Published online Jun 7, 2015. doi: 10.3748/wjg.v21.i21.6665
Peer-review started: October 20, 2014
First decision: December 26, 2014
Revised: January 5, 2015
Accepted: February 13, 2015
Article in press: February 13, 2015
Published online: June 7, 2015
Processing time: 234 Days and 6.1 Hours
AIM: To investigate whether the proportions of acetyl-histone-positive hepatocytes could be used as markers of deteriorating liver function.
METHODS: In total, 611 cirrhotic cases from 3701 patients who were diagnosed during the past 15 years were screened, and 152 follow-up cases were selected. Paraffin tissue microarray was prepared for immunohistochemistry to examine acetyl-histone expression. The proportions of positive hepatocytes were recorded, and their correlations to clinical and laboratory indicators were analyzed statistically.
RESULTS: The proportions of H2AK5ac+, H3K9/K14ac+ and H3K27ac+ hepatocytes gradually increased with deteriorating liver function and with increasing levels of serum markers of liver injury. In the follow-up cases, patients with > 70% H2AK5ac+, H3K9/K14ac+ or H3K27ac+ hepatocytes had statistically lower survival rates (P < 0.05). Furthermore, > 70% H2AK5ac+ or H3K27ac+ hepatocytes were strong independent predictors of overall survival (P < 0.05).
CONCLUSION: The proportions of acetyl-histone-positive hepatocytes are closely associated with the liver function and prognosis of cirrhotic patients.
Core tip: We previously divided hepatocytes into transcriptionally active and inactive cells based on their immunoreactivity to acetyl-histones. In the present study, we evaluated the expression of acetyl-histone markers in livers with progressive cirrhosis to investigate whether inactive hepatocytes can be activated for functional compensation and act as markers of liver function. We found that the proportions of acetyl-histone-positive hepatocytes were associated with liver function, which revealed a regeneration-independent compensatory mechanism of chronically damaged liver. In addition, assaying the proportions of acetyl-histone-positive hepatocytes may offer a novel strategy for evaluating patient liver function and prognosis.
- Citation: Zhou P, Xia J, Zhou YJ, Wan J, Li L, Bao J, Shi YJ, Bu H. Proportions of acetyl-histone-positive hepatocytes indicate the functional status and prognosis of cirrhotic patients. World J Gastroenterol 2015; 21(21): 6665-6674
- URL: https://www.wjgnet.com/1007-9327/full/v21/i21/6665.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i21.6665
Cirrhosis, which is the end stage of liver fibrosis, usually destroys the normal architecture and the synthetic and metabolic functions of the liver by increasing the fibrous tissues and regenerative nodules. Cirrhosis is also a consequence of an excessive healing response to various and continuous liver injuries[1]. In developing countries, chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are major risk factors for cirrhosis, although dietary exposure to aflatoxin B1 also plays an important role in cirrhosis[2]. Proliferative hepatocytes, bile ducts, pseudolobules, fibrous tissues and inflammatory cells constitute the major pathological and morphological characteristics of cirrhosis progression. Liver regeneration is commonly considered an adaptive process by which liver structure and function are recovered; however, cirrhotic patients can maintain their liver function at a normal level for many years, although the remnant hepatocytes are continuously lost[3-5]. In addition, in animal studies, rodents could survive on the remnant liver after a 70% partial hepatectomy even when liver regeneration was severely deprived. This evidence suggests that the remnant liver function can be compensated in a regeneration-independent manner; however, the underlying mechanism remains unknown.
Histone acetylation, which is a key modulator of chromatin structure and gene transcription[6,7], is regulated dynamically and reversibly by histone acetyltransferases (HATs) and by histone deacetylases (HDACs)[8-10]. HATs add an acetyl group to the lysine residues (Lys or K), thus neutralizing the positive charge, weakening the interaction between histone and DNA, and increasing the accessibility of transcription factors to their target genes to prime transcription and elongation[11]. Histone acetylation is widely considered a marker of active transcription[6-13]. In contrast, HDACs remove the acetyl group, which leads to chromatin compaction and to transcriptional repression[6]. The dysregulation of HDACs is also considered a crucial cause of various tumors, and HDAC inhibitors (HDACis) have been used preclinically and clinically as antitumor drugs[14-19].
Few studies have focused on the role of histone acetylation in non-tumorous lesions, such as cirrhosis. In our previous study, we observed that only a few hepatocytes in healthy livers of both mouse and human are acetyl-histone-positive and that these cells obtain a much higher gene transcription level than their negative counterparts. Therefore, we proposed a novel concept of “functional heterogeneity” of hepatocytes based on their entirely different acetyl-histone marker expression levels and subsequent transcriptional activity. As described previously, the extensive activation of inactive cells through histone acetylation is crucial for liver function maintenance after a partial hepatectomy, particularly when liver regeneration is severely inhibited. Furthermore, in a pilot study in cirrhotic patients, we found that the proportion of acetyl-histone-positive hepatocytes increases with the progression of chronic cirrhosis and that hepatocytes are almost completely positively stained in end-stage cirrhosis. Therefore, we hypothesized that our concept of hepatocytic functional heterogeneity might provide a novel explanation for the powerful compensatory capacity of the liver. Normally, active hepatocytes, which are characterized by acetyl-histone markers, are competent for routine physiological requirements, while inactive hepatocytes act as a functional reservoir for future activation to restore the liver function even independent of regeneration. With the continuous loss of hepatocytes in cirrhotic liver, the reserve hepatocytes are gradually activated, injured and exhausted, and then liver failure occurs[20].
In this study, we examined the expression of acetyl-histone markers in progressive cirrhosis in a large clinical sample to investigate whether the proportions of acetyl-histone-positive hepatocytes could act as markers of deteriorating liver function and to further confirm our hypothesis regarding the compensatory mechanism of the liver.
We collected 3701 cirrhotic cases that were diagnosed by biopsy between 1995 and 2009 from the Department of Pathology, West China Hospital, Sichuan University. We excluded the HBV-negative patients in this study to maintain consistency, and 611 cases were analyzed, ultimately obtaining follow-up data from 152 patients. Of the 3090 excluded cases, 421 cases were non-hepatitis B-associated cirrhosis, 1982 cases were core-needle biopsy tissues with insufficient tissue samples for investigation, and 687 cases lacked integrated clinical data. All human studies presented in our manuscript have been approved by the appropriate ethics committee and performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments.
All of the pathological slides of the 611 available cases were reviewed, and the corresponding clinical data and paraffin blocks were collected. The primary blocks were used to prepare tissue microarray (TMA) blocks and slides according to the typical morphological area of the primary pathological slides. Then, the TMA blocks were subjected to hematoxylin and eosin (HE) and immunohistochemical (IHC) staining. The EnVision staining method was used for IHC staining. Negative controls were prepared by replacing the primary antibodies with phosphate-buffered saline (PBS). Nuclear brown staining indicated a positive result. The IHC markers were H2AK5ac, H2BK5ac, H3K9/K14ac, H3K18ac, H3K23ac andH3K27ac antibodies (Table 1).
| Antibody | Clone | Vendor | Stain location |
| H2AK5ac | ab45152 rabbit monoclonal | Abcam | Nuclear |
| H2BK5ac | Ab40886 rabbit monoclonal | Abcam | Nuclear |
| H3K9/K14ac | 06-599 rabbit polyclonal | Millipore | Nuclear |
| H3K18ac | ab40888 rabbit monoclonal | Abcam | Nuclear |
| H3K23ac | ab61234 rabbit polyclonal | Ancam | Nuclear |
| H3K27ac | ab4729 rabbit polyclonal | Abcam | Nuclear |
The clinical data and laboratory test results from all 611 patients were collected. The patients were classified into grades A, B and C according to Child-Turcotte-Pugh (CTP) Score Classification[21,22]. The patient follow-up data were made available until the completion of this manuscript. The IHC-positive hepatocyte proportions were calculated to assess the acetyl-histone marker results.
Statistical analyses in this study were performed with SPSS16.0 software for Windows. The Kruskal-Wallis test was used to compare the liver function and the proportions of acetyl-histone-positive hepatocytes among the cases in different CTP grades. Correlations among the parameters of liver function, acetyl-histone-positive hepatocyte proportions and patient CTP grades were evaluated by Spearman’s correlation test. The Kaplan-Meier method was used in a univariate survival analysis to calculate the survival rates and to identify significant prognostic variables. The significance of the data was determined by the log-rank test. The COX proportional hazard regression model was used in a multivariate survival analysis to identify independent prognostic variables for overall survival (OS). A series of variables including age, gender, CTP grade, ascites status, and acetyl-histone-positive hepatocyte proportions were considered for inclusion in the multivariate logistic regression analysis to identify independent factors to predict mortality. The prognostic effect of different variables was expressed with the hazard ratio (HR) and 95% confidence interval (95%CI). All tests were two-tailed, and P < 0.05 was considered statistically significant.
In total, 3280 cases (88.6%) of liver cirrhosis induced by hepatitis B were screened from 3701 cases of liver cirrhosis during a 15-year period, among which, 1298 cases (35%) underwent surgical excision biopsy. Finally, 611 cases (16.5%) with available tissues and complete clinical data were analyzed.
The ratio of males to females was 2.53:1 (438:173). The mean age was 44.7 years (12-80 years). According to CTP score classification, 262 cases (42.9%) were considered grade A, 308 cases (50.4%) were considered grade B, and 41 cases (6.7%) were considered grade C. Upper gastrointestinal bleeding occurred in 242 cases (39.6%), ascites occurred in 131 cases (21.4%), jaundice appeared in 64 cases (10.5%), and hepatic encephalopathy occurred in 5 cases (0.8%).
Among the 152 (24.9%) follow-up patients, the median survival time was 84.5 mo (range, 1-211 mo). The ratio of males to females was 1.76:1 (97:55), and the mean age was 46.5 years (13-76 years). According to CTP score classification, 75 cases (49.3%) were considered grade A, 71 cases (46.7%) were considered grade B, and 6 cases (4%) were considered grade C. Of these cases, 57 cases (37.5%) had upper gastrointestinal bleeding histories, 31 cases (20.4%) had ascites, 16 cases (10.5%) were accompanied by hepatocellular carcinoma (HCC), 13 cases (8.6%) had jaundice, and 1 case (0.6%) had hepatic encephalopathy. Within the follow-up period, 114 cases (75%) survived with disease (1 patient accompanied by HCC), and 38 patients (25%) died of complications of cirrhosis (24 died of bleeding, and 14 died of HCC).
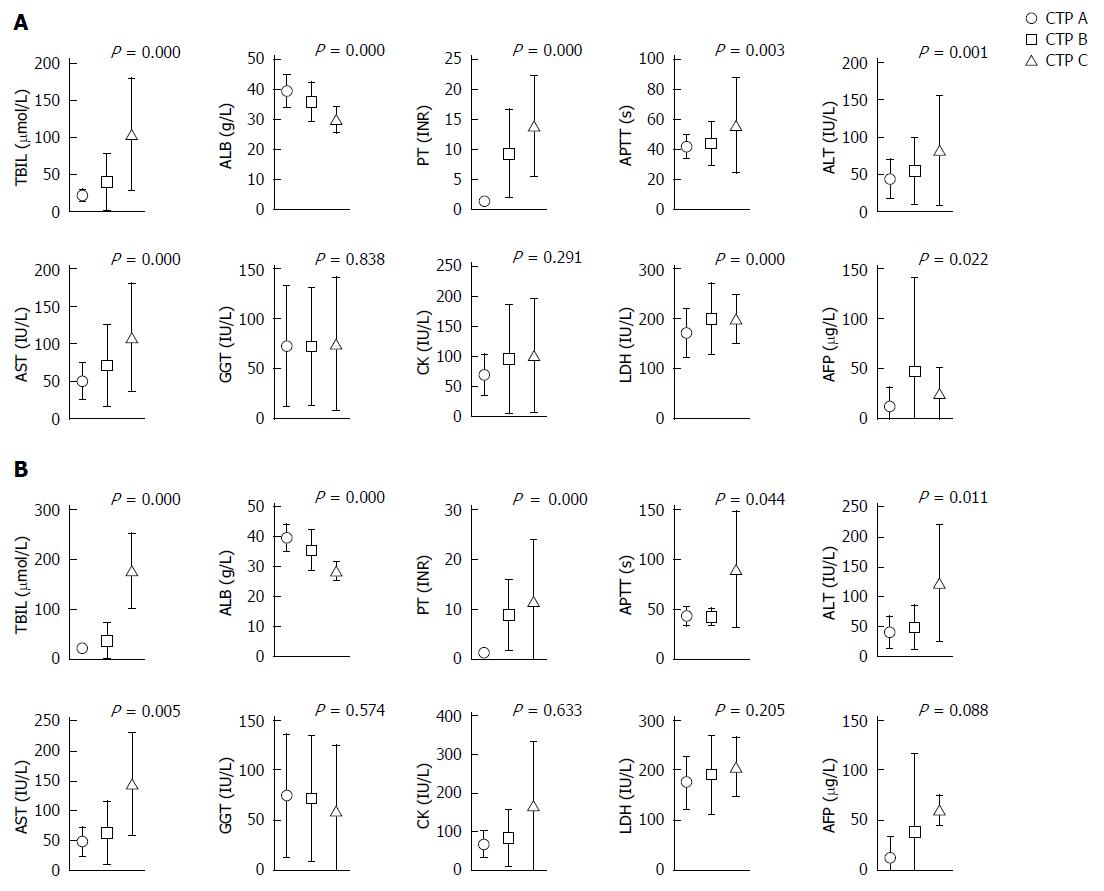
The serum values of total bilirubin (TBIL), albumin (ALB), prothrombin time (PT), activated partial thromboplastin time (APTT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactic dehydrogenase (LDH), and alpha fetoprotein (AFP) were statistically significant among each CTP grade (P < 0.05) of the 611 patients, except for γ-glutamyl transpeptidase (GGT) and creatine kinase (CK) (Figure 1). Considering the low follow-up rate, we compared the clinic characteristics and serum parameters of the 152 follow-up cases and of all 611 cases. No statistical significance (P > 0.05) was found for the patient age and TBIL, ALB, APTT, ALT, AST, GGT, CK, LDH and AFP values, suggesting that the follow-up cases were representative of all 611 included cases (Table 2).
| Follow-up (n = 152) | Total (n = 611) | P value | |
| Age (yr) | 46.52 ± 12.07 | 44.74 ± 11.81 | 0.099 |
| Total bilirubin (μmol/L) | 35.29 ± 41.09 | 36.72 ± 39.18 | 0.182 |
| Albumin (g/dL) | 3.73 ± 0.62 | 3.69 ± 0.64 | 0.497 |
| Prothrombin time (INR) | 5.21 ± 6.58 | 6.21 ± 7.11 | 0.002 |
| Activated partial thromboplastin time (s) | 44.72 ± 15.97 | 43.87 ± 14.20 | 0.862 |
| Alanine aminotransferase (IU/L) | 48.20 ± 28.95 | 52.06 ± 41.06 | 0.164 |
| Aspartate aminotransferase (IU/L) | 59.21 ± 46.12 | 64.59 ± 47.92 | 0.060 |
| γ-glutamyl transpeptidase (IU/L) | 72.55 ± 61.74 | 72.76 ± 59.84 | 0.630 |
| Creatine kinase (IU/L) | 78.49 ± 64.05 | 84.79 ± 73.36 | 0.266 |
| Lactic dehydrogenase (IU/L) | 183.01 ± 66.27 | 187.78 ± 62.43 | 0.203 |
| Alpha fetoprotein (μg/L) | 23.95 ± 52.26 | 29.22 ± 67.36 | 0.464 |
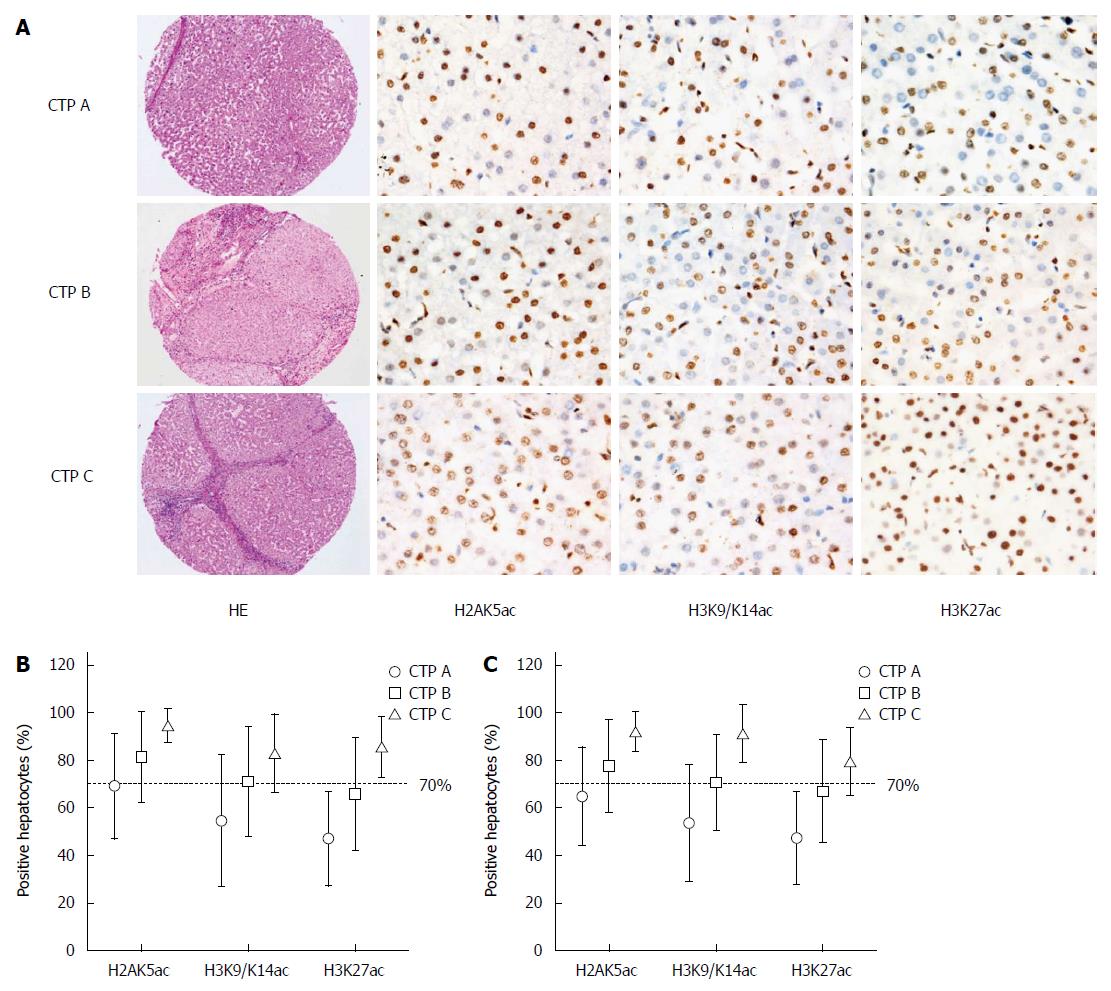
IHC staining of acetyl-histone markers was performed and the proportions of acetyl-histone-positive hepatocytes were calculated (Figure 2). H2BK5ac, H3K18ac and H3K23ac staining could not be calculated or interpreted because of unsatisfactory staining. However, the proportions of H2AK5ac+, H3K9/K14ac+ and H3K27ac+ hepatocytes statistically increased with the increase in CTP grades for both total and follow-up cases (P < 0.001) (Figure 2).
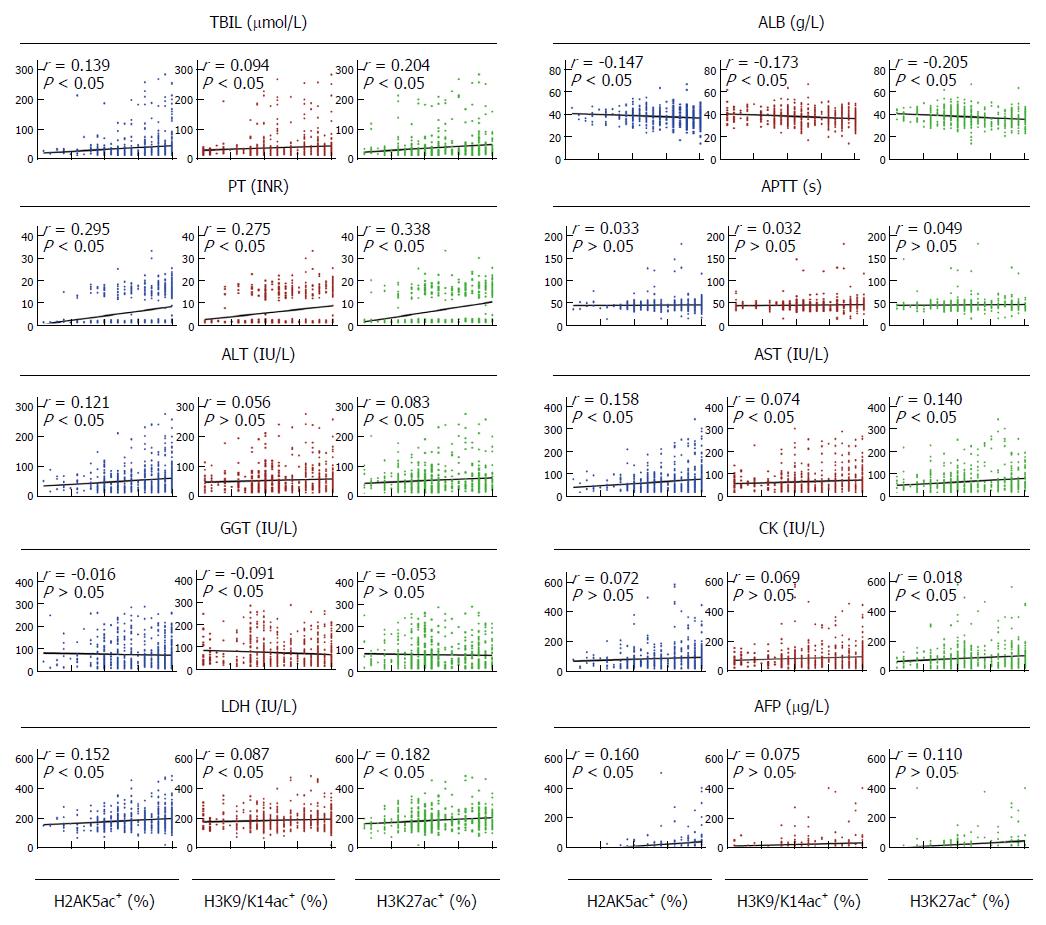
Among the 611 patients, the proportions of H2AK5ac+, H3K9/K14ac+ and H3K27ac+ hepatocytes were positively associated with the serum values of TBIL (P < 0.05), PT (P < 0.001) and LDH (P < 0.05) and were negatively associated with the serum values of ALB (P < 0.001). The proportions of H2AK5ac+ and H3K27ac+ hepatocytes were also positively associated with the serum values of both ALT (P < 0.05) and AST (P < 0.001). Additionally, the proportions of H2AK5ac+, H3K9/K14ac+ and H3K27ac+ hepatocytes were positively associated with the serum values of AFP (P < 0.05), GGT (P < 0.05) and CK (P < 0.01), respectively (Figure 3).
Among the 152 follow-up patients, the proportions of H2AK5ac+, H3K9/K14ac+ and H3K27ac+ cells were positively associated with the serum values of PT (P < 0.001), ALT (P < 0.05) and AST (P < 0.05), and the proportions of H2AK5ac+ and H3K27ac+ cells were also positively associated with the serum values of LDH (P < 0.005). Additionally, the proportions of H3K27ac+ and H3K9/K14ac+ cells were negatively associated with the serum values of ALB (P < 0.01), and the proportions of H3K27ac+ cells were positively associated with the serum values of TBIL (P < 0.001).
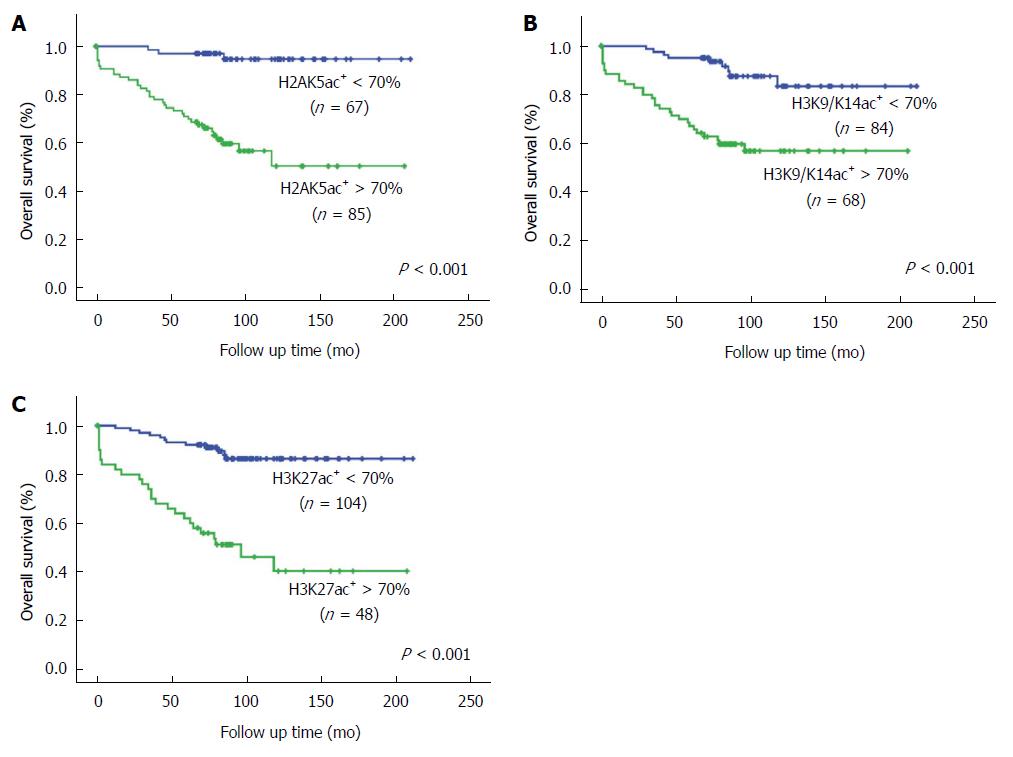
Because of the low percentage of CTP C cases in our follow-up patients, dividing the cases into three groups for prognostic analysis was inappropriate. Therefore, we used a cutoff value of 70% acetyl-histone-positive cells to divide the patients into two groups with lower or higher CTP grades (Figure 2). In univariate analysis, patients with > 70% H2AK5ac+, H3K9/K14ac+ or H3K27ac+ hepatocytes had significantly lower OS rates (P < 0.001) than did those with < 70% positive hepatocytes. The OS rates were 94.7% (95%CI: 88.6%-100.8%) and 50.3% (95%CI: 34.6%-66.0%) in the patients with < 70% and > 70% H2AK5ac+ hepatocytes, respectively. For patients with < 70% and > 70% H3K9/K14ac+ hepatocytes, the OS rates were 83.4% (95%CI: 72.2%-94.6%) and 56.9% (95%CI: 44.6%-69.2%), respectively, and for patients with < 70% and > 70% H3K27ac+ hepatocytes, the OS rates were 86.4% (95%CI: 79.1%-93.7%) and 40.4% (95%CI: 22.9%-57.8%), respectively. Additionally, patients with age > 60 years (P < 0.05) and CTP grade C (P < 0.05) had significantly lower OS rates compared with patients with age < 60 years and CTP grades A and B (Table 3 and Figure 4).
| Variable | Unitivariate analysis | Multivariate analysis | ||||
| OS (%) | 95%CI | P value | HR | 95%CI | P value | |
| Age (yr) | ||||||
| < 60 | 75.0 | 66.2-83.8 | 0.035 | 0.35 | 0.12-1.04 | 0.059 |
| > 60 | 49.4 | 24.1-74.7 | ||||
| Gender | 0.858-6.009 | |||||
| Female | 75.1 | 59.0-91.2 | 0.171 | 2.27 | 0.099 | |
| Male | 69.0 | 59.2-78.8 | ||||
| CTP grade | 0.26-8.56 | |||||
| A, B | 72.0 | 153.8-179.7 | 0.041 | 1.36 | 0.744 | |
| C | 50.0 | 10.0-89.9 | ||||
| Ascites | 0.31-2.60 | 0.843 | ||||
| No | 71.8 | 62.2-81.4 | 0.535 | 0.90 | ||
| Yes | 68.5 | 50.9-86.1 | ||||
| H2AK5ac | 1.68-24.49 | |||||
| < 70% | 94.7 | 88.6-100.8 | 0.000 | 6.41 | 0.007 | |
| > 70% | 50.3 | 34.6-66.0 | ||||
| H3K9/K14ac | 1.88 | 0.67-5.42 | ||||
| < 70% | 83.4 | 72.2-94.6 | 0.000 | 0.240 | ||
| > 70% | 56.9 | 44.6-69.2 | ||||
| H3K27ac | ||||||
| < 70% | 86.4 | 79.1-93.7 | 0.000 | 4.75 | 1.82-12.36 | 0.001 |
| > 70% | 40.4 | 22.9-57.8 | ||||
Multivariate analysis using the Cox proportional hazard model showed that > 70% H2AK5ac+ or H3K27ac+ hepatocytes were independent prognostic factors for OS rates (P = 0.007 and 0.001, respectively). Patients with > 70% H2AK5ac+ and H3K27ac+ hepatocytes had 6.41 (95%CI: 1.68-24.49) and 4.75 (95%CI: 1.82-12.36) times higher risks of mortality, respectively (Table 3).
In this study, we found that the proportions of acetyl-histone-positive hepatocytes increased gradually with decreasing liver function during the progression of cirrhosis. Patients with > 70% H2AK5ac+, H3K9/K14ac+ or H3K27ac+ hepatocytes had significantly lower OS rates, and > 70% H2AK5ac+ and H3K27ac+ hepatocytes are both strong independent predictors of overall survival. Our findings confirmed our hypothesis that acetyl-histone-negative hepatocytes act as a reservoir and that these cells will be activated for functional compensation if liver regeneration is unable to restore its architecture. In addition, our results also suggest that examining the hepatocytic acetyl-histone status is a potential strategy for revealing liver function or for evaluating reserve hepatocytes.
The liver possesses a unique regeneration capability to restore its histology and function upon various injuries[23-25]. However, in some cases, the liver can exert its full function in response to acute or chronic damage in both animal models and humans when the liver regeneration fails to restore the cell mass, suggesting that a small fraction of hepatocytes is sufficient to sustain the liver function and that inactive hepatocytes might function as a reservoir[26-28]. Histone acetylation is generally accepted as the marker of active gene transcription, and our previous work is the first direct evidence that acetyl-histone negative hepatocytes are transcriptionally inactive; thus, we hypothesized these cells might be reserve cells[20]. Indeed, we found that inactive hepatocytes are extensively activated after partial hepatectomy and that histone acetylation inhibition results in injured liver function and animal death. The present study demonstrates an unprecedented mechanism to explain the compensatory capability of the liver; however, whether this mechanism is identical in chronic liver cirrhosis remains unknown.
Liver cirrhosis is a chronic progressive change due to long lasting damage by various factors. Due to the increasing occurrence of viral hepatitis and alcohol abuse, the incidence of liver cirrhosis has increased continuously in many countries in recent decades[29,30]. In the present study, cirrhosis induced by hepatitis B accounted for 88.6% (3280/3701) of the examined cirrhotic liver biopsies, demonstrating that hepatitis B remains the most important cause of cirrhosis in China. To maintain consistency with the examined cases, we excluded the HBV-negative patients in the present study; other liver damage situations will be examined in further studies.
As expected, the proportions of hepatocytes expressing all examined acetyl-histone markers increased gradually with decreased liver function, as classified by CTP grade. In addition, some serum parameters closely correlated with the acetyl-histone expression levels. According to our hypothesis of hepatocytic functional heterogeneity, we demonstrated that the extensive transcriptional activation of the residual hepatocytes is helpful for maintaining hepatic function. Inactive hepatocytes, which act as a functional reservoir, are almost exhausted in end-stage cirrhosis, and then liver failure occurs. Our findings indicate that hepatocytic histone acetylation acts as a regulator that activates reserve cells for functional compensation.
The CTP score is a widely used system that predicts the long-term survival of cirrhotic patients[31]. In the present study, the CTP grade was not identified as an independent risk factor to predict long-term survival perhaps due to the low percentage of patients classified as CTP C. At least three reasons exist for this low percentage of CTP C patients. First, routine screenings during health examinations and effective therapy during early stages might delay the progression of cirrhosis and maintain most patients as CTP A or B for decades. Second, the patients that are classified as CTP C are often clearly diagnosed before biopsy. Third, CTP C patients are likely to avoid operation or biopsy because of their high risk of bleeding and other complications. In our survival analysis using a cutoff value of 70% acetyl-histone-positive hepatocytes, we found that patients with > 70% H2AK5ac+, H3K9/K14ac+ or H3K27ac+ hepatocytes were associated with a poor prognosis or with lower survival. Furthermore, > 70% H2AK5ac+ or H3K27ac+ hepatocytes were both strong independent predictors of patient OS rates. Taken together, these results indicate that a biopsy to evaluate the proportion of acetyl-histone-positive hepatocytes might offer a potential strategy to reveal hepatic functional reserves and to predict the prognosis of cirrhotic patients.
The present study has several shortcomings. First, considering such a high incidence of cirrhosis, the number of included cases is too small because most of the patients are definitively diagnosed by imaging examination. Even if the patients accept puncture biopsy, the amounts of tissues are insufficient for further IHC examination. Only a few patients received an open or laparoscopic biopsy, making the sampling bias unavoidable because the samples were not randomly selected. Nevertheless, additional cases should be included in future studies, particularly taking advantage of available puncture specimens. Second, the follow-up rate is extremely low, most likely because of active urbanization and population migration. Additionally, some patients changed their residential addresses and contact phone numbers after the large earthquake near our city in 2008. Fortunately, the follow-up cases are representative of the included cases. Third, not all of the acetyl-histone markers stained satisfactorily. Approximately five acetylation sites are at the N terminus of each histone (H2A, H2B, H3 and H4). At the beginning of our experiment, we performed IHC to examine the six most important markers. However, only three markers stained satisfactorily. In our previous work, we sorted the H2AK5+ cells and observed that other acetyl-histones including H2BK5, H3K9, H3K14, H3K27 and H3K9/14 are highly expressed simultaneously[20]. Therefore, we believe that these three markers can represent the global histone acetylation level of the cell.
In summary, our findings reveal a regeneration-independent compensatory mechanism in liver suffering chronic damage. In addition, examining the proportions of acetyl-histone-positive hepatocytes might offer a novel strategy for evaluating the liver function and patient prognosis, although the required invasive operation might limit its clinical implementation.
We wish to thank Fei Chen, Guo-Qing Cai, Jia Guo, and Feng-Yuan Li for their assistance with this experiment. We also thank Hong Feng for statistical assistance.
“Functional heterogeneity” of hepatocytes is a novel concept that has previously been proposed, which is based on the different expression level of acetyl-histone markers and the subsequent transcriptional activity. In a previous publication, the authors demonstrated the concept in the liver of rodent models after partial hepatectomy and in a small cohort of cirrhotic liver patients. Therefore, they speculate that the concept of hepatocytic functional heterogeneity might provide a novel explanation for the powerful compensatory capacity of the liver. Accordingly, the authors divided hepatocytes into transcriptionally active cells, which are in the minority but competent for routine physiological requirement, and inactive cells, which act as a reservoir for functional compensation after parenchymal loss in a regeneration-independent manner.
Liver regeneration is commonly considered an adaptive process by which liver structure and function are recovered; however, cirrhotic patients can maintain their liver function at a normal level for many years, although the remnant hepatocytes are continuously lost. In addition, in animal studies, rodents could survive on the remnant liver after a 70% partial hepatectomy even when liver regeneration was severely deprived. This evidence suggests that the remnant liver function can be compensated in a regeneration-independent manner, but the underlying mechanism remains unknown. Histone acetylation, which is a key modulator of chromatin structure and gene transcription, is regulated dynamically and reversibly by histone acetyltransferases (HATs) and HDACs. The dysregulation of HDACs is considered a crucial cause of various tumors in much research, and HDAC inhibitors have been used preclinically and clinically as antitumor drugs. But few studies have focused on the role of histone acetylation in non-tumorous lesions. The current research hotspot is to investigate whether the proportions of acetyl-histone-positive hepatocytes could reflect the liver function and be used as markers of deteriorating liver function.
“Functional heterogeneity” of hepatocytes was a novel concept that the authors proposed based on their previous study, and they hypothesized that this concept might provide a novel explanation for the powerful compensatory capacity of the liver. Normally, active hepatocytes, which are characterized by acetyl-histone markers, are competent for routine physiological requirements, while inactive hepatocytes act as a functional reservoir for future activation to restore the liver function independent of regeneration. In the present study, the authors examined the expression of acetyl-histone markers in progressive cirrhosis in a large clinical sample. The findings confirmed their hypothesis that acetyl-histone-negative hepatocytes act as a reservoir and that these cells will be activated for functional compensation if liver regeneration is unable to restore its architecture. In addition, the results also suggest that examining the hepatocytic acetyl-histone status is a potential strategy for revealing liver function or for evaluating reserve hepatocytes.
The study results suggest that examining the proportions of acetyl-histone-positive hepatocytes might offer a novel strategy for evaluating the liver function and patient prognosis.
Histone acetylation is an essential part of gene regulation, as is histone deacetylation. They are regulated dynamically and reversibly by HATs and by histone deacetylases (HDACs). Histone acetylation is the process by which HATs add an acetyl functional group to the lysine residues within the N-terminal tail protruding from the histone core of the nucleosome, thus neutralizing the positive charge, weakening the interaction between histone and DNA, and increasing the accessibility of transcription factors to their target genes to prime transcription and elongation.
This study investigates whether the proportion of acetyl-histone positive hepatocytes can be used as a marker of the deterioration of liver function in the context of cirrhosis. The conclusion of the study confirms that the proportions of acetyl-histone positive hepatocytes are closely associated with liver function and survival prognosis of cirrhotic patients. This is a very interesting study with a clear aim. The study methods and results successfully answered the question raised by the authors. In further studies, the authors may consider investigating the correlation between different causes of chronic liver disease and the extent of acetyl-histone-positive hepatocytes.
P- Reviewer: Omran DA, Romani A, Serrano MT S- Editor: Yu J L- Editor: Logan S E- Editor: Liu XM
| 1. | Iwaisako K, Brenner DA, Kisseleva T. What’s new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J Gastroenterol Hepatol. 2012;27 Suppl 2:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Kew MC. Hepatocellular carcinoma in developing countries: Prevention, diagnosis and treatment. World J Hepatol. 2012;4:99-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Boursier J, Cesbron E, Tropet AL, Pilette C. Comparison and improvement of MELD and Child-Pugh score accuracies for the prediction of 6-month mortality in cirrhotic patients. J Clin Gastroenterol. 2009;43:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Yan GZ, Duan YY, Ruan LT, Cao TS, Yuan LJ, Yang YL. Noninvasive quantitative testing of liver function using ultrasonography in patients with cirrhosis. Hepatogastroenterology. 2006;53:15-20. [PubMed] |
| 5. | Planas R, Ballesté B, Alvarez MA, Rivera M, Montoliu S, Galeras JA, Santos J, Coll S, Morillas RM, Solà R. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol. 2004;40:823-830. [PubMed] |
| 6. | Lo WS, Henry KW, Schwartz MF, Berger SL. Histone modification patterns during gene activation. Methods Enzymol. 2004;377:130-153. [PubMed] |
| 7. | Choi JK, Howe LJ. Histone acetylation: truth of consequences? Biochem Cell Biol. 2009;87:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, Zhao K. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019-1031. [PubMed] |
| 9. | Glozak MA, Seto E. Acetylation/deacetylation modulates the stability of DNA replication licensing factor Cdt1. J Biol Chem. 2009;284:11446-11453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Johnsson AE, Wright AP. The role of specific HAT-HDAC interactions in transcriptional elongation. Cell Cycle. 2010;9:467-471. [PubMed] |
| 11. | Khan SN, Khan AU. Role of histone acetylation in cell physiology and diseases: An update. Clin Chim Acta. 2010;411:1401-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Liang G, Lin JC, Wei V, Yoo C, Cheng JC, Nguyen CT, Weisenberger DJ, Egger G, Takai D, Gonzales FA. Distinct localization of histone H3 acetylation and H3-K4 methylation to the transcription start sites in the human genome. Proc Natl Acad Sci USA. 2004;101:7357-7362. [PubMed] |
| 13. | Racey LA, Byvoet P. Histone acetyltransferase in chromatin. Evidence for in vitro enzymatic transfer of acetate from acetyl-coenzyme A to histones. Exp Cell Res. 1971;64:366-370. [PubMed] |
| 14. | Shi B, Xu W. The development and potential clinical utility of biomarkers for HDAC inhibitors. Drug Discov Ther. 2013;7:129-136. [PubMed] |
| 15. | Huffman K, Martinez ED. Pre-clinical studies of epigenetic therapies targeting histone modifiers in lung cancer. Front Oncol. 2013;3:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Tang J, Yan H, Zhuang S. Histone deacetylases as targets for treatment of multiple diseases. Clin Sci (Lond). 2013;124:651-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 17. | Barneda-Zahonero B, Parra M. Histone deacetylases and cancer. Mol Oncol. 2012;6:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 354] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 18. | Atadja PW. HDAC inhibitors and cancer therapy. Prog Drug Res. 2011;67:175-195. [PubMed] |
| 19. | Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;280:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 322] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 20. | Shi Y, Sun H, Bao J, Zhou P, Zhang J, Li L, Bu H. Activation of inactive hepatocytes through histone acetylation: a mechanism for functional compensation after massive loss of hepatocytes. Am J Pathol. 2011;179:1138-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 22. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 23. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012;57:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [PubMed] |
| 25. | Madrahimov N, Dirsch O, Broelsch C, Dahmen U. Marginal hepatectomy in the rat: from anatomy to surgery. Ann Surg. 2006;244:89-98. [PubMed] |
| 26. | Nagy P, Teramoto T, Factor VM, Sanchez A, Schnur J, Paku S, Thorgeirsson SS. Reconstitution of liver mass via cellular hypertrophy in the rat. Hepatology. 2001;33:339-345. [PubMed] |
| 27. | Laconi S, Doratiotto S, Montisci S, Pani P, Laconi E. Repopulation by endogenous hepatocytes does not reconstitute liver mass in rats treated with retrorsine. Cell Transplant. 2008;17:1415-1421. [PubMed] |
| 28. | Pitzalis S, Doratiotto S, Greco M, Montisci S, Pasciu D, Porcu G, Pani P, Laconi S, Laconi E. Cyclin D1 is up-regulated in hepatocytes in vivo following cell-cycle block induced by retrorsine. J Hepatol. 2005;43:485-490. [PubMed] |
| 29. | Habib M, Mohamed MK, Abdel-Aziz F, Magder LS, Abdel-Hamid M, Gamil F, Madkour S, Mikhail NN, Anwar W, Strickland GT. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology. 2001;33:248-253. [PubMed] |
| 30. | Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, Richardson P, El-Serag HB. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182-1188.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 31. | Alsultan MA, Alrshed RS, Aljumah AA, Baharoon SA, Arabi YM, Aldawood AS. In-hospital mortality among a cohort of cirrhotic patients admitted to a tertiary hospital. Saudi J Gastroenterol. 2011;17:387-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |