disease genetics and treatment strategies: an update for the primary care pediatrician. Clin Pediatr (Phila). 2014;53:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Jiang Q, Ho YY, Hao L, Nichols Berrios C, Chakravarti A. Copy number variants in candidate genes are genetic modifiers of Hirschsprung disease. PLoS One. 2011;6:e21219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Sun M, Xing G, Yuan L, Gan G, Knight D, With SI, He C, Han J, Zeng X, Fang M. Neuroligin 2 is required for synapse development and function at the Drosophila neuromuscular junction. J Neurosci. 2011;31:687-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Suckow AT, Comoletti D, Waldrop MA, Mosedale M, Egodage S, Taylor P, Chessler SD. Expression of neurexin, neuroligin, and their cytoplasmic binding partners in the pancreatic beta-cells and the involvement of neuroligin in insulin secretion. Endocrinology. 2008;149:6006-6017. [PubMed] |
| 10. | Fu Z, Vicini S. Neuroligin-2 accelerates GABAergic synapse maturation in cerebellar granule cells. Mol Cell Neurosci. 2009;42:45-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Graf ER, Kang Y, Hauner AM, Craig AM. Structure function and splice site analysis of the synaptogenic activity of the neurexin-1 beta LNS domain. J Neurosci. 2006;26:4256-4265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Dinamarca MC, Weinstein D, Monasterio O, Inestrosa NC. The synaptic protein neuroligin-1 interacts with the amyloid β-peptide. Is there a role in Alzheimer’s disease? Biochemistry. 2011;50:8127-8137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Wouters MM, Boeckxstaens GE. Neuroimmune mechanisms in functional bowel disorders. Neth J Med. 2011;69:55-61. [PubMed] |
| 14. | Wang J, Mou Y, Zhang Q, Zhang F, Yang H, Zhang W, Li A. Expression and significance of neuroligins in myenteric cells of Cajal in Hirschsprung’s disease. PLoS One. 2013;8:e67205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Zhang Q, Wang J, Li A, Liu H, Zhang W, Cui X, Wang K. Expression of neurexin and neuroligin in the enteric nervous system and their down-regulated expression levels in Hirschsprung disease. Mol Biol Rep. 2013;40:2969-2975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Yang H, Niu J, Wang J, Zhang F, Zhang Q, Zhang W, Li A. The down-regulation of neuroligin-2 and the correlative clinical significance of serum GABA over-expression in Hirschsprung’s disease. Neurochem Res. 2014;39:1451-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, Liu X, Südhof TC, Powell CM. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 2009;8:114-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324-1328. [PubMed] |
| 19. | Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 755] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 20. | Levinson JN, El-Husseini A. Building excitatory and inhibitory synapses: balancing neuroligin partnerships. Neuron. 2005;48:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci USA. 2004;101:13915-13920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903-911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1497] [Cited by in RCA: 1381] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 23. | Biswas S, Reinhard J, Oakeshott J, Russell R, Srinivasan MV, Claudianos C. Sensory regulation of neuroligins and neurexin I in the honeybee brain. PLoS One. 2010;5:e9133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Du P, O’Grady G, Davidson JB, Cheng LK, Pullan AJ. Multiscale modeling of gastrointestinal electrophysiology and experimental validation. Crit Rev Biomed Eng. 2010;38:225-254. [PubMed] |
| 25. | Tam PK, Garcia-Barceló M. Genetic basis of Hirschsprung’s disease. Pediatr Surg Int. 2009;25:543-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 634] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 27. | Brooks AS, Bertoli-Avella AM, Burzynski GM, Breedveld GJ, Osinga J, Boven LG, Hurst JA, Mancini GM, Lequin MH, de Coo RF. Homozygous nonsense mutations in KIAA1279 are associated with malformations of the central and enteric nervous systems. Am J Hum Genet. 2005;77:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Shimotake T, Tomiyama H, Aoi S, Iwai N. Discrepancy between macroscopic and microscopic transitional zones in Hirschsprung’s disease with reference to the type of RET/GDNF/SOX10 gene mutation. J Pediatr Surg. 2003;38:698-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Catto-Smith AG, Trajanovska M, Taylor RG. Long-term continence after surgery for Hirschsprung’s disease. J Gastroenterol Hepatol. 2007;22:2273-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Kannaiyan L, Madabhushi S, Malleboyina R, Are NK, Reddy KR, Rao B. Calretinin immunohistochemistry: A new cost-effective and easy method for diagnosis of Hirschsprung’s disease. J Indian Assoc Pediatr Surg. 2013;18:66-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |