Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.533
Peer-review started: April 27, 2014
First decision: May 13, 2014
Revised: June 8, 2014
Accepted: July 11, 2014
Article in press: July 11, 2014
Published online: January 14, 2015
Processing time: 266 Days and 17 Hours
AIM: To evaluate the need for thrombomodulin (rTM) therapy for disseminated intravascular coagulation (DIC) in patients with acute cholangitis (AC)-induced DIC.
METHODS: Sixty-six patients who were diagnosed with AC-induced DIC and who were treated at our hospital were enrolled in this study. The diagnoses of AC and DIC were made based on the 2013 Tokyo Guidelines and the DIC diagnostic criteria as defined by the Japanese Association for Acute Medicine, respectively. Thirty consecutive patients who were treated with rTM between April 2010 and September 2013 (rTM group) were compared to 36 patients who were treated without rTM (before the introduction of rTM therapy at our hospital) between January 2005 and January 2010 (control group). The two groups were compared in terms of patient characteristics at the time of DIC diagnosis (including age, sex, primary disease, severity of cholangitis, DIC score, biliary drainage, and anti-DIC drugs), the DIC resolution rate, DIC score, the systemic inflammatory response syndrome (SIRS) score, hematological values, and outcomes. Using logistic regression analysis based on multivariate analyses, we also examined factors that contributed to persistent DIC.
RESULTS: There were no differences between the rTM group and the control group in terms of the patients’ backgrounds other than administration. DIC resolution rates on day 9 were higher in the rTM group than in the control group (83.3% vs 52.8%, P < 0.01). The mean DIC scores on day 7 were lower in the rTM group than in the control group (2.1 ± 2.1 vs 3.5 ± 2.3, P = 0.02). The mean SIRS scores on day 3 were significantly lower in the rTM group than in the control group (1.1 ± 1.1 vs 1.8 ± 1.1, P = 0.03). Mortality on day 28 was 13.3% in the rTM group and 27.8% in the control group; these rates were not significantly different (P = 0.26). Multivariate analysis identified only the absence of biliary drainage as significantly associated with persistent DIC (P < 0.01, OR = 12, 95%CI: 2.3-60). Although the difference did not reach statistical significance, primary diseases (malignancies) (P = 0.055, OR = 3.9, 95%CI: 0.97-16) and the non-use of rTM had a tendency to be associated with persistent DIC (P = 0.08, OR = 4.3, 95%CI: 0.84-22).
CONCLUSION: The add-on effects of rTM are anticipated in the treatment of AC-induced DIC, although biliary drainage for AC remains crucial.
Core tip: To evaluate the need for thrombomodulin (rTM) in the management of acute cholangitis (AC)-induced disseminated intravascular coagulation (DIC), we retrospectively compared patients treated with rTM (rTM group) and without rTM (control group). DIC resolution rates were higher in the rTM group (P < 0.01). Multivariate analysis identified only the absence of biliary drainage as significantly associated with persistent DIC (P < 0.01), while there was a trend towards an association between persistent DIC and a lack of rTM (P = 0.08). Therefore, the add-on effects of rTM are anticipated in the treatment of AC-induced DIC, although biliary drainage remains crucial.
- Citation: Suetani K, Okuse C, Nakahara K, Michikawa Y, Noguchi Y, Suzuki M, Morita R, Sato N, Kato M, Itoh F. Thrombomodulin in the management of acute cholangitis-induced disseminated intravascular coagulation. World J Gastroenterol 2015; 21(2): 533-540
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/533.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.533
In recent years, there have been several reports on the efficacy of recombinant human soluble thrombomodulin (rTM) for the treatment of disseminated intravascular coagulation (DIC) associated with infection[1-4]. Various disorders that cause infections were described in these reports, but none of the studies focused on a single disease. Treatment of the primary disease causing DIC remains the most important factor in the resolution of the pathological conditions that underlie infectious DIC[5], and the prognosis of patients with DIC may be markedly affected by the outcome of treatment of the primary disease. Thus, it is crucial to focus on the primary disease to accurately assess the treatment outcomes of patients with infectious DIC.
In acute cholangitis (AC)-induced DIC, the treatment for AC, including biliary drainage, can immediate resolve DIC. However, some patients still have poor outcomes, and further improvements in therapy are needed. The utility of rTM for the treatment of DIC remains unclear. Our PubMed search on rTM therapy for AC-induced DIC, using terms such as “disseminated intravascular coagulation”, “acute cholangitis”, and “thrombomodulin”, yielded only a single-arm case series that we previously reported[6]. We had reported favorable outcomes in patients who received a therapeutic regimen of rTM for AC-induced DIC. However, the prior series had a small sample size; in this study, we therefore compared a larger group of patients who were treated with and without rTM to evaluate the role of anti-DIC therapy with rTM for AC-induced DIC. This is the first comparative study of rTM in the treatment of AC-induced DIC.
Thirty consecutive patients who were diagnosed as having AC-induced DIC and who were treated with rTM at St. Marianna University School of Medicine Hospital between April 2010 and September 2013 were enrolled in this study (rTM group). They were compared to 36 patients with AC-induced DIC who were treated without rTM (before the introduction of rTM therapy at our hospital) between January 2005 and January 2010. Detailed data were available from medical records, which allowed these 36 patients to serve as historical controls for the analysis (control group).
The rTM group included 22 men and 8 women with a mean age ± SD of 77.0 ± 7.7 years. AC was diagnosed and graded according to the 2013 Tokyo Guidelines[7] for the management of AC. AC was severe in 28 patients and moderate in 2 patients, while no patients had mild AC. The primary diseases causing AC were choledocholithiasis in 20 patients, malignant biliary stricture in 9 (pancreatic carcinoma in 5 patients, cholangiocarcinoma in 2, lymph node metastasis of gastric cancer in 1, and malignant lymphoma in 1), and primary sclerosing cholangitis in 1. Based on the DIC diagnostic criteria defined by the Japanese Association for Acute Medicine[8] (Table 1), DIC was diagnosed when the DIC score was 4 or above. The mean DIC score ± SD at the time of DIC diagnosis was 5.4 ± 1.4. The dose of rTM was 380 units/kg per day in 26 patients, while 4 patients received rTM at a reduced dose of 130 units/kg per day, due to renal dysfunction. The duration of rTM treatment was 6 d in all patients. Other anti-DIC drugs used (besides TM) were antithrombin (AT) in 26 patients, gabexate mesilate (GM) in 14 patients, and nafamostat mesilate (NM) in 4 patients (including duplicate counts). The antibiotics used were meropenem (MEPM) in 19 patients, sulbactam/cefoperazone (CPZ/SBT) in 5 patients, doripenem in 5 patients, and tazobactam/piperacilin (TAZ/PIPC) in 1 patient. Biliary drainage was performed in 25 patients but not in 5 patients. Of the patients who did not undergo biliary drainage, 4 patients did not consent, and the presence of cholangitis after the clearance of bile duct stones precluded this procedure in 1 patient.
| Score | |
| Systemic inflammatory response syndrome criteria1 | |
| ≥ 3 | 1 |
| 0-2 | 0 |
| Platelet count (× 103/L) | |
| < 80 or > 50% decrease within 24 h | 3 |
| ≥ 80 and < 120; or > 30% decrease within 24 h | 1 |
| ≥ 120 | 0 |
| Prothrombin time (Value of patient/Normal value) | |
| ≥ 1.2 | 1 |
| < 1.2 | 0 |
| Fibrin/fibrinogen degradation products (mg/L) | |
| ≥ 25 | 3 |
| ≥ 10 and < 25 | 1 |
| < 10 | 0 |
| Diagnosis | |
| ≥ 4 points | DIC |
The control group included 21 men and 15 women with a mean age ± SD of 75.7 ± 9.4 years. AC was severe in 32 patients and moderate in 4 patients, while no patients had mild AC. The primary diseases causing AC were choledocholithiasis in 19 patients, malignant biliary stricture in 15 patients (pancreatic carcinoma in 6, cholangiocarcinoma in 5, gallbladder cancer in 2, and hepatocellular carcinoma in 2), bilio-jejunal anastomotic stricture in 1, and bile duct stricture due to a hepatic cyst in 1 patient. The mean DIC score ± SD at the time of DIC diagnosis was 5.2 ± 1.2. The anti-DIC drugs used were GM in 30 patients, NM in 18, AT in 16, and danaparoid sodium (DS) in 6 (including duplicate counts). The antibiotics used were MEPM in 14 patients, SBT/CPZ in 14, imipenem/cilastatin in 7, and TAZ/PIPC in 1. Biliary drainage was performed in 24 patients.
The rTM group of 30 patients and the control group of 36 patients were compared in terms of patient characteristics [including age, sex, primary disease (malignant/benign)], severity of cholangitis at the time of diagnosis, DIC score at the time of diagnosis, proportion of patients undergoing biliary drainage, and anti-DIC drugs, the DIC resolution rate, the DIC score, the systemic inflammatory response syndrome (SIRS) score, hematological values [platelet count (Plt), fibrin/fibrinogen degradation products (FDP), prothrombin time-international normalized ratio (PT-INR), fibrinogen (Fib), C-reactive protein (CRP), total bilirubin (T-bil)], and treatment outcomes. The day of DIC diagnosis and treatment initiation was designated as day 1, and hematological values were assessed on days 1, 3, 5, 7, and 9. Moreover, DIC resolution was defined as a decrease in the DIC score to 3 or less. The DIC and SIRS scores were expressed as mean ± SD, and hematological data were expressed as median values (quartiles).
A multinomial logistic regression analysis based on the univariate and multivariate analyses was used to identify factors that contributed to the failure of DIC resolution in patients with AC-induced DIC.
Written informed consent was obtained from all patients. This study was approved by the ethics committee of our hospital.
Statistical analyses were performed using the χ2 test, Fisher’s exact test, Welch’s t test, the Mann-Whitney U test or the Wilcoxon single rank test, as appropriate. Variables that were found to have a potentially significant association with persistent DIC (P < 0.2) by univariate analysis were selected for entry into a multiple logistic regression model. P values < 0.05 were regarded as statistically significant. Statistical analyses were performed using the Prism 5 program (Graph Pad Software, Inc., CA, United States) and SPSS (version 19; SPSS, Chicago, IL, United States).
There were no significant differences between the rTM group and the control group with respect to age, sex, primary disease, severity of cholangitis, DIC score, SIRS score, or the proportion of patients who underwent biliary drainage at the time of DIC diagnosis. With regards to anti-DIC agents other than rTM that were used, the proportion of patients who received AT was significantly higher in the rTM group, while a higher proportion of patients in the control group received GM, NM and DS were higher (Table 2).
| rTM group(n = 30) | Control group (n = 36) | P value | |
| Age (yr) | 77.0 ± 7.7 | 75.7 ± 9.4 | 0.554 |
| Sex (Male/Female) | 22/8 | 21/15 | 0.203 |
| Primary disease (Benign/Malignant) | 21/9 | 21/15 | 0.327 |
| Severity of cholangitis (Severe/Moderate) | 28/2 | 32/4 | 0.845 |
| DIC score | 5.4 ± 1.4 | 5.2 ± 1.2 | 0.523 |
| SIRS score | 2.4 ± 1.3 | 2.6 ± 1.0 | 0.599 |
| Biliary drainage | 25 | 24 | 0.123 |
| Anticoagulant drug | |||
| AT | 26 | 16 | < 0.001 |
| GM | 14 | 30 | 0.002 |
| NM | 4 | 18 | 0.004 |
| DS | 0 | 6 | 0.019 |
| Antibiotics | |||
| MEPM | 19 | 14 | 0.048 |
| IPM/CS | 0 | 7 | 0.031 |
| DRPM | 5 | 0 | 0.037 |
| SBT/CPZ | 5 | 14 | 0.047 |
| TAZ/PIPC | 1 | 1 | 0.556 |
The DIC resolution rate on day 9 was 83.3% (25/30) in the rTM group and 52.8% (19/36) in the control group (significantly higher in the rTM group; P = 0.009). The DIC resolution rates on day 7 were 76.7% (23/30) and 50.0% (18/36), respectively, and again, were significantly higher in the rTM group (P = 0.041).
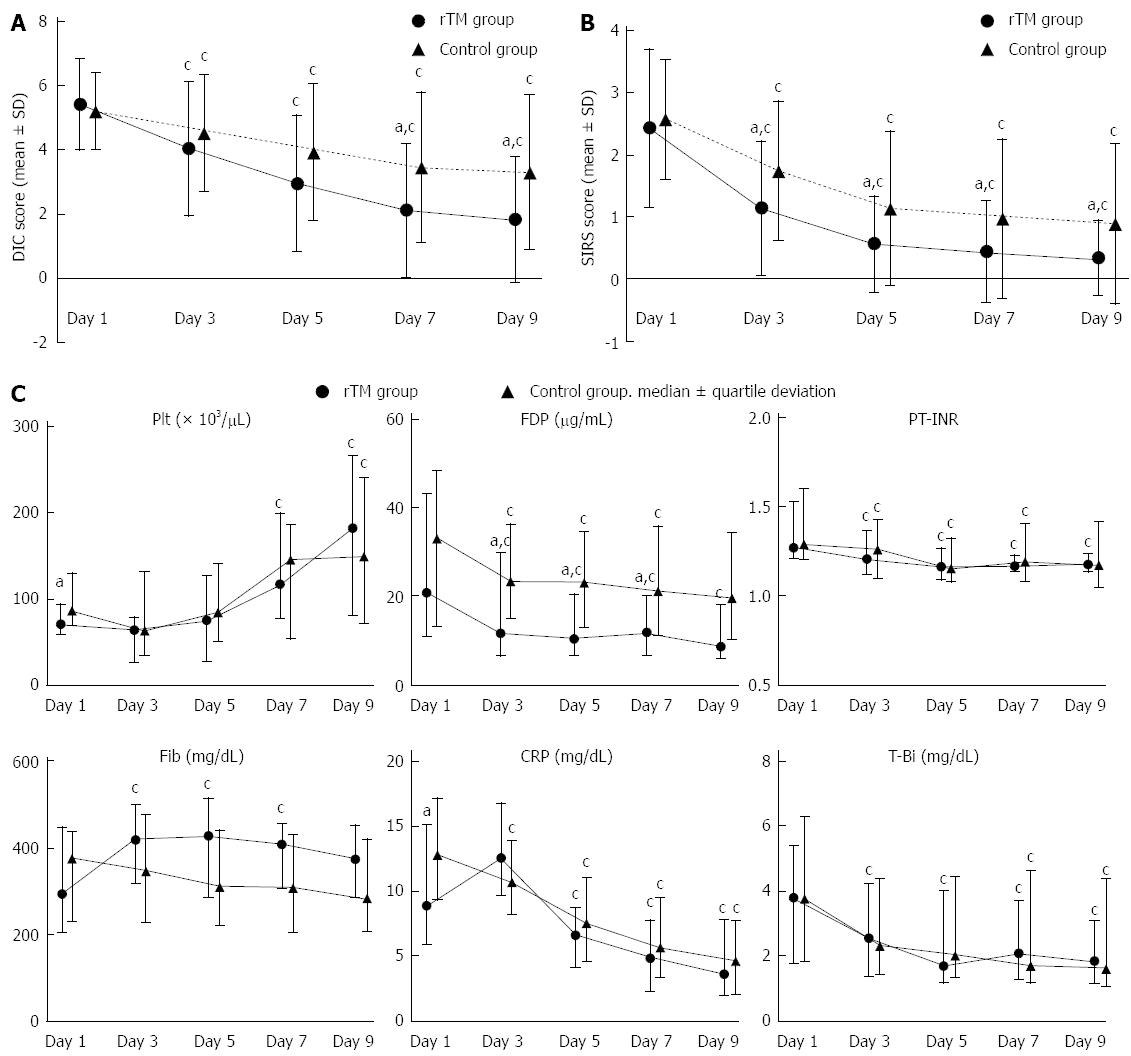
Both the rTM and control groups showed a significant decrease in DIC scores from day 3 onward, compared to those on day 1. The comparison between the rTM and control groups revealed no difference in the mean DIC scores at the time of diagnosis, which were 5.4 ± 1.4 in the rTM group and 5.2 ± 1.2 in the control group (P = 0.524). However, the mean DIC scores on day 7 were 2.1 ± 2.1 and 3.5 ± 2.3 (P = 0.018), and the mean DIC scores on day 9 were 1.8 ± 1.9 and 3.3 ± 2.4, respectively (P = 0.009). The mean DIC scores on days 7 and 9 were significantly lower in the rTM group (Figure 1A).
Compared to day 1, both the rTM and control groups showed a significant decrease in SIRS scores from day 3 onward. There were no differences between the rTM and control groups in terms of the mean SIRS scores at the time of diagnosis, which were 2.4 ± 1.3 in the rTM group and 2.6 ± 1.0 in the control group (P = 0.599). However, the scores on day 3 were 1.1 ± 1.1 and 1.8 ± 1.1 (P = 0.027), respectively, and were significantly lower in the rTM group. Subsequently, the mean SIRS scores in the rTM group remained significantly lower (Figure 1B).
The median hematological values (day 1/day 9) in the rTM group were as follows: Plt, 70.5 (58.8-94.0)/182.0 (80.5-266.5) × 103/μL (P < 0.001); FDP, 20.8 (10.8-43.2)/8.8 (5.9-17.9) μg/mL (P = 0.010); PT-INR, 1.27 (1.21-1.52)/1.18 (1.14-1.24) (P = 0.024); Fib, 293.5 (203.5-449.3)/373.0 (284.8-452.3) mg/dL (P = 0.092); CRP, 8.9 (5.9-15.1)/3.6 (2.0-7.8) mg/dL (P < 0.001); and T-bil, 3.8 (1.8-5.4)/1.9 (1.2-3.1) mg/dL (P = 0.023). The Plt, FDP, PT-INR, CRP, and T-bil values on day 9 showed significant improvement compared to those on day 1. In contrast, the median hematological values (day 1/day 9) in the control group were as follows: Plt, 88.5 (70.3-134.5)/155.0 (73.3-249.0) × 103/μL (P = 0.024); FDP, 35.4 (14.0-51.5)/21.0 (11.2-36.5) μg/mL (P = 0.155); PT-INR, 1.34 (1.24-1.67)/1.21 (1.08-1.47) (P = 0.054); Fib, 399.0 (243.0-464.0)/302.0 (219.0-445.5) mg/dL (P = 0.180); CRP, 13.6 (9.8-18.2)/4.8 (2.1-8.1) mg/dL (P < 0.001); and T-bil, 4.0 (1.9-6.7)/1.7 (1.1-4.7) mg/dL (P = 0.021). The Plt, CRP and T-bil values on day 9 showed significant improvement compared to the Day 1 values. A comparison of the median hematological values between the rTM and control groups showed that, although the levels of Plt on day 1 were significantly lower in the rTM group (P = 0.023), the levels of Plt on day 9 were higher in the rTM group; this difference did not reach statistical significance (P = 0.699). Although there was no difference in FDP on day 1 (P = 0.157) between the two groups, from day 3 onward (P = 0.045), the level of FDP was significantly lower in the rTM group. The fluctuations in median hematological values are shown in Figure 1C.
The mortality rate on day 28 was 13.3% (4/30) in the rTM group and 27.8% (10/36) in the control group; although mortality was higher in the control group, the difference did not reach statistical significance (P = 0.260). In the rTM group, all 4 deaths were classified as due to malignant tumors. Of the 10 deceased patients in the control group, cancer deaths occurred in 7 patients, and deaths due to worsening DIC were observed in the remaining 3 patients.
The univariate analysis identified primary disease (malignancy) (P = 0.003, OR = 5.3, 95%CI: 1.8-16), absence of biliary drainage (P < 0.001, OR = 16, 95%CI: 3.9-66), non-use of rTM (P = 0.010, OR = 4.5, 95%CI: 1.5-14), and non-use of NM (P = 0.016, OR = 0.26, 95%CI: 0.088-0.76) as factors that significantly contributed to persistent DIC (Table 3). A multivariate analysis was performed, incorporating the factors that were identified by univariate analysis, as well as the non-use of GM (P = 0.107) and Fib < 200 mg/dL (P = 0.186), both of which were factors with P values < 0.2 in the univariate analysis; the absence of biliary drainage (P = 0.003, OR = 12, 95%CI: 2.3-60) was the only factor that was found to contribute to persistent DIC (Table 4). Although the difference did not reach statistical significance, it was observed that primary disease (malignancies) (P = 0.055, OR = 3.9, 95%CI: 0.97-16) and non-use of rTM (P = 0.080, OR = 4.3, 95%CI: 0.84-22) tended to be associated with persistent DIC.
| Persistent DIC (n = 25) | Resolved DIC (n = 41) | P value | OR (95%CI) | |
| Age (> 80 yr) | 9 | 18 | 0.610 | 0.72 (0.26-2.0) |
| Female | 7 | 16 | 0.431 | 0. 61 (0.21-1.8) |
| Primary disease (Malignant) | 15 | 9 | 0.003 | 5.3 (1.8-16) |
| Severity of cholangitis (Severe) | 23 | 37 | 1.000 | 1.2 (0.21-7.3) |
| DIC score (> 6) | 12 | 15 | 0.442 | 1.6 (0.58-4.4) |
| SIRS score (> 3) | 11 | 24 | 0.313 | 0.56 (0.20-1.5) |
| Without biliary drainage | 14 | 3 | < 0.001 | 16 (3.9-66) |
| Without rTM | 19 | 17 | 0.010 | 4.5 (1.5-14) |
| Without AT | 12 | 13 | 0.203 | 2.0 (0.71-5.5) |
| Without GM | 5 | 17 | 0.107 | 0.35 (0.11-1.1) |
| Without NM | 12 | 32 | 0.016 | 0.26 (0.088-0.76) |
| Without DS | 22 | 37 | 1.000 | 0.79 (0.16-3.9) |
| Plt (< 80 × 103/μL) | 12 | 24 | 0.452 | 0.65 (0.24-1.8) |
| FDP (> 25 μg/mL) | 17 | 20 | 0.201 | 2.2 (0.79-6.3) |
| PT-INR | 10 | 12 | 0.426 | 1.6 (0.57-4.6) |
| Fib (< 200 mg/dL) | 7 | 5 | 0.186 | 2.8 (0.78-10) |
| CRP (> 15 mg/dL) | 7 | 14 | 0.786 | 0.75 (0.25-2.2) |
| T-Bil (> 10 mg/dL) | 4 | 3 | 0.412 | 0.49 (0.35-12) |
| P value | OR (95%CI) | |
| Primary disease (Malignant) | 0.055 | 3.9 (0.97-16) |
| Without biliary drainage | 0.003 | 12 (2.3-60) |
| Without rTM | 0.080 | 4.3 (0.84-22) |
| Without GM | 0.680 | 1.5 (0.25-8.5) |
| Without NM | 0.188 | 0.37 (0.083-1.6) |
| Fib (< 200 mg/dL) | 0.403 | 2.2 (0.35-14) |
Since May 2008, rTM has been available in Japan as a novel therapeutic agent for DIC. In recent years, there have been several reports on the efficacy of rTM, which binds to thrombin and activates protein C to exert an anticoagulant effect[9,10], for the treatment of infectious DIC[1-4]. In addition to this anticoagulant effect, rTM also elicits an indirect anti-inflammatory effect through activated protein C[9,11-13] and thrombin-activatable fibrinolysis[14,15]. Moreover, rTM exerting a direct anti-inflammatory effect by deactivating high mobility group box 1[16-18] and lipopolysaccharide[19] by binding to these molecules with the lectin-like domain of rTM. Thus, rTM has great potential as a drug for the treatment of infectious DIC.
However, the treatment of the underlying disease causing DIC is essential to achieve resolution of the pathological conditions that are associated with infectious DIC[5]. This is especially relevant in AC-induced DIC, where immediate biliary drainage can lead to prompt resolution of the DIC. Better therapies are needed, as there are still some DIC patients with poor outcomes; however, the usefulness of anti-DIC therapy with rTM remains unclear. Thus, we conducted the present study in patients with AC-induced DIC to evaluate the role of anti-DIC therapy with rTM by comparing outcomes between patients who did and did not receive rTM treatment.
Although there were no differences between the two groups in terms of age, sex, primary disease, severity of cholangitis, DIC score, or in the proportion of patients who underwent biliary drainage, the proportion of patients who received AT was significantly larger in the rTM group. However, the possibility of bias due to the therapeutic effects of AT must be taken into consideration when interpreting therapeutic outcomes in the rTM group. According to the Japanese guidelines for DIC treatment, which were prepared in 2009[5], AT is the most strongly recommended of all anti-DIC drugs. In the rTM group, which included patients who were treated in 2010 and thereafter, a higher frequency of AT use can be expected as a background condition. Because only a short time has elapsed since rTM became available, it is not included in the Japanese guidelines for DIC treatment. There have been many reports on the effectiveness of AT for the treatment of infectious DIC[20]. However, the KyberSept trial, reported in 2001[21], showed that the use of AT is not associated with decreased mortality, and the European guidelines for DIC treatment recommend restraint in the use of AT for the treatment of infectious DIC[22,23]. Our present univariate analysis identified only the use of rTM as a contributory factor in the successful treatment of DIC, while AT was not identified as such a factor. However, further studies are needed to determine the usefulness of AT for the treatment of AC-induced DIC; due to the retrospective nature of this study, we were unable to evaluate serum AT III values in our patients.
The DIC resolution rate was significantly higher in the rTM group than in the control group, suggesting that rTM is highly effective for the treatment of AC-induced DIC. Although significant decreases in the DIC and SIRS scores from day 1 to day 3 were observed in both the rTM group and in the control group, a comparison between these two groups revealed that the DIC and SIRS scores had been significantly lower since days 7 and 3, respectively, in the rTM group and that greater improvements in the scores were observed in this group. The SIRS scores in particular were significantly improved in the early phase of treatment in the rTM group, which may be attributable to the anti-inflammatory effect of rTM[9,11-19]. With respect to the hematological findings, the control group showed significant improvements in Plt, CRP, and T-bil from day 1 to day 9, whereas the rTM group showed significant improvements in coagulation markers, such as FDP and PT-INR, in addition to Plt, CRP and T-bil. Although Plt levels on day 1 were significantly lower in the rTM group than in the control group, the Plt values on day 9 were higher in the rTM group. However, these differences did not reach statistical significance. Although there was no difference in FDP between the two groups on day 1, the levels of FDP were significantly lower from day 3 onward in the rTM group. These results suggest that rTM exerts a favorable anticoagulant effect. Thus, it is possible that in patients with AC-induced DIC, earlier and more marked resolution of the pathological condition may occur with the use of rTM.
There was no statistically significant difference in the mortality rate on day 28 between the two groups. However, the causes of death in all 4 patients in the rTM group were classified as malignant tumors, but the causes of death in 3 of the 10 deceased patients in the control group were classified as being DIC-related. Based on these results, we can reasonably speculate that the resolution of DIC by rTM administration may have contributed to improved outcomes. In fact, there are reports on septic DIC describing reduced mortality at 28 d after the initiation of treatment with rTM[2,24,25]. In the present study, there were only 3 DIC-related deaths. To examine the effects of rTM on the improvement of the outcomes of patients with AC-induced DIC, multicenter studies with a larger sample size are needed.
In the present study, a multivariate analysis was performed to identify factors that contributed to persistent DIC. The absence of biliary drainage was identified as the only factor that contributed to persistent DIC. The treatment of the underlying disease causing DIC is considered to be the most important aspect of the treatment of infectious DIC[5], and the results of our study support this concept. Specifically, in patients with AC, a complete response is often achieved by biliary drainage[26,27], which is clearly the most important procedure for the clinical management of DIC. We advocate that biliary drainage be performed whenever possible. Furthermore, although the difference was not statistically significant, we observed that the non-use of rTM also tended to be associated with persistent DIC (P = 0.080, OR = 4.3, 95%CI: 0.84-22). It appears that treatment can be optimized by a combination of biliary drainage and the use of rTM. Moreover, our multivariate analysis revealed that the presence of malignant tumors also tended to be associated with persistent DIC, presumably because neoplastic as well as infectious DIC influenced the outcomes of patients in our study. Future studies are eagerly anticipated regarding the effects of rTM on neoplastic DIC due to solid cancers.
In conclusion, although biliary drainage for acute cholangitis is the most important treatment for AC-induced DIC, the use of rTM can lead to an earlier and more marked improvement in DIC and SIRS scores, which may improve clinical outcomes. However, to further examine the effects of rTM on the improvement of the outcomes of patients with AC-induced DIC, additional multicenter studies with a larger sample size are needed.
In acute cholangitis (AC)-induced disseminated intravascular coagulation (DIC), treatment for AC, including biliary drainage, can achieve resolution of the DIC. However, further improvements in treatment are needed, as there are still patients with poor outcomes.
There have been several reports on the efficacy of recombinant human soluble thrombomodulin (rTM) for DIC that is associated with infection. However, in AC-induced DIC, the usefulness of anti-DIC therapy with rTM remains unclear.
The authors compared patients treated with rTM (rTM group) and without rTM (control group) to evaluate the role of anti-DIC therapy with rTM for AC-induced DIC. DIC resolution rates were higher in the rTM group (P < 0.01), and DIC scores were lower in the rTM group (P < 0.01). Multivariate analysis identified only the absence of biliary drainage as a contributor to the failure of DIC resolution (P < 0.01), and the non-use of rTM also tended to contribute to failure of DIC resolution (P = 0.08).
The add-on effects of rTM are anticipated in the treatment of AC-induced DIC, although biliary drainage for AC remains crucial.
This paper is the first to demonstrate the effectiveness of rTM in cases of DIC due to acute cholangitis. Biliary drainage is the most effective procedure for the control of DIC, but rTM improves outcomes for patients. This retrospective study is original with solid data that is well analyzed.
P- Reviewer: Invernizzi P, Sakai Y S- Editor: Nan J L- Editor: A E- Editor: Ma S
| 1. | Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, Hirayama A, Matsuda T, Asakura H, Nakashima M. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost. 2007;5:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 2. | Yamakawa K, Fujimi S, Mohri T, Matsuda H, Nakamori Y, Hirose T, Tasaki O, Ogura H, Kuwagata Y, Hamasaki T. Treatment effects of recombinant human soluble thrombomodulin in patients with severe sepsis: a historical control study. Crit Care. 2011;15:R123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Aikawa N, Shimazaki S, Yamamoto Y, Saito H, Maruyama I, Ohno R, Hirayama A, Aoki Y, Aoki N. Thrombomodulin alfa in the treatment of infectious patients complicated by disseminated intravascular coagulation: subanalysis from the phase 3 trial. Shock. 2011;35:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Kato T, Sakai T, Kato M, Hagihara M, Hasegawa T, Matsuura K, Nakagawa T. Recombinant human soluble thrombomodulin administration improves sepsis-induced disseminated intravascular coagulation and mortality: a retrospective cohort study. Thromb J. 2013;11:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Wada H, Asakura H, Okamoto K, Iba T, Uchiyama T, Kawasugi K, Koga S, Mayumi T, Koike K, Gando S. Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb Res. 2010;125:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Nakahara K, Okuse C, Adachi S, Suetani K, Kitagawa S, Okano M, Michikawa Y, Takagi R, Shigefuku R, Itoh F. Use of antithrombin and thrombomodulin in the management of disseminated intravascular coagulation in patients with acute cholangitis. Gut Liver. 2013;7:363-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Kiriyama S, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Yokoe M. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2013;20:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 8. | Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, Mayumi T, Murata A, Ikeda T, Ishikura H. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 459] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 9. | Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4217] [Cited by in RCA: 3823] [Article Influence: 159.3] [Reference Citation Analysis (0)] |
| 10. | Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 696] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 11. | Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 591] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 12. | Yuksel M, Okajima K, Uchiba M, Horiuchi S, Okabe H. Activated protein C inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production by inhibiting activation of both nuclear factor-kappa B and activator protein-1 in human monocytes. Thromb Haemost. 2002;88:267-273. [PubMed] |
| 13. | Kurosawa S, Esmon CT, Stearns-Kurosawa DJ. The soluble endothelial protein C receptor binds to activated neutrophils: involvement of proteinase-3 and CD11b/CD18. J Immunol. 2000;165:4697-4703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Myles T, Nishimura T, Yun TH, Nagashima M, Morser J, Patterson AJ, Pearl RG, Leung LL. Thrombin activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation. J Biol Chem. 2003;278:51059-51067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Declerck PJ. Thrombin activatable fibrinolysis inhibitor. Hamostaseologie. 2011;31:165-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, Uchimura T, Ida N, Yamazaki Y, Yamada S. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Ito T, Kawahara K, Okamoto K, Yamada S, Yasuda M, Imaizumi H, Nawa Y, Meng X, Shrestha B, Hashiguchi T. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol. 2008;28:1825-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Nagato M, Okamoto K, Abe Y, Higure A, Yamaguchi K. Recombinant human soluble thrombomodulin decreases the plasma high-mobility group box-1 protein levels, whereas improving the acute liver injury and survival rates in experimental endotoxemia. Crit Care Med. 2009;37:2181-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Shi CS, Shi GY, Hsiao HM, Kao YC, Kuo KL, Ma CY, Kuo CH, Chang BI, Chang CF, Lin CH. Lectin-like domain of thrombomodulin binds to its specific ligand Lewis Y antigen and neutralizes lipopolysaccharide-induced inflammatory response. Blood. 2008;112:3661-3670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Kienast J, Juers M, Wiedermann CJ, Hoffmann JN, Ostermann H, Strauss R, Keinecke HO, Warren BL, Opal SM. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J Thromb Haemost. 2006;4:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 290] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, Chalupa P, Atherstone A, Pénzes I, Kübler A. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286:1869-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 884] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 22. | Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 689] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 23. | Di Nisio M, Baudo F, Cosmi B, D’Angelo A, De Gasperi A, Malato A, Schiavoni M, Squizzato A. Diagnosis and treatment of disseminated intravascular coagulation: guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res. 2012;129:e177-e184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Yamakawa K, Ogura H, Fujimi S, Morikawa M, Ogawa Y, Mohri T, Nakamori Y, Inoue Y, Kuwagata Y, Tanaka H. Recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Intensive Care Med. 2013;39:644-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Ogawa Y, Yamakawa K, Ogura H, Kiguchi T, Mohri T, Nakamori Y, Kuwagata Y, Shimazu T, Hamasaki T, Fujimi S. Recombinant human soluble thrombomodulin improves mortality and respiratory dysfunction in patients with severe sepsis. J Trauma Acute Care Surg. 2012;72:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Lai EC, Mok FP, Tan ES, Lo CM, Fan ST, You KT, Wong J. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;326:1582-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 328] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Miura F, Takada T, Strasberg SM, Solomkin JS, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Yoshida M, Mayumi T. TG13 flowchart for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |