Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.423
Peer-review started: August 14, 2014
First decision: September 27, 2014
Revised: October 12, 2014
Accepted: November 11, 2014
Article in press: November 11, 2014
Published online: January 14, 2015
Processing time: 157 Days and 4.9 Hours
Rectal cancer classification is important to determine the preoperative chemoradiation therapy and to select appropriate surgical technique. We reviewed the Western and Japanese rectal cancer classification and we propose our new classification based of Magnetic resonance imaging (MRI). We determine the relation of the tumor to fixed parameters in MRI, which are peritoneal reflection and levator ani muscle. Then, we classify the rectal cancer into four levels based on tumor distal margin and invasion to MRI parameters. We applied all three classifications to 60 retrospectively collected patients of different rectal cancer distance and we compared our classifications to the others. Based on each level we standardize our surgical approach. For stages I-III, We found that level I where tumor distal margin is located above the peritoneal reflection and all of them were received low anterior resection (LAR) without chemoradiation. Level II where tumor distal margin is located from the peritoneal reflection and above the levator ani insertion on the rectum. 90% of them were received LAR ± chemoradiation. Level III where tumor distal margin is located at the level of levator ani insertion or invading any part of the levator ani. 60% of them had ULAR + coloanal anastomosis ± chemoradiation. Level IV where the tumor distal margin is located below the levator ani insertion; 77% were received APR ± chemoradiation. The overall kappa for all levels between surgeons and radiologist was 0.93 (95%CI: 0.87-0.99), which is indicating almost perfect agreement. We concluded that the management of rectal tumors differed among each tumor level and our new MRI based classification might facilitate the prediction of surgical and chemoradiation management with better communication among a multidisciplinary team comparing to other classifications.
Core tip: We reviewed the current rectal cancer classification and we propose a new rectal cancer classification based on new radiological parameters that might lead to change in the future decision making and management. We provide a comparison between our new novel classification, Western and Japanese one.
- Citation: Alasari S, Lim D, Kim NK. Magnetic resonance imaging based rectal cancer classification: Landmarks and technical standardization. World J Gastroenterol 2015; 21(2): 423-431
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/423.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.423
At the latter half of the 20th century, surgical therapy for rectal cancer underwent vital changes[1]. However, the complication and death rates from rectal cancer still remain high. This finding might be explained by variable application of the available therapies and surgeon decision at time of surgery. The variation in therapy use has been shown in rectal cancer compared with many other diseases[2-5]. Heald et al[6,7] and MacFarlane et al[8] started a “total mesorectal excision” technique as a method to reduce local recurrence rates (4%-8%) following rectal resection for rectal cancer, without adjuvant therapy. Surgeons with different types of training and institutions with variety of cancer patient’s volumes provide rectal cancer management. Similar patients with similar tumors might receive different treatments depending on where and from whom they seek treatment; some of these treatment variations may represent suboptimal patient care[1].
An approach to management of rectal cancer patients using a multidisciplinary team (MDT) might provide better communication and facilitate high-quality management. It is proved in literature that the MDT could improve patient’s 3- and 5-year survival[9,10]. The treatment strategy was altered after discussed at MDT meeting in 58.33% of colorectal cancer patients before operation especially in the matter of the sphincter-preservation and local control (P = 0.049)[10]. The issue of variability in surgical decisions among surgeons, particularly in low rectal cancer, to preserve the sphincter or perform advanced surgery remains unresolved worldwide. Several limitations present in the previous studies that have tried to found the nature of therapy variations. Large population-based studies (i.e., the National Cancer Data Base reports from the American College of Surgeons Committee on Cancer and the American Cancer Society) show the variations in rectal cancer therapy over time without clinical interpretation of these variations[1].
The decision about surgery or chemoradiation treatment depends on several factors, one of them are radiologic evaluation of the tumor. Among those radiologic investigations, magnetic resonance imaging (MRI) commonly used to determine the status of perirectal node or the circumferential tumor margins[11-13]. However, small number of studies reported the relation between rectal cancer and peritoneal reflection or levator ani muscle by MRI[11,12,14,15]. Determination of the best preoperative surgical approach are depend on several factors one of them are tumor location in which, some authors depend on the height from anal verge while the others on radiologic relation to peritoneal reflection[14,15].
We reviewed the current tumor location classification and we propose a one based on the relationship of the tumor to fixed parameters on MRI imaging. Furthermore, we suggest the possible surgical approach based on the new classification.
The management of rectal cancer poses many challenges to both surgeons and oncologists.
Knowledge of rectal anatomy is a key for medical and surgical management and important for the selection of appropriate imaging modality. The rectum begins immediately following the sigmoid colon, and ends at the anal canal. Based on distance from the anal verge; the rectum is divided into the upper (11-15 cm), the middle (7-10 cm), and the lower thirds (0-6 cm). The upper 1/3 is covered by peritoneum. The peritoneum covers only anteriorly at the middle rectum while the lower 1/2 is completely extraperitoneal[16,17]. The mesorectum behind the rectum separated from the presacral fascia by mesorectal fascia which its lack at the distal third of the rectum just before its entry in the pelvic floor muscles. In regards to the upper rectum, Benzoni et al[15] found that the relation between tumor location and peritoneal reflection is a prognostic factor in rectal cancer. The tumor located at the extra-peritoneal part of the rectum is more aggressive than those at the intra-peritoneal even when treated by neoadjuvant chemoradiotherapy[15,18,19].
The lower part of the rectum where the mesorectum end and levator ani muscle insert is an important part mainly for treatment decision, which is different from the part with mesorectum and above the levator ani insertion site. The tumor at this level can easily goes outside the rectal wall to the levator ani muscle or to the sphincters below this level, which might lead to change in chemoradiation and surgical approach.
To optimize the treatment strategy on an individual basis, we need detailed information about primary tumor location, local extension, potential nodal-stage, potential circumferential resection margin involvement and extra-mural venous invasion[20]. The complexity of the anatomy and relationship of the tumor to adjacent structures, i.e., bone and muscles-might lead to difficulty in prediction and management decisions regarding the type of surgical approach and chemoradiation use. Radiology plays a key role in tumor management. It provides a vital knowledge about the tumor diagnosis and preoperative staging. Of all the radiologic modalities that evaluate the rectal cancer, MRI is a superior modality that provides a better anatomical visualization comparing to computed tomography (CT) and endorectal ultrasound (EUS)[21]. In addition, it provides high accuracy in detection of tumor location, tissue characterization, detailed anatomical relation to the tumor and tumor staging. So, preoperative MRI is useful modality to determine the surgical approach and need for neoadjuvant or adjuvant therapy[21].
The benefit of MRI for surgical treatment decisions was investigated retrospectively by Shihab et al[22] who found that MRI could objectively confirm the clinical impression by delineation of the local extent of the tumor and its relationship to the levator ani and the intersphincteric plane.
The accuracy of predicting tumor extent beyond the muscularis propria was within 0.5 mm tolerance in the mid or upper rectum, and suggests MRI can accurately predict ultimate outcome. MRI can also accurately measure the distance between the anorectal junction and/or and the distal part of the tumor and the luminal length of the tumor, circumferential resection margin particularly in the mid-rectum, involvement of the levator in the low rectum and the extramural depth of invasion[20,23].
Surgical approach and chemoradiation therapy decisions in treatment of rectal cancer were determined by multiple factors. Tumor location and preoperative stage are the most important clinical elements. In population-based studies, the information of the tumor location is rarely available. However, in the institution-specific studies, which usually provide more clinical data, cannot reflect the practices in a general population. A consensus statement for tumor location and how it affects surgical decisions differ between Japan and Western countries.
Currently, the tumor distance from the anal verge (upper, middle, and lower)[24,25] as adopted by Western and most others countries, or the relationship of the tumor to the peritoneal reflection (Ra, Rb, and P)[26] as proposed by Japanese surgeons, are used to determine tumor locations. Peritoneal reflection separates the Ra and Rb border, which approximately corresponds to the level of the middle Houston valve.
In regard to western classification, some studies reported that the tumor height from the anal verge might have beneficial on the radiotherapy of rectal tumors[1]. However, measurements of distances from the anal verge are still unclear due to the methods provides to date like digital rectal examination or rigid sigmoidoscopy, are rather vague and subjective and the reported distances from the anal verge to the levator ani insertion and peritoneal reflection are variable[27]. Accordingly, based on this landmark we cannot measure the exact location of the peritoneal reflection or level of levator ani muscle insertion. We considered that if the peritoneal reflection and levator ani insertion could be clearly visualized and localized radiologically, that would provide a more objective localization method rather than the distances from the anal verge measurement.
In regard to Japanese classification, it is based on the relation with respect to peritoneal reflection. However, still it is difficult to determine preoperatively the exact location of the peritoneal reflection. Furthermore, in relation to the mesorectum, the definitions of extra-peritoneal and intra-peritoneal locations are vague[27]. The start of the P level, which is the anal canal, is not clearly defined preoperatively by specific fixed landmarks.
Preoperative evaluations are vital to determine the treatment options for rectal cancer. Moreover, the decision about the preoperative chemoradiotherapy and type of surgery is dependent on tumor location, tumor invasion, nodal status, involvement of the mesorectal fascia, and distant metastasis[11-13,20]. Due to the changes of the surgical approach of the rectal cancer over several years, new rectal classification to predict the best approach are needed. In the era of sphincter preservation, technical innovations and improvement in the radiological modalities, the surgical approach for each tumor location is not clearly defined.
Previously rectal cancer was treated either by anterior resection (AR) or abdominoperineal resection (APR). Then low anterior resection (LAR) proves non-inferiority results to APR with better quality of life. Currently, the low rectal cancer can be approached by ultra low anterior resection with coloanal anastomosis, partial or complete intersphincteric resection, tailored levator ani excision (hemilevator excision) or even local excision. Those approaches proved to be alternative to APR with better sphincter saving and quality of life. However, preoperative MRI might predict which of those approaches are more likely to be used but lacking of specific parameters encourage us to propose a new rectal classification.
High-resolution pelvic MRI is now routinely used in Korea as well as in United Kingdom and Europe as a preoperative staging and selection tool for the use of preoperative chemoradiation. MRI can easily localize the tumor above or below the peritoneal reflection and strongly predicts the likelihood of involvement of the circumferential resection margin, involvement of the levator ani muscle in the low rectum and the extramural depth of invasion. Furthermore, it can identify patients at risk of the surgeon being unable to achieve an R0 resection[23].
Long course chemoradiation or short course radiation therapy are routinely used for locally advanced rectal cancer “T3, T4 tumors or any TN+ (stage II, III)” as defined by National Comprehensive Cancer Network guidelines, however; recent improvements in the quality of surgery, i.e., TME, MRI and pathological reporting of the operative specimen, lead most of the investigators to question both these approaches[20]. Most of the time long course chemoradiation used for low rectal cancer in a goal to preserve the sphincter but definition of low rectal cancer are variable and some patients considered as low rectal cancer based on tumor distance from anal verge while the exact location of the tumor are above the levator ani where using short course radiation or TME alone may be better to avoid radiation complications. The same thing can be applied to peritoneal reflection, which is an important since most of the tumor above the peritoneal reflection less likely to have local recurrence where the radiation can be omitted. But the exact location of peritoneal reflection needs to be determined by MRI and not by only measuring the distance from anal verge where from this point we propose our new classification.
Our proposed classification depends on division of the rectum into levels which is depends on a fixed parameters seen on preoperative MRI. Those parameters are peritoneal reflection and levator ani insertion on the rectum.
In a study by Jung et al[27] found that based on the location of the peritoneal reflection, the subdivision of the rectum by MRI is more objective and anatomical than other classification methods and could facilitate treatment planning. However, his classification do not cover the whole rectum for that we add a levator ani insertion as a parameter for lower rectal classification.
MRI (sagittal and coronal views) was used to determine fixed, tumor-related anatomical parameters and so initiate a change in the management plan. These parameters were peritoneal reflection and levator ani muscle insertion.
To locate the tumor in the rectum, we determined two factors - the tumor “distal margin” from the parameters and tumor tethering “radiologically the tumor closely in contact with adjacent structure and we cannot clearly define a separate margin” or “invasion” to those parameters.
We then defined each rectal division “class” and performed a retrospective study of a 60 rectal cancer patients selected randomly based on their tumor distance from anal verge and comparison between our classification and those used by Western countries and Japan were performed too. We showed the advantage of our classification in the prediction of the exact surgical procedure over the others.
Data were analyzed using the SPSS statistical software (Statistical Product and Service Solutions version 18 for Windows; SPSS Inc., Chicago, IL, United States). A P value less than or equal to 0.05 was deemed to indicate statistical significance. The tumor level on MRI compared to sigmoidoscopy by the 1 radiologist and 2 colorectal surgeons. The inter-observer agreement between surgeon and surgeon and surgeons and radiologist was evaluated using Cohen’s Kappa statistics. Kappa statistic was tested for overall levels and each level separately. Weighted Kappa < 0 indicate (no agreement); Kappa = 0.0 - 0.20 (slight agreement); Kappa = 0.21 - 0.40 (fair agreement); Kappa = 0.41 - 0.60 (moderate agreement); Kappa = 0.61 - 0.80 (substantial agreement); Kappa = 0.81 - 1.00 (almost perfect agreement). Statistical analyses were performed using Stata-MP 10.1 (Stata Corp, College Station, Tex).
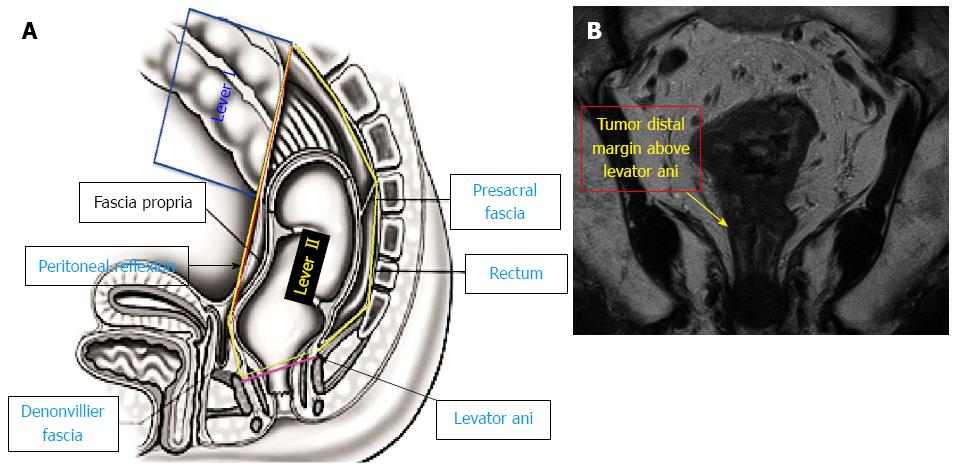
Based on the MRI sagittal view, the first parameter was the rectal peritoneal reflection, which is the line connecting the lowest point of the peritoneal reflection anteriorly to the highest point of the sacral promontory posteriorly (Figure 1).
Based on the MRI coronal view, the second parameter was the levator ani insertion on the rectum (anorectal ring) (Figure 1).
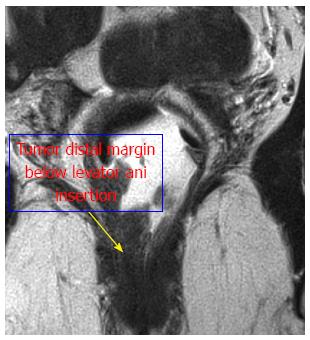
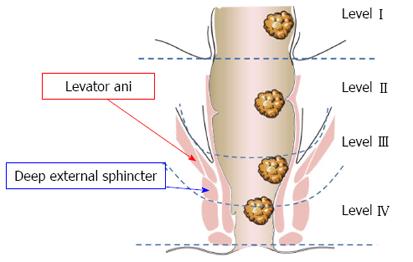
Thus, based on those parameters, we divided the rectum into the following four levels: (1) Level I: the tumor “distal margin” is located “above” the peritoneal reflection on the sagittal MRI view (Figure 1A); (2) Level II: the tumor “distal margin” is located “from” the peritoneal reflection and “above” the levator ani insertion on the rectum (anorectal ring) on MRI sagittal and coronal views (Note: the tumor should not invade the levator ani at this level) (Figure 1); (3) Level III: the tumor “distal margin” is located “at” the level of levator ani insertion on the rectum (anorectal ring) or the tumor margin is invading any part of the levator ani from its origin to its insertion (Figure 2); and (4) Level IV: the tumor “distal margin” is located “below” the levator ani insertion on the rectum (Figures 3 and 4).
To apply our classification clinically, we performed a retrospective comparative analysis of 60 randomly selected patients diagnosed with rectal cancer at various locations within the rectum. Thirty-eight (63%) of these patients were male, and 22 (36%) were female. Their mean age was 59.18 ± 12.66 years. Twenty-seven patients (45%) received neoadjuvant chemoradiotherapy. The preoperative tumor stage was determined. A total of six patients (10%) had a disease of stage I, 10 (16%) had stage II, and 44 (73%) had stage III. The postoperative stage was also reported. A total of 6 (10%) patients had stage 0 (complete response) disease, 9 (15%) had stage I, 15 (25%) had stage II, and 30 (50%) had stage III.
Then, we compared the tumor location on MRI and sigmoidoscopy for each patient. Of 60 patients, 12 showed a difference of 2 cm or more. However, this finding was not statistically significant (P = 0.64). Therefore, we depended on the MRI view, which could show not only the tumor distance from the anal verge but also the anatomical landmarks and relationships of the tumor to the parameters.
We next compared our tumor location level to the Western and Japanese rectal location divisions. For the Western division, we selected random cases to cover all parts of the ano-rectum (upper, middle, lower) from 1 to 15 cm. For the Japanese division, we divided the rectum based on the location above the peritoneal reflection (Ra), below the peritoneal reflection (Rb), and at the anatomical anal canal (P).
For upper rectal cancer (Table 1), the tumor distal margin of 19 patients (31%) was 11-15 cm. Eight of them were Ra, and 11 were Rb. Four of them were level I, and 15 were level II. Clinically, 17 (89%) had stage III disease, and two (10%) had stage II disease. Six patients had neoadjuvant chemoradiation. Six patients received open surgery, two received robotic surgery, and eleven received laparoscopic surgery. Eighteen (94%) patients had lower anterior resection (LAR), whereas one (5%) patient underwent the Hartmann procedure due to tumor invasion of other organs; this was level II. Postoperatively, one patient (5%) was stage I, 6 (31%) were stage II, and 12 (63.1%) were stage III. Technically, we found that all level I patients received LAR.
| Age | Sex | CCRT | CM | R | L | Pre OP TS | TNM | O/L/R | OP | Post OP TS | TNM |
| 67 | M | Y | 11 | Rb | 2 | III | T3N+ | L | LAR | III | YPT3N1 |
| 68 | F | Y | 11 | Rb | 2 | III | T3N+ | O | LAR | III | YPT3N1 |
| 56 | F | N | 11 | Rb | 2 | III | T3N+ | L | LAR | III | PT3N1 |
| 70 | F | N | 11 | Rb | 2 | III | T2N+ | L | LAR | III | PT3N1 |
| 67 | F | Y | 12 | Rb | 2 | III | T3N+ | L | LAR | II | YPT3N0 |
| 48 | M | Y | 12 | Rb | 2 | III | T3N+ | L | LAR | II | YPT3N0 |
| 49 | F | N | 12 | Rb | 2 | III | T4N+ | O | LAR | III | PT4N1 |
| 50 | F | N | 12 | Rb | 2 | III | T3N+ | L | LAR | I | PT1N0 |
| 52 | M | Y | 13 | Ra | 2 | II | T3N0 | L | LAR | II | YPT3N0 |
| 60 | M | N | 13 | Ra | 2 | III | T3N+ | O | LAR | II | PT3N0 |
| 64 | M | N | 13 | Rb | 2 | III | T3N+ | R | LAR | III | PT3N1 |
| 74 | M | N | 13 | Rb | 2 | III | T3N+ | L | LAR | III | PT3N1 |
| 54 | F | N | 14 | Ra | 2 | III | T3N+ | O | LAR | III | PT3N1 |
| 67 | M | N | 14 | Ra | 2 | III | T3N+ | L | LAR | III | PT4N1 |
| 63 | M | N | 14 | Ra | 1 | III | T3N+ | L | LAR | II | PT3N0 |
| 44 | F | Y | 15 | Rb | 2 | III | T4N+ | O | Hartm. | III | YPT4N1 |
| 66 | M | N | 15 | Ra | 1 | III | T4N+ | O | LAR | III | PT3N1 |
| 60 | M | N | 15 | Ra | 1 | III | T3N+ | L | LAR | III | PT3N2 |
| 73 | M | N | 15 | Ra | 1 | II | T3N0 | R | LAR | II | PT3N0 |
For middle rectal cancer (Table 2), the tumor distal margin in 16 patients (26%) was 7-10 cm. All of these patients were Rb located in level II. Clinically, 1 (6%) patient was stage I, 4 (25%) were stage II, and 11 (68%) were stage III. Half of the patients (50%) received neoadjuvant chemoradiation therapy. Four patients underwent surgery using an open approach, and six underwent each laparoscopic and robotic surgery. All patients (100%) had LAR. Postoperatively, one patient was each of stage 0 and I. Six patients were stage II, and eight were stage III.
| Age (yr) | Sex | CCRT | CM | R | L | Pre OP TS | TNM | O/L/R | OP | Post OP TS | TNM |
| 64 | F | N | 7.0 | Rb | 2 | II | T3N0 | O | LAR | II | PT3N0 |
| 68 | F | N | 7.0 | Rb | 2 | III | T3N+ | O | LAR | II | PT3N0 |
| 50 | M | Y | 7.2 | Rb | 2 | III | T3N+ | L | LAR | III | YPT3N2 |
| 85 | M | Y | 7.8 | Rb | 2 | III | T3N+ | R | LAR | III | YPT3N1 |
| 66 | M | N | 8.0 | Rb | 2 | III | T3N+ | O | LAR | III | PT3N1 |
| 73 | M | N | 8.0 | Rb | 2 | III | T3N+ | L | LAR | III | PT3N1 |
| 49 | M | Y | 8.5 | Rb | 2 | III | T3N+ | R | LAR | 0 | YPT0N0 |
| 58 | M | Y | 8.8 | Rb | 2 | III | T3N0 | L | LAR | II | YPT3N0 |
| 78 | M | N | 9.0 | Rb | 2 | II | T3N0 | L | LAR | III | PT4N1 |
| 52 | F | N | 9.3 | Rb | 2 | III | T3N+ | R | LAR | III | PT3N2 |
| 61 | F | Y | 9.5 | Rb | 2 | II | T3N0 | L | LAR | III | YPT3N1 |
| 73 | F | Y | 9.5 | Rb | 2 | III | T3N+ | R | LAR | II | YPT3N0 |
| 48 | M | Y | 10.0 | Rb | 2 | I | T2N0 | R | LAR | I | YPT2N0 |
| 74 | F | Y | 10.0 | Rb | 2 | III | T2N+ | R | LAR | II | YPT3N0 |
| 47 | F | N | 10.0 | Rb | 2 | III | T3N+ | O | LAR | III | PT3N2 |
| 82 | M | N | 10.0 | Rb | 2 | II | T3N0 | L | LAR | II | PT3N0 |
For lower rectal cancer (Table 3), the tumor distal margin in 26 (43%) patients was 1-6 cm. All patients were P according to the Japanese classification. According to our classification, 11 (42%) patients were at level II, five (19%) were at level III, and 10 (38%) were at level IV. Clinically, five (19%) patients were stage I, four (15%) were stage II, 16 (61%) were stage III, and one (3%) was stage IV. Half (50%) of the patients received neoadjuvant chemoradiation. Nine patients underwent robotic and open surgery each, whereas eight underwent laparoscopic surgery. Because three levels exist in those considered to have low-rectal tumors, the procedures also differed. Nine (34%) patients had LAR and APR each. Six (23%) patients had ultra-low anterior resection and hand-sewn coloanal anastomosis (CAA). Two (7%) patients had ultra-low anterior section with intersphincteric resection (ISR) and hand-sewn CAA. Complete pathologic response (stage 0) was achieved in 5 (20%) patients. Seven (26%) showed a stage I disease, 3 (11%) had stage II, and 10 (40%) had stage III.
| Age (yr) | Sex | CCRT | CM | R | L | Pre OP TS | TNM | O/L/R | OP | Post OP TS | TNM |
| 51 | M | Y | 1.0 | P | 4 | III | T4N+ | O | APR | III | YPT2N1 |
| 57 | M | Y | 1.0 | P | 4 | III | T3N+ | R | ULAR +ISR | 0 | YPT0N0 |
| 49 | M | N | 1.0 | P | 4 | III | T3N+ | O | APR | III | PT4N2 |
| 75 | M | N | 1.3 | P | 4 | II | T3N0 | O | APR | I | PT2N0 |
| 37 | M | Y | 2.0 | P | 4 | III | T4N+ | O | APR | III | YPT3N1 |
| 62 | F | Y | 2.0 | P | 4 | III | T3N+ | O | ULAR +ISR | 0 | YPT0N0 |
| 54 | M | N | 2.0 | P | 4 | II | T3N0 | O | APR | II | PT3N0 |
| 48 | M | N | 2.0 | P | 4 | II | T3N0 | O | APR | III | PT3N1 |
| 83 | F | Y | 3.0 | P | 3 | IV | T4N+ | O | APR | II | YPT3N0 |
| 75 | F | Y | 3.5 | P | 2 | III | T3N+ | R | LAR | 0 | YPT0N0 |
| 54 | M | N | 3.5 | P | 3 | I | T2N0 | R | ULAR + CAA | I | PT1N0 |
| 72 | M | N | 3.7 | P | 4 | III | T3N+ | L | APR | III | PT3N2 |
| 37 | M | Y | 4.0 | P | 3 | III | T3N+ | L | ULAR + CAA | 0 | YPT0N0 |
| 47 | F | Y | 4.0 | P | 2 | III | T3N+ | L | LAR | 0 | YPT0N0 |
| 60 | M | N | 4.2 | P | 2 | I | T1N0 | L | LAR | I | PT1N0 |
| 27 | F | N | 4.3 | P | 2 | II | T3N0 | R | LAR | I | PT2N0 |
| 64 | F | Y | 5.0 | P | 2 | III | T2N+ | R | LAR | II | YPT3N0 |
| 65 | M | N | 5.0 | P | 2 | I | T2N0 | L | ULAR + CAA | I | PT2N0 |
| 37 | M | Y | 5.5 | P | 3 | III | T3N+ | L | ULAR + CAA | III | YPT3N2 |
| 71 | F | N | 5.6 | P | 2 | III | T2N+ | R | LAR | I | PT1N0 |
| 50 | M | Y | 5.8 | P | 3 | III | T3N+ | R | LAR | III | YPT2N2 |
| 40 | M | N | 6.0 | P | 2 | I | T2N0 | L | ULAR + CAA | I | PT2N0 |
| 56 | M | N | 6.0 | P | 2 | I | T2N0 | L | LAR | III | PT3N2 |
| 44 | M | Y | 6.1 | P | 2 | III | T3N+ | R | ULAR + CAA | III | YPT3N1 |
| 56 | M | Y | 6.3 | P | 2 | III | T3N+ | R | LAR | III | YPT3N1 |
Overall procedures for level I 4 (100%) patients had LAR, for level II 38 (90%) patients had LAR, 3 (7%) ULAR + CAA and 1 (2%) had Hartman procedure. For level III 3 (60%) patients had ULAR + CAA and 1 (20%) had LAR and APR each. For level IV 7 (77%) patients had APR and 2 (22%) had ULAR + ISR.
The overall kappa for all levels between surgeons and radiologist was 0.93 and confidence interval (CI: 0.87-0.99), which is indicating almost perfect agreement. The kappa for level I was 1 (100%), which is, indicate a perfect agreement between surgeons and radiologist. Regarding level II the kappa between surgeons was 1 (100%) but between surgeons and radiologist was 97.61% with overall average kappa of 0.98 (98.41%) and still indicates a perfect agreement. For level III the kappa between surgeons was 1 (100%) but between surgeons and radiologist was 80% with overall average kappa of 0.86 (86.66%), which is indicate a perfect agreement. For level IV the kappa was 1(100%) between all observers and indicates almost perfect agreement.
Our new rectal parameters and classification could provide a common understanding among individuals in a multidisciplinary team. Whenever the tumor level is identified, the most likely procedure and chemoradiation choice can be determine directly.
Regarding upper rectal cancer, the term “upper rectal cancer” does not indicate the location of the tumor above or below the peritoneal reflection, so decisions regarding chemoradiation therapy cannot be made based only on this term. Additionally, even with 11-15 cm, the peritoneal reflection is located at a variable distance from the anal verge from patient to patient, and many radiation oncologists do not recommend administration of radiation to tumors above the peritoneal reflection.
The Ra values (indicating tumors above the peritoneal reflection and that are level I) for both the Japanese and our classifications were similar, indicating a tumor above the peritoneal reflection that is less likely to be treated with radiation therapy unless a T4 lesion is evident. However, Ra defined as a tumor above peritoneal reflection. Some surgeons include those tumors with distal margin invading the peritoneal reflection, which might denial the use of chemoradition. Those tumor invading the peritoneal reflection considered high risk for recurrence, therefore; in our classification we include them in the level II.
Concerning middle rectal cancer, most advanced cases at this level would receive chemoradiation therapy. However, determining the exact distance of tumors located at 7-11 cm had no much impact on the surgical decision.
Regarding the Japanese and our classifications, level II was most similar to Rb, but we limited our level to tumors with distal margins above the levator ani insertion. We found that all patients at level II had LAR with or without chemoradiation.
Thus, all lesions at level II can be treated using LAR with or without chemoradiation. However, some lesions with invasion to other organs (T4) and not responding to chemoradition therapy should be treated using pelvic exenteration, if possible.
Regarding lower rectal cancer, most advanced cases at this level require chemoradiation for sphincter preservation and to reduce local recurrence. However, determining the exact distance of tumors located at 1-6 cm did not reflect the surgical procedure performed unless the surgeons were unfamiliar with sphincter preservation, in which case the patients underwent APR. Moreover, the distance of the levator ani insertion to the rectum differed among patients. Some patients with a tumor distance of 5 or 6 cm had LAR, whereas APR or CAA with or without ISR was performed in others. Those differences were due to the variability in the location of the levator ani. If the tumor is located at 5 or 6 cm above the levator ani, LAR is highly possible with stapler anastomosis. However, if the tumor is located at or below the levator ani, stapler anastomosis could not be performed, and perineal dissection was conducted instead.
In the Japanese classification, all tumors located at the anal canal are referred to as P. However, in our classification, we subdivided this area into two levels due to the variability of the technique: levels III and IV. Level III; in which tumors are located at the level of the levator ani or are invading it (level of the dentate line) require careful dissection to achieve safe circumferential margins. If there is no invasion to the levator ani, CAA with or without ISR is the treatment of choice; otherwise, partial (or tailored) levator excision or APR is used for invasive tumors. Additionally, this area is technically challenging due to the muscular structure and location of the anorectal ring.
For level IV, in which the distal margin of the tumor is below the levator ani (the tumor originates from the rectum but extends down to the anal canal), most early stage tumors can easily undergo CAA with or without ISR. However, in cases of sphincter invasion, APR is the procedure of choice. Level IV is not technically challenging and is easier than level III due to the proximity to the anal verge; additionally, circumferential margins can be achieved easily.
Thus, in tumors with a distal margin located at or below the level of levator ani, stapling anastomosis is less likely to be possible, and perineal phase dissection must be conducted whether hand-sewn anastomosis is performed with or without ISR, or with APR.
In our study, of 25 patients with a tumor located at 1-6 cm, 17 (68%) had a tumor located at 3-6 cm. Although the tumors were located below 6 cm (low rectal cancer), 11 (64%) were level II. Eight (72%) of these 11 tumors were treated using LAR, whereas the other three (27%) underwent hand-sewn CAA. Those three cases underwent colo-anal anastomosis directly because the surgeon did not attempt to locate the tumor intraoperatively after complete rectal mobilization.
After the localization of the levator ani and its relationship to the tumor has been determined, whether a procedure should be performed above (LAR with stabling anastomosis) or below (APR/CAA ± ISR) it can be determined.
Neoadjuvant chemoradiation therapy can change the tumor characteristics and might lead to tumor partial or complete response. Also it leads to increase the rate of sphincter saving procedure. In addition to that the tumor location can be changed. With all those factors we with Jung et al[27] and we need to go with tumor level to know which procedure will be suitable for each individual patient based on restaging MRI. Summary of the Management of Rectal Tumors: (1) for early stage lesions, either local excision is used or the guidelines of radical therapy are followed; (2) for radical resection of tumors at stages T1-3, N±: Level I: LAR, - chemoradiation; (3) for radical resection of tumors at stages T1-3, N±: Level II: LAR/± chemoradiation; (4) for radical resection of tumors at stages T1-3, N±: Level III: CAA ± ISR, partial levator ani or sphincter resection, APR/± chemoradiation; and (5) for radical resection of tumors at stages T1-3, N±: Level IV: APR or CAA ± ISR/± chemoradiation.
Management of rectal tumors differed among levels. However, our new radiological classification based on MRI facilitates determination of the most appropriate tumor management technique at each level of the rectum, and might increase communication among individuals in a multidisciplinary team. Evaluation of this classification in a prospective study is warranted.
Authors thank the research assistant Mr. Dong-Su Jang, Department of Anatomy, Yonsei University College of Medicine, Seoul, South Korea for his contribution in diagrammatic illustration.
P- Reviewer: Liu H, Li YM S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Schroen AT, Cress RD. Use of surgical procedures and adjuvant therapy in rectal cancer treatment: a population-based study. Ann Surg. 2001;234:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Beart RW, Steele GD, Menck HR, Chmiel JS, Ocwieja KE, Winchester DP. Management and survival of patients with adenocarcinoma of the colon and rectum: a national survey of the Commission on Cancer. J Am Coll Surg. 1995;181:225-236. [PubMed] |
| 3. | McArdle CS, Hole D. Impact of variability among surgeons on postoperative morbidity and mortality and ultimate survival. BMJ. 1991;302:1501-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 384] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Kelly JV, Hellinger FJ. Physician and hospital factors associated with mortality of surgical patients. Med Care. 1986;24:785-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Steele RJ. The influence of surgeon case volume on outcome in site-specific cancer surgery. Eur J Surg Oncol. 1996;22:211-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 1912] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 7. | Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1054] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 8. | MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1219] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 9. | MacDermid E, Hooton G, MacDonald M, McKay G, Grose D, Mohammed N, Porteous C. Improving patient survival with the colorectal cancer multi-disciplinary team. Colorectal Dis. 2009;11:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Du CZ, Li J, Cai Y, Sun YS, Xue WC, Gu J. Effect of multidisciplinary team treatment on outcomes of patients with gastrointestinal malignancy. World J Gastroenterol. 2011;17:2013-2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Brown G, Kirkham A, Williams GT, Bourne M, Radcliffe AG, Sayman J, Newell R, Sinnatamby C, Heald RJ. High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR Am J Roentgenol. 2004;182:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Iafrate F, Laghi A, Paolantonio P, Rengo M, Mercantini P, Ferri M, Ziparo V, Passariello R. Preoperative staging of rectal cancer with MR Imaging: correlation with surgical and histopathologic findings. Radiographics. 2006;26:701-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008;191:1827-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Memon S, Keating JP, Cooke HS, Dennett ER. A study into external rectal anatomy: improving patient selection for radiotherapy for rectal cancer. Dis Colon Rectum. 2009;52:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Benzoni E, Terrosu G, Bresadola V, Cerato F, Cojutti A, Milan E, Dado G, Bresadola F. Analysis of clinical outcomes and prognostic factors of neoadjuvant chemoradiotherapy combined with surgery: intraperitoneal versus extraperitoneal rectal cancer. Eur J Cancer Care (Engl). 2006;15:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Lahaye MJ, Lamers WH, Beets GL, Beets-Tan RGH. MR Anatomy of the rectum and the mesorectum. Benign Anorectal Diseases: Diagnosis with Endoanal and Endorectal Ultrasound and New Treatment Options. New York, NY: Springer 2006; 67-77. [DOI] [Full Text] |
| 17. | Rosenberg R, Maak M, Schuster T, Becker K, Friess H, Gertler R. Does a rectal cancer of the upper third behave more like a colon or a rectal cancer? Dis Colon Rectum. 2010;53:761-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Lewander A, Gao J, Adell G, Zhang H, Sun XF. Expression of NF-κB p65 phosphorylated at serine-536 in rectal cancer with or without preoperative radiotherapy. Radiol Oncol. 2011;45:279-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Mihaylova I, Parvanova V, Velikova C, Kurteva G, Ivanova D. Degree of tumor regression after preoperative chemo-radiotherapy in locally advanced rectal cancer-Preliminary results. Rep Pract Oncol Radiother. 2011;16:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Glynne-Jones R. Neoadjuvant treatment in rectal cancer: do we always need radiotherapy-or can we risk assess locally advanced rectal cancer better? Recent Results Cancer Res. 2012;196:21-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Ho ML, Liu J, Narra V. Magnetic resonance imaging of rectal cancer. Clin Colon Rectal Surg. 2008;21:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Shihab OC, How P, West N, George C, Patel U, Quirke P, Heald RJ, Moran BJ, Brown G. Can a novel MRI staging system for low rectal cancer aid surgical planning? Dis Colon Rectum. 2011;54:1260-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 329] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 24. | Gordon PH, Nivatvongs S. Principle and practice of surgery for the colon, rectum, and anus. 3rd ed. New York: Informa Health Care 2006; . |
| 25. | Townsend CM, Beauchamp RD, Evers BM, Mattox K. Textbook of surgery: The biologic basis of modern surgical practice. 17th ed. Phyladelphia: Elselvier Saunders 2004; . |
| 26. | Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal carcinoma. Tokyo: Kanehara Co., LTD 1997; p4-5. |
| 27. | Jung EJ, Ryu CG, Kim G, Kim SR, Nam SE, Park HS, Kim YJ, Hwang DY. Is rectal MRI beneficial for determining the location of rectal cancer with respect to the peritoneal reflection? Radiol Oncol. 2012;46:296-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |