Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.644
Peer-review started: April 21, 2014
First decision: May 29, 2014
Revised: June 30, 2014
Accepted: July 30, 2014
Article in press: July 30, 2014
Published online: January 14, 2015
Processing time: 271 Days and 23.4 Hours
AIM: To measure biochemical parameters in stomach biopsies and test their suitability as diagnostic biomarkers for gastritis and precancerous lesions.
METHODS: Biopsies were obtained from the stomachs of two groups of patients (n = 40) undergoing fiber-optic endoscopy due to upper gastrointestinal symptoms. In the first group (n = 17), only the corpus region was examined. Biopsies were processed for microscopic examination and measurement of mitochondrial O2 consumption (cellular respiration), cellular adenosine triphosphate (ATP), glutathione (GSH), and caspase activity. In the second group of patients (n = 23), both corpus and antral regions were studied. Some biopsies were processed for microscopic examination, while the others were used for measurements of cellular respiration and GSH level.
RESULTS: Microscopic examinations of gastric corpus biopsies from 17 patients revealed normal mucosae in 8 patients, superficial gastritis in 7 patients, and chronic atrophic gastritis in 1 patient. In patients with normal histology, the rate (mean ± SD) of cellular respiration was 0.17 ± 0.02 μmol/L O2 min-1 mg-1, ATP content was 487 ± 493 pmol/mg, and GSH was 469 ± 98 pmol/mg. Caspase activity was detected in 3 out of 8 specimens. The values of ATP and caspase activity were highly variable. The presence of superficial gastritis had insignificant effects on the measured biomarkers. In the patient with atrophic gastritis, cellular respiration was high and ATP was relatively low, suggesting uncoupling oxidative phosphorylation. In the second cohort of patients, the examined biopsies showed either normal or superficial gastritis. The rate of cellular respiration (O2.μmol/L min-1 mg-1) was slightly higher in the corpus than the antrum (0.18 ± 0.05 vs 0.15 ± 0.04, P = 0.019). The value of GSH was about the same in both tissues (310 ± 135 vs 322 ± 155, P = 0.692).
CONCLUSION: The corpus mucosa was metabolically more active than the antrum tissue. The data in this study will help in understanding the pathophysiology of gastric mucosa.
Core tip: Using small gastric mucosal biopsies obtained from patients with upper gastrointestinal symptoms, several cellular bioenergetic and dynamic parameters were measured and correlated with the histopathological features of the gastric mucosa.
- Citation: Alfazari AS, Al-Dabbagh B, Al-Dhaheri W, Taha MS, Chebli AA, Fontagnier EM, Koutoubi Z, Kochiyi J, Karam SM, Souid AK. Profiling cellular bioenergetics, glutathione levels, and caspase activities in stomach biopsies of patients with upper gastrointestinal symptoms. World J Gastroenterol 2015; 21(2): 644-652
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/644.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.644
The gastric mucosa of the normal human stomach includes numerous tubular epithelial glands. In the corpus region, each gland is lined by a heterogeneous population of cells secreting mucus, acid, pepsinogen, and various hormones and peptides[1]. In the antrum, the glands produce mainly mucus, hormones, and peptides. Analysis of gastric mucosal tissues from patients undergoing endoscopic examination (for recurrent upper gastrointestinal symptoms) and comparing them with gastric cancer tissues obtained from three different regions (safe margin, tumor edge, and tumor center) revealed that these tissues represent the multistep process of gastric carcinogenesis[2]. The sequential changes in the morphology of the gastric glands coincide with increased proliferating stem/progenitor cells during progression from normal to gastritis, into metaplasia, and finally into adenocarcinoma. Indeed when a stem cell-specific marker (Oct4) was used, the labeling pattern and the measurement of Oct4 protein content supported the central role of stem cells in driving precancerous and cancerous changes[3]. Since proliferation of gastric stem/progenitor cells and alteration of cellular dynamics is an important event in carcinogenesis, measurement of the cellular bioenergetics of gastric mucosal biopsies would be an emerging need.
Cellular bioenergetics reflects the biochemical processes involved in the energy metabolism (energy conversion or transformation). Cellular respiration implies the delivery of O2 and metabolic fuels to the mitochondria, the oxidation of reduced metabolic fuels with passage of electrons to O2, and the synthesis of adenosine triphosphate (ATP)[4]. Impaired bioenergetics therefore entails disturbances in these processes.
Cellular mitochondrial O2 consumption is a highly sensitive biomarker for detecting tissue derangements[5]. Impairments in cellular membranes, mitochondria, or metabolic enzymes are expected to disrupt energy kinetics within the cell. Cells with intact bioenergetics are more capable of repairing damage. Furthermore, apoptosis with activation of caspases is more likely to result in cell death if associated with impaired cellular bioenergetics[6]. Therefore, energy metabolism has a significant impact on the fate of the cell. This notion stems from the dependency of human biological systems on aerobic metabolism. Cancer cells, on the other hand, may survive on anaerobic metabolism, a phenomenon commonly referred to as aerobic glycolysis or the Warburg effect[7].
Several human and animal studies have demonstrated that bioenergetics of the gastric epithelium are affected by various diseases (e.g., ischemia) and toxins (e.g., acetylsalicylic acid and non-steroidal anti-inflammatory drugs)[8-11]. Similarly, gastric tissue deficient in superoxide dismutase (a parietal cell enzyme that prevents the accumulation of superoxides) has mitochondrial dysfunction and perturbed energy metabolism, which manifests via reduced ATP and increased apoptosis[9].
Cellular bioenergetics has been used as a biomarker for metabolic diseases[12]. In the present study, cells and tissues obtained from patients were used to diagnose impaired cellular bioenergetics. The main aim of the present study was to show the feasibility of performing the same measurements [cellular respiration, ATP, glutathione (GSH), and caspase activity] on small gastric mucosal biopsies. The results here demonstrate the feasibility of measuring cellular mitochondrial O2 consumption, ATP, GSH, and apoptosis in small mucosal biopsies from the stomach of patients.
Pd(II) complex of meso-tetra-(4-sulfonatophenyl)-tetrabenzoporphyrin (Pd phosphor) was purchased from Porphyrin Products (Logan, UT). Monobromobimane (mBBr, MW 271.111) was purchased from Molecular Probes (Eugene, Oregon). A lyophilized powder of caspase inhibitor I [N-benzyloxycarbonyl-Val-Ala-Asp(O-methyl)-fluoromethylketone; zVAD-fmk; MW 467.5; pan-caspase inhibitor] was purchased from Calbiochem (La Jolla, CA). Ac-DEVD-AMC (N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin; MW 675.64; caspase-3 substrate) was purchased from Axxora LLC (San Diego, CA). Recombinant human active caspase-3 was purchased from BD Pharmingen™ (Becton Dickinson & Company, Franklin Lakes, NJ, United States). Glucose, 5,5’-dithio-bis(2-nitrobenzoic acid) [DTNB, MW 396.35, molar extinction coefficient at 412 nm 13.6 × 103], GSH (MW 307.43; pKa 8.7), HPLC-grade methanol, dichloromethane, trifluoroacetic acid (TFA), methanesulfonic acid (MSA), and remaining reagents were purchased from Sigma-Aldrich (St. Louis, MO).
GSH was prepared in dH2O and its concentration was measured by Ellman’s reagent[9]. The GS-bimane derivative (GSH standard), sodium methane sulfonate (NaMS), mBBr, and DTNB solutions were prepared and stored as described[13-15]. DTNB working solution was 0.2 mmol/L DTNB in 100 mmol/L Tri-Cl (pH 8.0). GSH standard (2 μmol/L) was used to generate a calibration curve with each analytical run, which was linear from 10 to 200 picomoles (R≥ 0.982).
zVAD-fmk (2.14 mmol/L) and Ac-DEVD-AMC (7.4 mmol/L) were prepared in dimethyl sulfoxide and stored at -20 °C. Pd phosphor (2.5 mg/mL = 2 mmol/L), sodium cyanide (CN, 1.0 mol/L), and glucose oxidase (10 mg/mL) were prepared in dH2O and stored at -20 °C.
This work is compliant with the Declaration of Helsinki (2000) of the World Medical Association. The study was approved by the Institutional Review Board for the protection of human subjects, Al Ain Medical District Human Research Ethics Committee (Protocol No. 12/49 CRD 199). All patients provided informed written consent.
The first cohort involved 17 patients who were admitted to the Endoscopy Unit of Tawam Hospital (Al Ain City, Abu Dhabi) for diagnostic fiber-optic endoscopy due to recurrent upper gastrointestinal symptoms (dyspepsia, abdominal pain, and heartburn) (Table 1). After collecting samples for standard patient care, five to eight additional mucosal biopsies were collected for the purpose of this study. The samples (7.7-30 mg) varied in dimensions from 1 mm × 1 mm to 2 mm × 3 mm. Tissue samples were processed for histological examination and measurements of cellular respiration, caspase activity, ATP, and GSH. Values of the measured biomarkers were expressed per specimen as wet weight (in mg). For consistency, studied samples were obtained from the gastric corpus (body) midway along the greater curvature. In a separate cohort of 23 patients, samples were obtained from the corpus and the antrum; these additional samples were processed for histology, cellular respiration, and GSH only (due to limited sample availability) (Table 2).
| Patients | Age (yr) | Gender | Medications | Clinical findings | H. pylori | Histology | kc | ATP | GSH | AMC |
| 1 | 48 | F | Mebeverine | Hysterectomy | - | Normal | 0.18 | 1547 ± 5.6 | 396 | 0 |
| 2 | 38 | F | PPI | Peptic ulcer | ND | Normal | 0.19 | 13 ± 0.4 | 404 | 0 |
| Thyroxin | Thyroid cancer | |||||||||
| 3 | 52 | F | PPI | IBS | + | Normal | 0.13 | 267 ± 0.3 | 517 | 0 |
| NSAID | Thyroid neoplasm | |||||||||
| Progesterone | ||||||||||
| 4 | 20 | F | - | Hiatal hernia | - | Normal | 0.18 | 492 ± 5.0 | 570 | 0 |
| 5 | 50 | M | PPI, Losartan, Prednisolone | Acromegaly | - | Normal | 0.16 | 336 ± 3.8 | 360 | 0 |
| Hypertension | ||||||||||
| 6 | 34 | M | Morbid obesity | Morbid obesity | + | Normal | 0.16 | 495 ± 7.0 | 638 | 9 |
| 7 | 23 | M | PPI | Hypertension | - | Normal | 0.18 | 14 ± 1.2 | 461 | 36 |
| Aspirin | Dyslipidemia | |||||||||
| 8 | 52 | F | PPI | - | - | Normal | 0.19 | 731 ± 5.2 | 403 | 63 |
| Calcium, vitamin D Atorvastatin | ||||||||||
| mean ± SD | 40 ± 13 | 0.17 ± 0.02 | 487 ± 493 | 469 ± 98 | ||||||
| 9 | 38 | F | antacid | Mesenteric cyst | - | Superficial gastritis | 0.15 | 1525 ± 8.7 | 476 | 95 |
| 10 | 22 | M | PPI | - | + | Superficial gastritis | 0.20 | 90 ± 4.1 | 347 | 14 |
| 11 | 46 | F | PPI | - | - | Superficial gastritis | 0.18 | 949 ± 1.3 | 373 | 14 |
| 12 | 28 | F | PPI | IBS | + | Superficial gastritis | 0.18 | 11 ± 0.2 | 830 | 13 |
| Hyperthyroidism | ||||||||||
| Depression | ||||||||||
| 13 | 65 | M | PPI | - | - | Superficial gastritis | 0.21 | 14 ± 0.9 | 496 | 0 |
| 14 | 62 | F | PPI | - | + | Superficial gastritis | 0.14 | 56 ± 2.0 | 366 | 0 |
| 15 | 55 | F | PPI | Breast cancer | - | Superficial gastritis | 0.17 | ND | ND | ND |
| Tamoxifen | ||||||||||
| mean ± SD | 47 ± 18 | 0.18 ± 0.04 | 370 ± 563 | 481 ± 182 | ||||||
| 16 | 72 | F | PPI | Diabetes mellitus | + | Atrophic gastritis | 0.27 | 275 ± 4.2 | 606 | 2 |
| Hypertension | ||||||||||
| Dyslipidemia | ||||||||||
| Breast cancer | ||||||||||
| 17 | 36 | M | - | Morbid obesity | + | Inadequate | 0.14 | 37 ± 0.1 | 1138 | 105 |
| Patients | Age (yr) | GI presentation | Gender | Gastric corpus | Gastric antrum | ||
| kc | GSH | kc | GSH | ||||
| 18 | 27 | Liver lesion | F | 0.11 | 357 | 0.12 | 378 |
| 19 | 71 | Hypertension, diabetes, dyslipidemia, dyspepsia | M | 0.13 | 627 | 0.16 | 379 |
| 20 | 22 | Dyspepsia | M | 0.13 | 366 | 0.25 | 331 |
| 21 | 18 | Dyspepsia | F | 0.14 | 319 | 0.19 | 260 |
| 22 | 30 | Dyspepsia | M | 0.16 | 733 | 0.21 | 853 |
| 23 | 34 | Thyroidectomy, dyspepsia | F | 0.14 | 449 | 0.18 | 408 |
| 24 | 24 | Familial Mediterranean fever, dyspepsia | M | 0.17 | 256 | 0.14 | 243 |
| 25 | 66 | Diabetes, gastritis | M | 0.15 | 243 | 0.12 | 317 |
| 26 | 69 | Prostate cancer, aortic aneurysm, dyspepsia | M | 0.22 | 269 | 0.13 | 470 |
| 27 | 22 | Morbid obesity, dyspepsia | M | 0.15 | 307 | 0.13 | 256 |
| 28 | 30 | Dyspepsia | M | 0.15 | 351 | 0.17 | 347 |
| 29 | 46 | Obesity, dyspepsia | F | 0.22 | 256 | 0.14 | 356 |
| 30 | 21 | Dyspepsia | F | 0.15 | 260 | 0.12 | 342 |
| 31 | 49 | Hypertension, diabetes, ovarian, and cervical cancers, dyspepsia | F | 0.18 | 202 | 0.10 | 104 |
| 32 | 44 | Hypertension, diabetes, dyslipidemia, dyspepsia | F | 0.18 | 180 | 0.17 | 166 |
| 33 | 34 | Dyspepsia | F | 0.20 | 218 | 0.11 | 256 |
| 34 | 18 | Thalassemia major, s/p BMT, dyspepsia | F | 0.15 | 219 | 0.12 | 193 |
| 35 | 81 | GERD, esophagitis, hiatal hernia | M | 0.19 | 279 | 0.11 | 219 |
| 36 | 62 | Dyspepsia | F | 0.28 | 258 | 0.19 | 554 |
| 37 | 40 | Morbid obesity, dyslipidemia, chronic renal failure, dyspepsia | F | 0.13 | 301 | 0.11 | 326 |
| 38 | 26 | Data not available | F | 0.30 | 305 | 0.18 | 177 |
| 39 | 39 | Data not available | M | 0.23 | 214 | 0.13 | 260 |
| 40 | 32 | Data not available | F | 0.21 | 166 | 0.12 | 211 |
| mean ± SD | 40.4 ± 19.8 | 0.18 ± 0.05 | 310 ± 135 | 0.15 ± 0.04 | 322 ± 155 | ||
For histological examination, tissue samples were processed as previously described[2]. Helicobacter pylori (H. pylori) infection was detected using Warthin-Starry stain[16] or urease-based test (campylobacter-like organism test, Ptonto DryTM, Medical Instruments Corporation, Brignais, France).
Within 20 min of sample collection, the specimens were transferred to 1.0 mL RPMI containing 0.5% fat-free bovine albumin and 3 μmol/L Pd phosphor and processed for O2 measurements at 37 °C as previously described[17-19].
For measuring cellular ATP, a specimen from each patient was immediately homogenized in 0.5 mL of ice-cold 2% trichloroacetic acid for 2 min. The supernatants were collected by centrifugation (1000 g at 4 °C for 5 min) and stored at -20 °C until analysis as previously described[17-19].
For GSH labeling with mBBr, the reaction solution containing the gastric specimen (7.7-30 mg) was incubated at 25 °C for 15 min. The reaction was stopped with 100 μL of 70% perchloric acid and diluted with 400 μL of 10 mmol/L Tris-MSA. The tissue was vortexed, homogenized, and centrifuged. The supernatant was stored at -20 °C until HPLC analysis[13-15].
For measuring caspase activity, two specimens from each patient were used. They were immediately placed in 1.0 mL RPMI containing 37 μmol/L Ac-DEVD-AMC with and without 32 μmol/L zVAD-fmk as previously described[17-19].
The reversed-phase HPLC system (Waters, Milford, MA, United States) was used. Ultrasphere IP column, 4.6 mm × 250 mm (Beckman, Fullerton, CA, United States) was operated at 25 °C at 1.0 mL/min. For GSH determination, solvent A was 0.1% (v/v) trifluoroacetic acid/water and solvent B was HPLC-grade methanol. The flow rate was 1.0 mL/min. The employed gradient was: 0 min, 10% B; 5 min 10% B; 13 min, 100% B; 15 min, 10% B; 20 min, re-inject. The excitation and emission wavelengths were 390 nm and 480 nm, respectively. The injection volume was 50 μL.
For AMC detection, the excitation wavelength was 380 nm and the emission wavelength 460 nm. Solvents A and B was HPLC-grade methanol: dH2O 1:1 (isocratic). The run time was 15 min and the injection volume was 50 μL.
Data were analyzed using SPSS statistical package (version 19). The nonparametric Mann-Whitney test (2 independent variables) was used to compare samples.
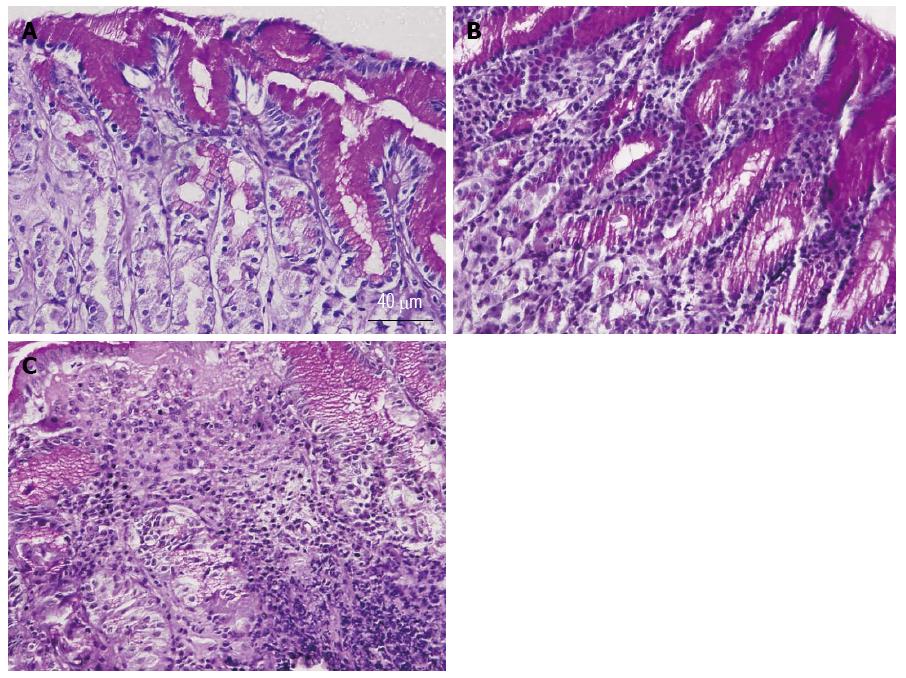
Gastric corpus specimens were collected from the first 17 patients; their results are summarized in Table 1. The patients’ age averaged 44 ± 16 years; 11 patients (65%) were females. All patients had recurrent upper gastrointestinal symptoms (dyspepsia, abdominal pain, and heartburn). Twelve (71%) patients were receiving proton pump inhibitors (PPI). Biopsies of 7 patients (41%) tested positive for H. pylori (Table 1). Microscopic examination of 5-micron-thick gastric mucosal sections revealed that 8 patients had normal gastric mucosa (Figure 1A). The biopsies of 7 patients had chronic superficial gastritis with infiltration of the luminal side of the mucosa with some inflammatory cells (Figure 1B). The gastric mucosa of only one patient (Patient 16) revealed evidence of chronic atrophic gastritis with massive infiltration with inflammatory cells (Figure 1C). The biopsy of one patient was inadequate for microscopic examination.
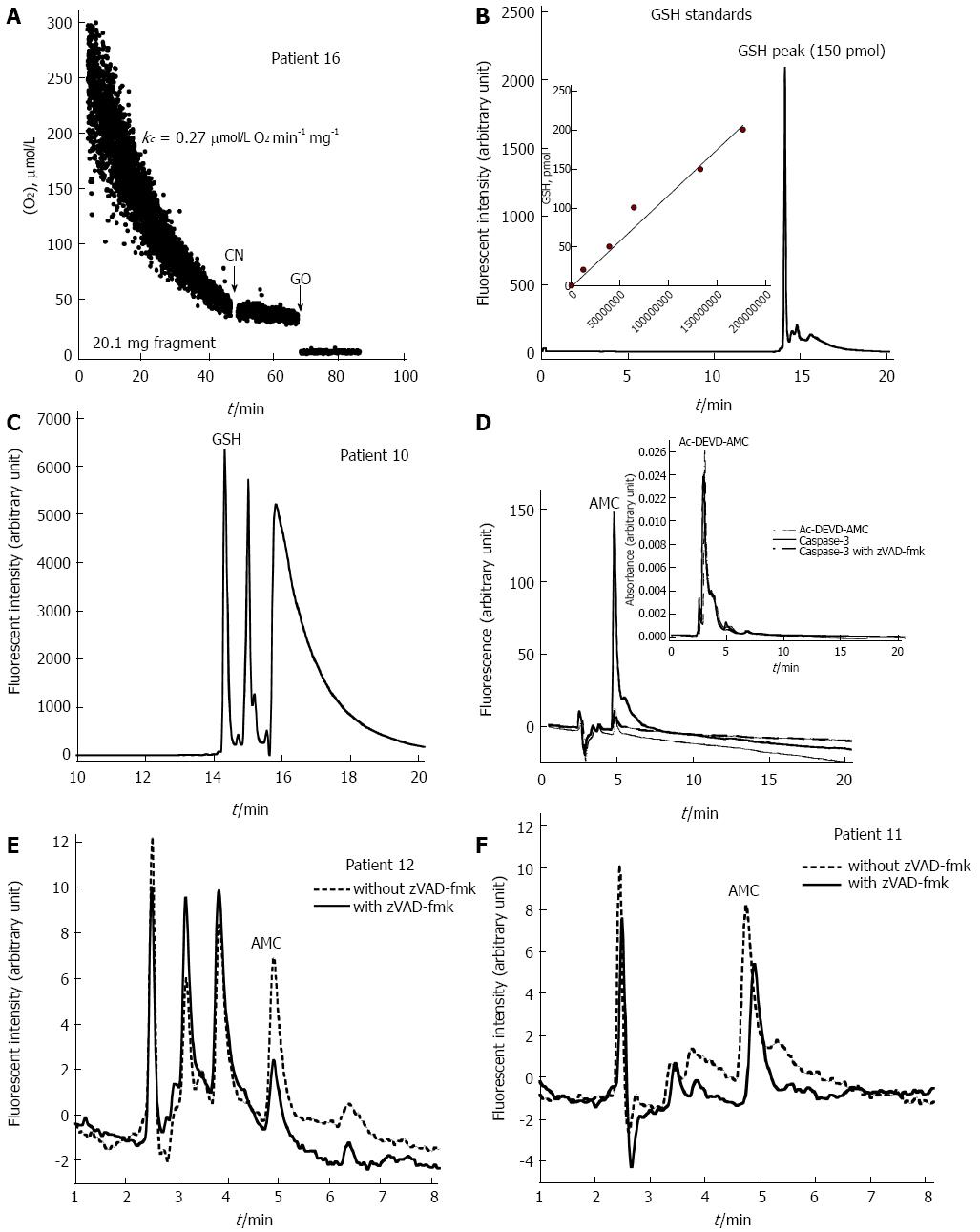
O2 consumption by the stomach biopsy of Patient 16 (a patient with atrophic gastritis) is shown in Figure 2A. The rate of cellular respiration was the highest (0.27 μmol/L O2 mg-1 min-1), but cellular ATP was below the average (275 pmol/mg) (Table 1). This result suggested uncoupling oxidative phosphorylation (a state of high mitochondrial O2 consumption with low cellular ATP) as a mechanism of the enhanced respiration. O2 consumption was completely inhibited by cyanide, confirming that the oxidation occurred in the mitochondrial respiratory chain. The addition of glucose oxidase (which catalyzes the reaction of D-glucose + O2 to D-glucono-δ-lactone + H2O2) depleted the remaining O2 in the solution.
The rates of cellular mitochondrial O2 consumption (kc, μmol/L O2 mg-1 min-1) were 0.17 ± 0.02 for the 8 normal histology patients and 0.18 ± 0.03 for the 7 superficial gastritis patients (P = 0.867). The corresponding values for cellular ATP were 487 ± 493 and 370 ± 563, respectively (P = 0.573). The large variation in cellular ATP was likely due to sample processing. Nevertheless, the data show that superficial gastritis was not associated with bioenergetic changes in the gastric mucosa.
Representative GSH standard HPLC run and GSH standard curve are shown in Figure 2B; of note, GSH labeling with mBBr was blocked by N-ethylmaleimide (data not shown). Representative GSH run of acid-soluble supernatant of the stomach biopsy of Patient 10 (a patient with superficial gastritis) is shown in Figure 2C. Cellular GSH for the 8 patients with normal histology was 469 ± 98, and 481 ± 182 for the 7 patients with superficial gastritis (P = 0.662) (Table 1). Consistently, superficial gastritis was not associated with GSH changes in the gastric mucosa.
Ac-DEVD-AMC cleavage by the recombinant human active caspase-3 is shown in Figure 2D. The reaction, in 1.0 mL RPMI, contained 100 ng caspase-3 with and without 32 μmol/L zVAD-fmk (pan-caspase inhibitor). The mixtures were incubated at 37 °C for 10 min. Ac-DEVD-AMC (37 μmol/L) was then added and the incubation continued at 37 °C for an additional 20 min. Ac DEVD-AMC was detected by absorbance at 380 nm with a retention time of approximately 3 min (Figure 2D). The product AMC was detected by fluorescence (380 nm excitation and 460 nm emission) with a retention time of about 5 min (Figure 2D). The cleavage reaction was inhibited by zVAD-fmk (Figure 2D). Caspase activity was set as the AMC peak area without zVAD-fmk minus the AMC peak area with zVAD-fmk. Representative HPLC runs of caspase-3 activity in the gastric corpus of Patients 12 and 11 are shown in Figure 2E and F, respectively. Caspase activity was detected in 3 of 8 (38%) patients with normal histology and 5 of 7 (71%) patients with abnormal histology (Table 1).
H. pylori had no significant effect on the rate of respiration, level of ATP, cellular GSH, or intracellular caspase activity (P > 0.121). Non-significant effects were also noted with respect to the use of PPI (P > 0.104).
The second cohort involved gastric corpus and antrum specimen collection from 23 additional patients. Due to limited sample availability, these biopsies were processed only for histology, cellular respiration, and GSH measurements (Table 2). Tissue samples for histology, however, were only available for 7 out of 23 (30%) patients; all had either normal or varying degrees of superficial (mild) gastritis. The patients’ age averaged 40.4 ± 19.8 years; 14 patients (61%) were females. The rate of respiration (μmol/L O2 mg-1 min-1) was slightly higher in the corpus than the antrum (0.18 ± 0.05 vs 0.15 ± 0.04, P = 0.019). The value of GSH was about the same in both tissues (310 ± 135 vs 322 ± 155, P = 0.692).
Bioenergetic studies on the gastric epithelium are relatively limited, especially with respect to investigating human stomach diseases and the use of compound biomarkers[8,10,11,20-30]. The main purpose of this study was to examine the suitability of using biochemical parameters (cellular respiration, ATP, GSH, and caspase activity) as biomarkers for the gastric mucosa. The success of these measurements relies on the appropriate processing of the samples at the site of tissue collection. For O2 measurements, the tissue should be immediately placed in ice-cold RPMI medium saturated with 95% O2 and 5% CO2. The sample should then be transferred to the laboratory on wet-ice and processed for the O2 measurement within a few minutes of collection. For ATP, the tissue should be immediately quenched (at the procedure site) with acidic solution (freshly-made) to prevent ATP hydrolysis by cellular ATPases. For GSH, the tissue should be immediately immersed (at the procedure site) in thiol derivatization reaction that contains a large excess of mBBr (5 mmol/L). The GS-bimane derivatives are stable and can be stored until HPLC analysis. For caspase activity, the sample should be immediately placed in the Ac-DEVD-AMC cleavage reaction at the procedure site.
Having adhered to these experimental procedures, the values for the rate of cellular respiration (CV ≤ 17%) and GSH content (CV ≤ 48%) were reasonably consistent within the studied biopsies (Tables 1 and 2). These results were noted despite the wide-spectrum of clinical and histological variations among the patients and samples. Thus, cellular O2 consumption and GSH are relatively preserved in the gastric mucosa. Cellular ATP (CV = 120%) and caspase activity (CV = 108%) were markedly varied however, likely due sample processing (Table 1).
We do identify that there are limitations to this study, as the sample size is relatively small and includes patients with minor gastric pathology. The clinical significance of these measurable biomarkers needs to be explored in future studies in patients with various pathologies, such as H. pylori infection, and the use of PPI.
Patient 3 had the lowest rate of respiration (0.13 μmol/L O2 mg-1 min-1); she had benign thyroid neoplasm and was taking multiple medications, including thyroxin, PPI, diclofenac, and medroxyprogesterone. Nevertheless, the cellular ATP, GSH, and caspase activity were not significantly different (Table 1).
Patient 16 had atrophic gastritis. Her rate of respiration was the highest (0.27 μmol/L O2 mg-1 min-1). She also had other complicated clinical problems (e.g., diabetes mellitus, hypertension, dyslipidemia, and breast cancer) and was on PPI. While the rate of cellular respiration was the highest, the cellular ATP level was below average (275 pmol/mg) (Table 1), suggesting uncoupling oxidative phosphorylation.
Bioenergetics of the gastric epithelium was investigated in specimens collected from animal and human tissues[8,10,11,20]. In the bullfrog gastric mucosa, cellular mitochondrial O2 consumption was increased and cellular ATP was decreased in the presence of acetylsalicylic acid[8]. Deficits in gastric cellular bioenergetics are also documented in shock and ischemia[10]. Non-steroidal anti-inflammatory drugs (NSAID) are shown to uncouple mitochondrial oxidative phosphorylation (lowering cellular ATP) in the gastric tissue[11]. Patient 6 was on aspirin and his cellular ATP was low (14 pmol/mg) (Table 1).
Activation of the mitochondrial apoptotic pathway is essential for H. pylori-induced apoptosis in gastric epithelial cells[21]. The H. pylori vacuolating cytotoxin A (vacA) causes direct mitochondrial disturbances and alterations in the bioenergetics of gastric epithelial cells[24]. Here, H. pylori had no noticeable effects on kc, ATP, GSH, or caspase activity. Nevertheless, the impact of H. pylori on the studied biomarkers requires a much larger sample size and appropriately selected control group.
Oxidative phosphorylation was measured in permeabilized corpus mucosal biopsies[22]. Cellular respiration was about 2-fold lower in patients with atrophic gastritis compared to non-atrophic gastritis. This effect was attributed to a deficiency of complex I of the respiratory chain[22]. Furthermore, limiting cellular bioenergetics was proposed to cause dysfunction of the zymogenic mucosal cells[23]. These studies demonstrate that stomach mucosal diseases can be associated with altered oxidative phosphorylation[23].
Activation of caspases permeabilizes (uncouples) the inner mitochondrial membrane, resulting in the collapse of the proton motive force, loss of electrochemical potential, and uncoupling of oxidative phosphorylation[25]. These processes lead to the rapid depletion of cellular nutrients, metabolic fuels, and ATP. The gastric mucosa is an intensely energy-consuming tissue. This demand is met by the mitochondria-rich acid producing parietal cells, which secrete the gastric acid and initiate the process of digestion. To prevent self-destruction, the columnar epithelium makes gastric mucosal barriers that resist the highly acidic and proteolytic gastric juice[26]. It is believed that mitochondrial dysfunctions impact gastric mucosal integrity, and thus measuring cellular mitochondrial O2 consumption in gastric biopsies is justified.
Oxidative stress is induced in the stomach as a result of gastric insults, including chronic infections. GSH is a major detoxifying thiol which protects against oxidative stress. In indomethacin-treated rats, cellular GSH and mitochondrial enzymes are reduced. Esomeprazole, a proton pump inhibitor, was able to reserve GSH levels and mitochondrial enzyme activities[27]. Due to its γ-glutamyl transpeptidase, H. pylori can also reduce gastric epithelial GSH, exposing the bacterium, as well as the gastric epithelium, to oxidative stress[28].
ATP is produced in the mitochondria via oxidative phosphorylation by the proton-motive force that is used by ATP synthase to catalyze ADP phosphorylation[29]. The mitochondria are also the target of self-generated reactive oxygen species. Premalignant atrophic gastritis and gastric carcinoma are both associated with decreased respiratory capacity and mitochondrial complex I deficiency[22,30]. Therefore, investigating metabolic biomarkers in the gastric mucosa is much needed and future studies should determine whether they can be used to explore the mechanisms of diseases involving the gastric mucosa.
Since proliferation of gastric stem/progenitor cells and alteration of cellular dynamics are important events in carcinogenesis, the measurement of cellular bioenergetics of gastric mucosal biopsies would be an emerging need.
Cellular bioenergetics has been used as a biomarker for some diseases. Whether it can be useful as a diagnostic tool for some gastric diseases is not known yet. In this study, the authors have demonstrated that various cellular bioenergetic and dynamic parameters could be measured and found useful for small gastric mucosal biopsies.
Recent reports have highlighted the importance of cellular dynamics and bioenergetics as diagnostic tools for some gastrointestinal and metabolic diseases. In this study, the authors report that cellular bioenergetics and other biochemical parameters could be useful tools for investigating stomach diseases.
By demonstrating the possible use of small mucosal biopsies for bioenergetic measurements, this study may represent a future strategy for the investigation and diagnosis of patients with upper gastrointestinal problems.
Following microscopic examination, gastric mucosal biopsies were categorized as superficial (mild) or sever according to the Sydney classification criteria.
This manuscript “Profiling cellular bioenergetics, glutathione levels, and caspase activities in stomach biopsies of patients with upper gastrointestinal symptoms” is very interesting study.
P- Reviewer: Hoensch HP, Karatapanis S S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Liu XM
| 1. | Karam SM, Straiton T, Hassan WM, Leblond CP. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells. 2003;21:322-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Al-Awadhi H, John R, Al-Marzooqi F, Vincze A, Branicki F, Karam SM. Sequential alterations in gastric biopsies and tumor tissues support the multistep process of carcinogenesis. Histol Histopathol. 2011;26:1153-1164. [PubMed] |
| 3. | Al-Marzoqee FY, Khoder G, Al-Awadhi H, John R, Beg A, Vincze A, Branicki F, Karam SM. Upregulation and inhibition of the nuclear translocation of Oct4 during multistep gastric carcinogenesis. Int J Oncol. 2012;41:1733-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Madeira VM. Overview of mitochondrial bioenergetics. Methods Mol Biol. 2012;810:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Tao Z, Jones E, Goodisman J, Souid AK. Quantitative measure of cytotoxicity of anticancer drugs and other agents. Anal Biochem. 2008;381:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2418] [Cited by in RCA: 2546] [Article Influence: 121.2] [Reference Citation Analysis (0)] |
| 7. | Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26:877-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 786] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 8. | Spenney JG, Bhown M. Effect of acetylsalicylic acid on gastric mucosa. II. Mucosal ATP and phosphocreatine content, and salicylate effects on mitochondrial metabolism. Gastroenterology. 1977;73:995-999. [PubMed] |
| 9. | Jocelyn PC. Spectrophotometric assay of thiols. Methods Enzymol. 1987;143:44-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 222] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Menguy R, Masters YF. Gastric mucosal energy metabolism and “stress ulceration”. Ann Surg. 1974;180:538-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Mahmud T, Rafi SS, Scott DL, Wrigglesworth JM, Bjarnason I. Nonsteroidal antiinflammatory drugs and uncoupling of mitochondrial oxidative phosphorylation. Arthritis Rheum. 1996;39:1998-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Al-Jasmi F, Penefsky HS, Souid AK. The phosphorescence oxygen analyzer as a screening tool for disorders with impaired lymphocyte bioenergetics. Mol Genet Metab. 2011;104:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Souid AK, Newton GL, Dubowy RL, Fahey RC, Bernstein ML. Determination of the cytoprotective agent WR-2721 (Amifostine, Ethyol) and its metabolites in human blood using monobromobimane fluorescent labeling and high-performance liquid chromatography. Cancer Chemother Pharmacol. 1998;42:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Souid AK, Fahey RC, Dubowy RL, Newton GL, Bernstein ML. WR-2721 (amifostine) infusion in patients with Ewing’s sarcoma receiving ifosfamide and cyclophosphamide with mesna: drug and thiol levels in plasma and blood cells, a Pediatric Oncology Group study. Cancer Chemother Pharmacol. 1999;44:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Souid AK, Fahey RC, Aktas MK, Sayin OA, Karjoo S, Newton GL, Sadowitz PD, Dubowy RL, Bernstein ML. Blood thiols following amifostine and mesna infusions, a pediatric oncology group study. Drug Metab Dispos. 2001;29:1460-1466. [PubMed] |
| 16. | Warthin AS, Chronister AC. A more rapid and improved method of demonstrating spirochetes in tissues (Warthin and Starry’s cover-glass method). Am J Syphil. 1920;4:97-103. |
| 17. | Alfazari AS, Al-Dabbagh B, Almarzooqi S, Albawardi A, Souid AK. A preparation of murine liver fragments for in vitro studies: liver preparation for toxicological studies. BMC Res Notes. 2013;6:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Alfazari AS, Al-Dabbagh B, Almarzooqi S, Albawardi A, Souid AK. Bioenergetic study of murine hepatic tissue treated in vitro with atorvastatin. BMC Pharmacol Toxicol. 2013;14:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Tao Z, Goodisman J, Penefsky HS, Souid AK. Caspase activation by anticancer drugs: the caspase storm. Mol Pharm. 2007;4:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Jones MK, Zhu E, Sarino EV, Padilla OR, Takahashi T, Shimizu T, Shirasawa T. Loss of parietal cell superoxide dismutase leads to gastric oxidative stress and increased injury susceptibility in mice. Am J Physiol Gastrointest Liver Physiol. 2011;301:G537-G546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Potthoff A, Ledig S, Martin J, Jandl O, Cornberg M, Obst B, Beil W, Manns MP, Wagner S. Significance of the caspase family in Helicobacter pylori induced gastric epithelial apoptosis. Helicobacter. 2002;7:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Gruno M, Peet N, Tein A, Salupere R, Sirotkina M, Valle J, Peetsalu A, Seppet EK. Atrophic gastritis: deficient complex I of the respiratory chain in the mitochondria of corpus mucosal cells. J Gastroenterol. 2008;43:780-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Gruno M, Peet N, Seppet E, Kadaja L, Paju K, Eimre M, Orlova E, Peetsalu M, Tein A, Soplepmann J. Oxidative phosphorylation and its coupling to mitochondrial creatine and adenylate kinases in human gastric mucosa. Am J Physiol Regul Integr Comp Physiol. 2006;291:R936-R946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Kimura M, Goto S, Wada A, Yahiro K, Niidome T, Hatakeyama T, Aoyagi H, Hirayama T, Kondo T. Vacuolating cytotoxin purified from Helicobacter pylori causes mitochondrial damage in human gastric cells. Microb Pathog. 1999;26:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Ricci JE, Muñoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, Yadava N, Scheffler IE, Ellisman MH, Green DR. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 470] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 26. | Demitrack ES, Aihara E, Kenny S, Varro A, Montrose MH. Inhibitors of acid secretion can benefit gastric wound repair independent of luminal pH effects on the site of damage. Gut. 2012;61:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Pastoris O, Verri M, Boschi F, Kastsiuchenka O, Balestra B, Pace F, Tonini M, Natale G. Effects of esomeprazole on glutathione levels and mitochondrial oxidative phosphorylation in the gastric mucosa of rats treated with indomethacin. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr. 2012;50:35-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Fernández-Vizarra E, Tiranti V, Zeviani M. Assembly of the oxidative phosphorylation system in humans: what we have learned by studying its defects. Biochim Biophys Acta. 2009;1793:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 30. | Puurand M, Peet N, Piirsoo A, Peetsalu M, Soplepmann J, Sirotkina M, Peetsalu A, Hemminki A, Seppet E. Deficiency of the complex I of the mitochondrial respiratory chain but improved adenylate control over succinate-dependent respiration are human gastric cancer-specific phenomena. Mol Cell Biochem. 2012;370:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |