Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.6008
Peer-review started: November 24, 2014
First decision: January 8, 2015
Revised: February 1, 2015
Accepted: April 3, 2015
Article in press: April 3, 2015
Published online: May 21, 2015
Processing time: 178 Days and 1.4 Hours
AIM: To investigate middle hepatic vein (MHV) management in adult living donor liver transplantation and safer remnant volumes (RV).
METHODS: There were 59 grafts with and 12 grafts without MHV (including 4 with MHV-5/8 reconstructions). All donors underwent our five-step protocol evaluation containing a preoperative protocol liver biopsy Congestive vs non-congestive RV, remnant-volume-body-weight ratios (RVBWR) and postoperative outcomes were evaluated in 71 right graft living donors. Dominant vs non-dominant MHV anatomy in total liver volume (d-MHV/TLV vs nd-MHV/TLV) was constellated with large/small congestion volumes (CV-index). Small for size (SFS) and non-SFS remnant considerations were based on standard cut-off- RVBWR and RV/TLV. Non-congestive RVBWR was based on non-congestive RV.
RESULTS: MHV and non-MHV remnants showed no significant differences in RV, RV/TLV, RVBWR, total bilirubin, or INR. SFS-remnants with RV/TLV < 30% and non-SFS-remnants with RV/TLV ≥ 30% showed no significant differences either. RV and RVBWR for non-MHV (n = 59) and MHV-containing (n = 12) remnants were 550 ± 95 mL and 0.79 ± 0.1 mL vs 568 ± 97 mL and 0.79 ± 0.13, respectively (P = 0.423 and P = 0.919. Mean left RV/TLV was 35.8% ± 3.9%. Non-MHV (n = 59) and MHV-containing (n = 12) remnants (34.1% ± 3% vs 36% ± 4% respectively, P = 0.148. Eight SFS-remnants with RVBWR < 0.65 had a significantly smaller RV/TLV than 63 non-SFS-remnants with RVBWR ≥ 0.65 [SFS: RV/TLV 32.4% (range: 28%-35.7%) vs non-SFS: RV/TLV 36.2% (range: 26.1%-45.5%), P < 0.009. Six SFS-remnants with RV/TLV < 30% had significantly smaller RVBWR than 65 non-SFS-remnants with RV/TLV ≥ 30% (0.65 (range: 0.6-0.7) vs 0.8 (range: 0.6-1.27), P < 0.01. Two (2.8%) donors developed reversible liver failure. RVBWR and RV/TLV were concordant in 25%-33% of SFS and in 92%-94% of non-SFS remnants. MHV management options including complete MHV vs MHV-4A selective retention were necessary in n = 12 vs n = 2 remnants based on particularly risky congestive and non-congestive volume constellations.
CONCLUSION: MHV procurement should consider individual remnant congestive- and non-congestive volume components and anatomy characteristics, RVBWR-RV/TLV constellation enables the identification of marginally small remnants.
Core tip: Prevention of liver failure in middle hepatic vein (MHV) inclusive right graft donors involves consideration of both congestive and non-congestive remnant volumes. MHV management should be individually based on MHV anatomy characteristics. Non-congestive volumes represent an important safety parameter in MHV management, especially in the setting of small for size remnants. The remnant-volume-body-weight ratios - remnant volumes/total liver volume constellation seems to have a synergistic (complementary) capacity for the identification of marginally small remnants with the highest risk potential of postoperative liver failure.
- Citation: Radtke A, Sgourakis G, Molmenti EP, Beckebaum S, Cicinnati VR, Schmidt H, Peitgen HO, Broelsch CE, Malagó M, Schroeder T. Risk of venous congestion in live donors of extended right liver graft. World J Gastroenterol 2015; 21(19): 6008-6017
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/6008.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.6008
The precise determination of graft and remnant volumes constitutes the most important parameter to prevent postoperative donor and recipient liver failure in adult live donor liver transplantation (ALDLT)[1-3]. Middle hepatic vein (MHV)-containing grafts are associated with small remnants whose function may be further impaired by early postoperative venous congestion of their medial sector (segment 4A/B)[1,4,5]. The occurrence of small-for-size syndrome (SFSS) in donors as a result of inadequate functional remnant volume is a constant reminder of the controversy surrounding venous congestion and MHV management. The commonly accepted definitions for small-for-size-(SFS)-remnants do not even consider remnant volume values[4,6-11]. To date, there are no published reports correlating the extent of functional impairment and parenchymal congestion in non-MHV containing remnants, and remnant volume limits for safe MHV inclusion with the right graft are still undefined.
In the present series, we evaluated our experience with liver failure in right graft donors. Our goal was to analyse the impact of MHV-containing right grafts on remnant volume (RV) and function. We considered the ratios remnant-volume-body-weight-ratio (RVBWR) and remnant volume percentage of total liver volume (RV/TLV) as a way to discriminate between SFS- and non-SFS remnants based on commonly accepted cut off values[4,8]. The following queries were addressed: (1) How concordant are these ratios in assessing SFS-remnants and determining their volume limits? (2) Is MHV procurement with right grafts associated with substantial loss of remnant volume? (3) Does inclusion of the MHV in right grafts impact remnant liver function and donor morbidity as a result of venous congestion? and (4) Does MHV anatomy affect venous outflow (= congestive volume) and thereby influence MHV management?
We finally considered “reasonable” criteria for procurement of right grafts with/without complete MHV vs selective MHV-4A preservation in remnants based both on our own experience with donors without evidence of steatosis as well as on that of others[4,6-8,11-16].
From January 2003 to October 2007, 71 consecutive live donors (36 females and 35 males, mean age 37 ± 10.1 years) underwent right graft hepatectomy at the University Hospital Essen, Germany. There were 59 grafts with and 12 grafts without MHV (including 4 with MHV-5/8 reconstructions). All donors underwent our five-step protocol evaluation containing a preoperative protocol liver biopsy as previously described[14,17]. Biopsy results in all resected donors showed less than 10% steatosis and no evidence of hepatopathologic changes.
Sixty eight out of 71 right graft recipients (28 females and 43 males, mean age 50 ± 11.0 years) suffered from liver cirrhosis classified for Child-A score; n = 22, Child-B score; n = 33, Child-C score; n = 13, while in the remaining n = 3 cases with no cirrhosis the indication for liver transplantation were neuroendocrine liver metastases (n = 2) as well as liver metastases from insulinoma (n = 1, Table 1). The overall “Model of End-Stage Liver Diesease”-score (MELD) was of mean of 14 ± 8 (range: 11-40).
| Parameter | Number |
| Total | 71 |
| Male | 43 |
| Female | 28 |
| Autoimmune hepatitis | 5 |
| Hepatitis B | 4 |
| Hepatitis B associated with hepatocellular carcinoma (HCC) | 7 |
| Hepatitis C | 8 |
| Hepatitis C associated with HCC | 10 |
| Alcoholic | 7 |
| Alcoholic + associated with HCC | 6 |
| Morbus Wilson | 2 |
| Primary biliary sclerosis (PBC) | 2 |
| Primary sclerosing cholangitis (PSC) | 7 |
| HCC | 4 |
| Cryptogenic | 6 |
| Others1 | 3 |
Computed tomography (CT) imaging was performed using a 16-row-Multidetector-CT-Scanner (Sensation16®, Siemens, Erlangen, Germany) as originally published by our group[18].
CT images were analyzed with the software assistant HepaVision® (MeVis institute, Bremen, Germany)[19,20].
RV: Congestive and non-congestive volumes.
Congestive volume: Venous congestion volume resulting from the detachment of left sided MHV-(4A/B) tributaries draining the left medial sector.
Non-congestive volume: Volume safely drained by the left hepatic vein (LHV) tributaries.
Congestive volume-index: Percentage of volume with venous congestion.
Donor RV/TLV: Remnant volume percentage of total liver volume considering the remnant volume with intact bi-sectorial venous outflow via the middle (MHV)- and LHV tributaries.
Donor RVBWR: (Safely drained by MHV and LHV) vs non-congestive RVBWR (safely drained by LHV) were calculated according to the Heinemann formula[9].
The “carving” transection plane followed the course of the MHV, exposing it on the resection surface of either graft (MHV-procurement) or remnant (MHV-retention) livers[21]. The MHV trunk served as a reproducible surgical landmark for the exact extrapolation (by means of color doppler scanning, IOUS) of the 3-D liver model onto the operative field.
We evaluated the correlation between RVBWR and RV/TLV as a way to distinguish between SFS- and non-SFS remnant status based on the following cut off values: SFS-remnant: RVBWR < 0.65 vs non-SFS-remnant RVBWR ≥ 0.65[8]. SFS-remnant: RV/TLV< 30% vs non-SFS-remnant RV/TLV ≥ 30%[4].
SFSS was defined as either poor initial remnant function or prolonged remnant dysfunction as a result of inadequate functional liver mass in the absence of other causative factors. This definition was based on criteria for both LDLT donors and recipients likewise tumor hepatectomy patients[22,23]. SFSS was characterised by the presence of at least two of the following symptoms within the first four post-operative weeks: encephalopathy (stage ≥ 2), progressive intrahepatic cholestasis [Bilirubin > 5.0 (reference value: 0.2-1.2)], prolonged severe coagulopathy (INR > 2.2), excessive intractable ascites (> 3 L/d).
Hepatic vein with the largest percentage of total liver volume (TLV) as originally classified by our group[20].
The non-parametric Sign test was used for two variables lacking normal distribution. The non-parametric Wilcoxon matched pairs test was applied to test the hypothesis that two variables (lacking normal distribution) were drawn from the same distribution. The Mann-Whitney U Test was used to test the significance of the difference between two independent samples of an ordinal variable as well as differences in the shape of the distributions (not just the location of the ranks) of the two groups. Significance was considered at a level < 0.05. Statistical release 7 (Statsoft) was used for statistical analysis.
Mean overall RV and RVBWR were 565 ± 97 mL and 0.79 ± 0.12, respectively. RV and RVBWR for non-MHV (n = 59) and MHV-containing (n = 12) remnants were 550 ± 95 mL and 0.79 ± 0.1 mL vs 568 ± 97 mL and 0.79 ± 0.13, respectively (P = 0.423 and P = 0.919, Mann Whitney U test).
Mean left RV/TLV was 35.8% ± 3.9%. Non-MHV (n = 59) and MHV-containing (n = 12) remnants (34.1% ± 3% vs 36% ± 4% respectively, P = 0.148 Mann Whitney U test) showed no significant differences.
We assessed the concordance between RVBWR < 0.65 and RV/TLV < 30% in all 71 right graft donors (Table 2).
| Remnant status defined by Rvbwr | Remnant status defined by Rv/tlv | ||
| SFS | Non SFS | SFS | Non SFS |
| RVBWR < 0.65 | RVBWR ≥ 0.65 | RV/TLV < 30% | RV/TLV ≥ 30% |
| n = 8 | n = 63 | n = 6 | n = 65 |
| RV/TLV < 30% | RV/TLV < 30% | RVBWR < 0.65 | RVBWR < 0.65 |
| Concordant | Non-concordant | Concordant | Non-concordant |
| 2/8 | 4/63 | 2/61 | 5/652 |
| 25% | 6% | 33% | 8% |
| RV/TLV ≥ 30% | RV/TLV ≥ 30% | RVBWR ≥ 0.65 | RVBWR ≥ 0.65 |
| Non-concordant | Concordant | Non-concordant | Concordant |
| 6/8 | 59/63 | 4/6 | 60/65 |
| 75% | 94% | 67% | 92% |
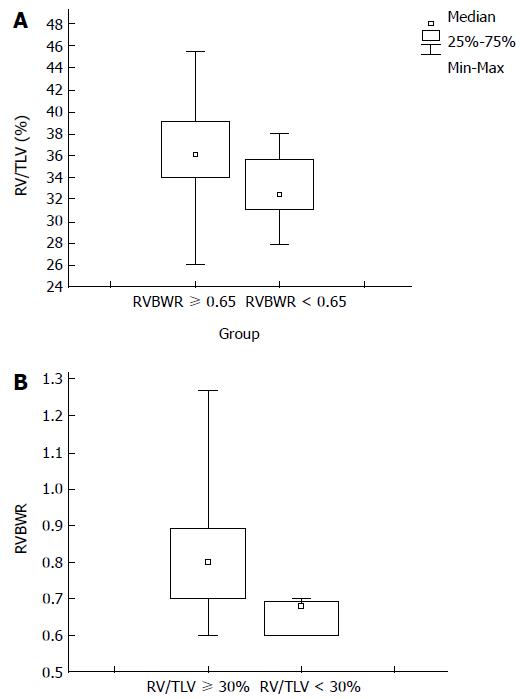
Twenty-five percent (n = 2/8) of SFS-remnants had RVBWR < 0.65 with RV/TLV < 30%. 94% (n = 59/63) of non-SFS-remnants had RVBWR ≥ 0.65 and RV/TLV ≥ 30%. Eight SFS-remnants with RVBWR < 0.65 had a significantly smaller RV/TLV than 63 non-SFS-remnants with RVBWR ≥ 0.65 [SFS: RV/TLV 32.4% (range: 28%-35.7%) vs non-SFS: RV/TLV 36.2% (range: 26.1%-45.5%), P < 0.009, Mann Whitney U test] Figure 1A.
Thirty-three percent (n = 2/6) of SFS-remnants had RV/TLV < 30% with RVBWR < 0.65. 92% (n = 60/65) of non-SFS-remnants had RV/TLV ≥ 30% and RVBWR ≥ 0.65. Six SFS-remnants with RV/TLV < 30% had significantly smaller RVBWR than 65 non-SFS-remnants with RV/TLV ≥ 30% [0.65 (range: 0.6-0.7) vs 0.8 (range: 0.6-1.27), P < 0.01, Mann Whitney U test] Figure 1B.
Mean overall congestive volume (CV) was 209.2 ± 77.6 mL (range: 40-459 mL) with a CV-index of 36.9 ± 11.6 %RV (range: 6.1-70.2 %RV). Mean non-congestive [safely drained by the left hepatic vein (LHV)] donor RVBWR (0.48 ± 0.12, range: 0.2-0.79) was significantly smaller than the corresponding donor RVBWR (safely drained by both MHV and LHV) (0.79 ± 0.12, range: 0.6-1.27, P < 0.0001, Wilcoxon’s signed ranks test).
Non-MHV containing remnants had a higher (although P values were very close) peak total bilirubin and INR than MHV-containing remnants (potentially suggesting a “negative effect” of venous congestion in the early postoperative liver function) (Table 3).
| Remnants | Remnants | Remnants | Remnants | |
| Peak (mean ± SD) | MHV | Non-MHV | RV/TLV ≥ 30% | RV/TLV < 30% |
| n = 12 | n = 59 | n = 65 | n = 6 | |
| Bilirubin (0.2-1.2) | 4.26 ± 2.86 | 5.54 ± 7.89 | 4.39 ± 3.92 | 5.1 ± 2.9 |
| P = 0.544 | P = 0.27 | |||
| INR (1.0) | 1.99 ± 0.52 | 2.15 ± 0.83 | 1.87 ± 0.44 | 2.02 ± 0.57 |
| P = 0.9 | P = 0.587 | |||
There were no donor deaths. Overall postoperative donor morbidity was 15.5% (n = 11), including 6 (8.4%) grade III-IV Dindo-Clavien complications[23]. There was no significant difference among remnants with (n = 3, 25%) or without (n = 8, 13.6%) MHV under their diverse volume conditions (P = 0.4077, chi-square). Five medical complications included: 2 pleural effusions (1 in an MHV- and 1 in a non-MHV remnant) requiring drainage (D-II), 1 pneumonia in a non-MHV remnant (D-II), and 2 reversible liver failures (D-IVA). Six surgical morbidities included 2 bile leaks (1 in a non-MHV- and 1 in an MHV remnant) associated with bilomas and treated with percutaneous drainage (D-IIIA), 1 IVC thrombosis treated surgically in a non-MHV remnant (D-IIIB), 1 subphrenic abscess drained operatively in a non MHV remnant (D-IIIB), and 2 superficial wound infections (D-IA).
Two (2.8%) donors developed reversible liver failure (see SFSS definition). Neither of them had a history of liver disease, experienced any adverse intraoperative events, or developed surgical/medical complications. Postoperative color doppler ultrasonography confirmed intact porto-arterial inflow and hepatic venous outflow.
Case-1: 40 year old female, BMI 26, liver biopsy < 10% steatosis, normal preoperative LFTs. MHV-containing remnant with safely (MHV + LHV)-drained-RVBWR of 0.63 (RV = 434 mL, RV/TLV = 35%). Postoperatively developed grade 2°encephalopathy, with peak Bilirubin of 26.5mg/dl and INR of 3.7. Recovered completely after two courses of plasmapheresis.
Case 2: 44-year-old male, BMI 27, liver biopsy < 10% steatosis, normal preoperative LFTs. Non-MHV-containing remnant with RVBWR of 0.65 (RV = 584 mL = RV/TLV 31%). RV safely drained by LHV of 344 mL (CV-index = 40.2%), with safely (LHV)-drained-RVBWR of 0.39. Postoperatively developed grade 2° encephalopathy, with peak bilirubin of 19.8 mg/dL and INR of 2.5. Recovered spontaneously after a hospital stay of 26 d.
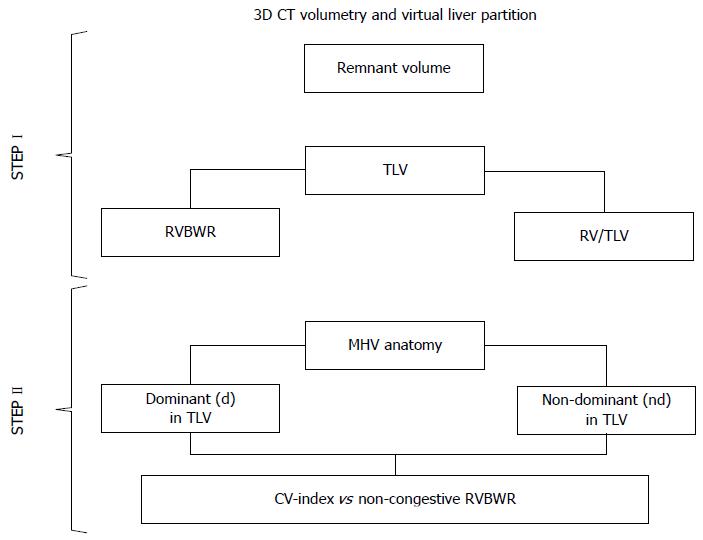
Our stepwise 3D-CT volumetry combined estimated left remnant congestive- and non-congestive volumes following virtual liver partition (Figure 2). Based on the experience of the Kyoto and Nagoya groups[12,13], the extremely low (25%-33%) concordance between donor RVBWR and RV/TLV, and the two reversible remnant liver failures in our series, we differentiated between right grafts inclusive of complete MHV and left remnants with selective MHV-4A retention by considering individual MHV anatomy patterns[16].
In 12 donors, the MHV was completely retained with the left remnants, providing an intact two-sectorial venous (MHV + LHV) drainage. In 10 cases, a risky dominant (d)-MHV type was preserved because of its particularly large congestive volume when compared to the non-dominant (nd)-MHV (d-MHV mean CV-index 41.2 ± 6.6 %RV vs nd-MHV mean CV-index 36.1 ± 12.2 %RV, P = 0.07, Mann-Whitney U test). In 2 donors with nd-MHV, the decision to retain the MHV with the left remnant was based on their small donor RVBWR-RV/TLV constellation (0.6/28.2% and 0.63/35%, Table 4).
| Donor | Remnant | Remnant | Remnant | Remnant | Remnant | Remnant CV-index | Remnant nc-RVBWR | SFSS |
| n = 71 | Total MHV | MHV-4A | volume | RV/TLV | RVBWR | (LHV) | ||
| preserved | preserved | |||||||
| 1 | Yes | Yes | 434 mL | 35% | 0.63 | 37.9% | 0.38 | Yes |
| 2 | No | No | 584 mL | 31% | 0.65 | 40.2% | 0.39 | Yes |
| 3 | No | No | 512 mL | 32.9% | 0.62 | 40.0% | 0.40 | No |
| 4 | No | No | 500 mL | 31.7% | 0.64 | 37.2% | 0.38 | No |
| 5 | No | Yes | 429 mL | 35.1% | 0.60 | 67.9% | 0.20 | No |
| 6 | No | No | 506 mL | 38% | 0.65 | 14.8% | 0.57 | No |
| 7 | No | Yes | 536 mL | 35.6% | 0.62 | 43.4% | 0.27 | No |
| 8 | No | No | 505 mL | 32% | 0.63 | 39.4% | 0.49 | No |
| 9 | No | No | 389 mL | 28% | 0.60 | 24.4% | 0.41 | No |
| 10 | Yes | Yes | 464 mL | 28.2% | 0.60 | 55.4% | 0.20 | No |
The left sided MHV-4A drainage territory was preserved in 4 of 59 donors who underwent procurement of MHV-containing grafts as originally described by our group[15]. This decision was based on an extremely small non-congestive-RVGWR (0.2-0.27) (safely drained by LHV) in 2 cases (Table 4) and on the anatomical characteristics of the MHV-4A/MHV-8 confluence into the MHV trunk in the other 2 instances.
Two (20%) of ten donors with estimated very small RVBWR ≤ 0.65 (inclusive of two with RV/TLV < 30%) developed reversible liver failure. The MHV was retained in two remnants (one with liver failure). Eight remnants (one with liver failure) had no MHV. In three non-liver failure remnants with extremely low non-congestive-RVBWR < 0.3 (safely drained by LHV), the MHV was completely retained or the MHV-4A drainage was preserved in the remnant liver (Table 4).
Although a RV/TLV of at least 30%-35% is usually required to avoid small-for-size syndrome (SFSS)[1,4,8], successful outcomes with RV/TLV < 30% have been reported in the setting of optimal liver quality[6,7,11,24]. Inclusion of the MHV with right grafts, which has been reported to optimize graft function[1,4,7,25] but to potentially impair remnant recovery[1,26-28], has both supporters and detractors[2,7,8,27,29-34]. Currently, many centres encourage a selective MHV management policy based on individual graft/remnant characteristics[4,7,12].
Our series allowed us to conclude that procurement of right grafts including complete MHV itself did not cause a significant volume loss in remnants. Indeed, there were no significant RV, RV/TLV and RVBWR differences between remnants with and without MHV. We attributed this result to our “carving” liver partitioning technique, in which the transection plane exposed the MHV trunk on the resection surface of either graft (MHV-harvest) or remnant (MHV-retention) livers. There was no difference in donor morbidity attributable to SFS-remnant-status or MHV inclusion (even with RVBWR and RV/TLV below the respective marginal limits of < 0.6 and < 30%).
Our overall donor morbidity of 15.5% including 8.4% of Dindo-Clavien III-IV type complications were comparable with the data reported in the literature[5,7,12].
In the Kyoto series overall 10% of donors suffered morbidity with similar incidence of complications who required treatment between (-) MHV (13%) vs (+) MHV (9%) remnants[12]. Comparable, in our donors the incidence of postoperative interventions did not considerably differ between the non-MHV (5.1%) and the MHV-contained (8.3%) remnants. In line with the cited reports, all our donors returned to their pre-donation lifestyles. The subgroup analysis of the Istambul series[7] revealed a much higher overall complication rate in non-MHV (22.4%) vs MHV-contained remnants (7.8%) mirroring our own experience with the tendency for (-) MHV remnants to have more complications than (+) MHV ones (25% vs14%).
Furthermore, our experience as well as that of others did not reveal any late complications attributable to remnant size or MHV-status[7,35]. Our virtual data however, showed that the “sacrifice” of the left sided MHV-4A/B drainage in remnants due to MHV inclusion with the graft was associated with large congestive volumes (CV-index of 36.9 ± 11.6 %RV) that resulted in a significant reduction of non-congestive volumes (non-congestive-RVBWR) safely drained by LHV. We also observed a potentially (not statistically significant) detrimental effect of venous congestion in non-MHV remnants as illustrated by their elevated liver function markers (INR, total bilirubin) in the early postoperative period. These observations strongly correlate with previous published reports.
In the study of the Clischy group[5] segment IV congestion was never seen on the postoperative CT in donors who underwent a standard MHV-exclusive right graft harvest, while in the setting of the extended right graft inclusive of MHV procurements 84% remnants revealed venous congestion with the morbidity rate of 37%. Yaprak et al[11] had observed that RV/TLV ≤ 30% impacted donor outcome (especially postoperative hyperbilirubinemia and major complications) irrespective of donor RVBWR (< 0.6 or > 0.6). In their experience, RVBWR < 0.6 significantly affected liver function but not donor morbidity. In a prospective study by Dayangac et al[7], procurement of right grafts inclusive of MHV was not associated with any additional donor risk except in SFS-remnants with RV/TLV < 30% (57% complication rate and prolonged postoperative hyperbilirubinemia). Others showed an association between small remnant volume and donor morbidity[1,11,36,37]. In our series the slightly higher bilirubin and INR levels in SFS-remnants probably resulted from a small RV.
The impact of remnant volume and remnant MHV-status on remnant regeneration has been extensively investigated. Belghiti et al[1] observed that a small RV accelerated early tissue regeneration, decreasing the proportion of functional liver tissue and increasing the risk of liver failure. Dayangac et al[7] showed that small non-MHV remnants had a significantly higher volume increase after the first postoperative week when compared to MHV remnants (76% and 50%, respectively). Similarly to our data, studies from several other groups showed that the volume regeneration rate of the total remnant liver (TLV) did not significantly differ among extended and regular right graft hepatectomies[7,38,39]. However, the observed compensatory lateral hyper-growth effect attributable to transient venous congestion in the MHV drainage area seems to reflect a competition between sectors, with the lateral one dominating regardless of remnant MHV status[40]. The development of a procoagulant state induced by the intense remnant regeneration as described by the Paris group might help explain the IVC thrombosis in one of our donors[1].
Our study revealed a very poor concordance between donor RVBWR and RV/TLV cut offs in SFS remnants (25%-33% in our series). Preoperative volume assessment based solely on RV/TLV can be misleading, particularly when compared to RVBWR of remnant volume and donor BMI[41]. RVBWR was also found to be more specific than RV/TLV as a predictor of postoperative outcomes in hepatic resections with SFS remnants[6]. Yigitler et al[42] observed a poor correlation between RV/TLV ≤ 30% and RVBWR < 0.6 for SFS remnants after major hepatic resections. In a retrospective analysis by Yaprak et al[11], remnants with marginal RVBWR < 0.6 and RV/TLV ≤ 30% constellation had the highest (52.2%) donor morbidity. However, their observation was not reproduced in our marginally small remnants.
Reversible liver failure occurred in MHV-inclusive as well as in non-MHV remnants with remnant volumes much above the commonly accepted limits (RVBWR 0.63-0.65 and RV/TLV 31%-35%). A retrospective analysis of virtual and clinical data confirming a non-steatosis in all donors on preoperative liver biopsy suggested that extensive venous congestion (CV-index of 40.2% RV) likely accounted for liver failure in case-2 [a non-MHV remnant with a tightly calculated functional reserve (non-congestive-RVBWR) of 0.39]. On the contrary, in case-1 (liver failure in an MHV-inclusive-remnant with intact bi-sectorial venous drainage via MHV + LHV, no plausible explanation could be found. A small-for-size syndrome (SFSS) is a multifactorial process primarily associated with insufficient functional liver mass that constitutes a life-threatening condition for both donors and recipients[22]. Although, a “safe” donor RVBWR-RV/TLV constellation seems to be the most effective parameter in donor selection and remnant MHV management, “liver quality” and “remnant volumes” are by no means dogmatic parameters[11,43]. The “venous congestion” and vice versa“non-congestive volume” association is potentially a strong additional factor[44].
The main goal of our study was to evaluate MHV management safety parameters to prevent life-threatening liver failure in MHV inclusive-right graft donors. As venous congestion in the drainage territories of MHV-4A/B branches can occur after procurement of right grafts containing MHV, congestive and non-congestive volume characteristics for each remnant should be carefully considered when making a decision on safe MHV management in donors.
Our study also showed that 10 of 12 retained MHV remnants had risky dominant d-MHV anatomy, with considerably large CV-index when compared to nd-MHV, that required complete preservation of the MHV in the left remnants. Based on our learning curve experience (including two lethal SFSS grafts[14] and two reversible SFSS remnants) and the experiences of other groups[12,13], we followed an “exclusion” scheme aimed at identifying high risk donors unsuitable for MHV-inclusive grafts. The main finding distinguishing our series from previous ones is that MHV inclusion with right grafts is not (by itself) associated with prohibitively small remnant volumes. We individualized MHV management by determining MHV-4A/B drained congestive and safely LHV drained non-congestive volume components.
All donors with (extremely small) non-congestive-RVBWR < 0.3 underwent successfully either complete MHV- or MHV-4A remnant-preserving right graft procurements. The two donors with reversible liver failure in our series portray an enormous risk potential. Further validation of our findings with a systematic prospective clinical study will be required.
Our final conclusions include: (1) prevention of liver failure in MHV inclusive right graft donors involves consideration of both congestive and non-congestive remnant volumes; (2) MHV management should be individually based on MHV anatomy characteristics; (3) non-congestive volumes represent an important safety parameter in MHV management, especially in the setting of SFS remnants; and (4) the RVBWR-RV/TLV constellation seems to have a synergistic (complementary) capacity for the identification of marginally small remnants with the highest risk potential of postoperative liver failure.
The accurate magnitude of graft and remnant volumes comprises the most critical parameter to preclude postoperative donor and recipient liver failure in adult live donor liver transplantation (ALDLT). Middle hepatic vein (MHV)-containing grafts are correlated with small remnants whose function may be further compromised by immediate postoperative venous congestion of their medial sector (segment 4A/B). The incident of small-for-size syndrome (SFSS) in donors as a result of ineffective functional remnant volume is a steady notice of the dispute encompassing venous congestion and MHV management.
Current virtual data, disclosed that the “sacrifice” of the left sided MHV-4A/B drainage in remnants due to MHV inclusion with the graft was related with large congestive volumes (CV-index of 36.9 ± 11.6 %RV) that gave rise to a significant reduction of non-congestive volumes (non-congestive-RVBWR) securely drained by LHV. The authors likewise noted a potentially harmful outcome of venous congestion in non-MHV remnants as demonstrated by their elevated liver function tests (INR, total bilirubin) in the early postoperative period.
As yet, there are no published studies connecting the magnitude of functional impairment and parenchymal congestion in non-MHV containing remnants, and remnant volume limits for secure MHV enclosure with the right graft are still indeterminate.
MHV management in adult live donor liver transplantation should be individually based on MHV anatomy characteristics.
This is a good paper, which investigates the outcome of RL-LDLT donors with remnant liver with or without the MHV trunk. It also analyzes the consistency between RVBWR and remnant volumes/total liver volume.
P- Reviewer: Vitale A, Wang WT, Zamani F S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Belghiti J, Liddo G, Raut V, Zappa M, Dokmak S, Vilgrain V, Durand F, Dondéro F. “Inherent limitations” in donors: control matched study of consequences following a right hepatectomy for living donation and benign liver lesions. Ann Surg. 2012;255:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Marcos A. Right lobe living donor liver transplantation: a review. Liver Transpl. 2000;6:3-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Mittler J, Pascher A, Jonas S, Pratschke J, Neumann UP, Langrehr JM, Neuhaus P. Adult living donor liver transplantation: living donation of the right liver lobe. Langenbecks Arch Surg. 2007;392:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Fan ST. Live donor liver transplantation in adults. Transplantation. 2006;82:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Scatton O, Plasse M, Dondero F, Vilgrain V, Sauvanet A, Belghiti J. Impact of localized congestion related to venous deprivation after hepatectomy. Surgery. 2008;143:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Chun YS, Ribero D, Abdalla EK, Madoff DC, Mortenson MM, Wei SH, Vauthey JN. Comparison of two methods of future liver remnant volume measurement. J Gastrointest Surg. 2008;12:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Dayangac M, Taner CB, Balci D, Memi I, Yaprak O, Akin B, Duran C, Killi R, Ayanoglu O, Yuzer Y. Use of middle hepatic vein in right lobe living donor liver transplantation. Transpl Int. 2010;23:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Fan ST, Lo CM, Liu CL, Yong BH, Chan JK, Ng IO. Safety of donors in live donor liver transplantation using right lobe grafts. Arch Surg. 2000;135:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 336] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Heinemann A, Wischhusen F, Püschel K, Rogiers X. Standard liver volume in the Caucasian population. Liver Transpl Surg. 1999;5:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317-1321. [PubMed] |
| 11. | Yaprak O, Guler N, Altaca G, Dayangac M, Demirbas T, Akyildiz M, Ulusoy L, Tokat Y, Yuzer Y. Ratio of remnant to total liver volume or remnant to body weight: which one is more predictive on donor outcomes? HPB (Oxford). 2012;14:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Asakuma M, Fujimoto Y, Bourquain H, Uryuhara K, Hayashi M, Tanigawa N, Peitgen HO, Tanaka K. Graft selection algorithm based on congestion volume for adult living donor liver transplantation. Am J Transplant. 2007;7:1788-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Kamei H, Fujimoto Y, Nagai S, Suda R, Yamamoto H, Kiuchi T. Impact of non-congestive graft size in living donor liver transplantation: new indicator for additional vein reconstruction in right liver graft. Liver Transpl. 2007;13:1295-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Radtke A, Sgourakis G, Molmenti EP, Beckebaum S, Cicinnati V, Broelsch CE, Peitgen HO, Malagó M, Schroeder T. Computer-assisted surgical planning in adult-to-adult live donor liver transplantation: how much does it help? A single center experience. Transplantation. 2012;94:1138-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Radtke A, Sgourakis G, Molmenti EP, Schroeder T, Cicinnati VR, Beckebaum S, Peitgen HO, Broelsch CE, Malagó M. The “carving” liver partitioning technique for graft hepatectomy in live donor liver transplantation: a single-center experience. Surgery. 2013;153:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Radtke A, Sotiropoulos GC, Sgourakis G, Molmenti EP, Schroeder T, Saner FH, Beckebaum S, Broelsch CE, Broering DC, Malago M. Hepatic venous drainage: how much can we learn from imaging studies? Anatomic-functional classification derived from three-dimensional computed tomography reconstructions. Transplantation. 2010;89:1518-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Valentín-Gamazo C, Malagó M, Karliova M, Lutz JT, Frilling A, Nadalin S, Testa G, Ruehm SG, Erim Y, Paul A. Experience after the evaluation of 700 potential donors for living donor liver transplantation in a single center. Liver Transpl. 2004;10:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Schroeder T, Nadalin S, Stattaus J, Debatin JF, Malagó M, Ruehm SG. Potential living liver donors: evaluation with an all-in-one protocol with multi-detector row CT. Radiology. 2002;224:586-591. [PubMed] |
| 19. | Fasel JH, Selle D, Evertsz CJ, Terrier F, Peitgen HO, Gailloud P. Segmental anatomy of the liver: poor correlation with CT. Radiology. 1998;206:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Radtke A, Schroeder T, Sotiropoulos GC, Molmenti E, Schenk A, Paul A, Nadalin S, Lang H, Saner F, Peitgen HO. Anatomical and physiological classification of hepatic vein dominance applied to liver transplantation. Eur J Med Res. 2005;10:187-194. [PubMed] |
| 21. | Malago M, Molmenti EP, Paul A, Nadalin S, Lang H, Radtke A, Liu C, Frilling A, Biglarnia R, Broelsch CE. Hepatic venous outflow reconstruction in right live donor liver transplantation. Liver Transpl. 2005;11:364-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Clavien PA, Oberkofler CE, Raptis DA, Lehmann K, Rickenbacher A, El-Badry AM. What is critical for liver surgery and partial liver transplantation: size or quality? Hepatology. 2010;52:715-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] |
| 24. | Liu CL, Fan ST. Adult-to-adult live-donor liver transplantation: the current status. J Hepatobiliary Pancreat Surg. 2006;13:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Malagó M, Testa G, Frilling A, Nadalin S, Valentin-Gamazo C, Paul A, Lang H, Treichel U, Cicinnati V, Gerken G. Right living donor liver transplantation: an option for adult patients: single institution experience with 74 patients. Ann Surg. 2003;238:853-862; discussion 862-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | de Villa VH, Chen CL, Chen YS, Wang CC, Lin CC, Cheng YF, Huang TL, Jawan B, Eng HL. Right lobe living donor liver transplantation-addressing the middle hepatic vein controversy. Ann Surg. 2003;238:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Fan ST, Lo CM, Liu CL, Wang WX, Wong J. Safety and necessity of including the middle hepatic vein in the right lobe graft in adult-to-adult live donor liver transplantation. Ann Surg. 2003;238:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Sano K, Makuuchi M, Miki K, Maema A, Sugawara Y, Imamura H, Matsunami H, Takayama T. Evaluation of hepatic venous congestion: proposed indication criteria for hepatic vein reconstruction. Ann Surg. 2002;236:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Akamatsu N, Sugawara Y, Kaneko J, Sano K, Imamura H, Kokudo N, Makuuchi M. Effects of middle hepatic vein reconstruction on right liver graft regeneration. Transplantation. 2003;76:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Cattral MS, Molinari M, Vollmer CM, McGilvray I, Wei A, Walsh M, Adcock L, Marks N, Lilly L, Girgrah N. Living-donor right hepatectomy with or without inclusion of middle hepatic vein: comparison of morbidity and outcome in 56 patients. Am J Transplant. 2004;4:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Chan SC, Lo CM, Liu CL, Wong Y, Fan ST, Wong J. Tailoring donor hepatectomy per segment 4 venous drainage in right lobe live donor liver transplantation. Liver Transpl. 2004;10:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Kasahara M, Takada Y, Fujimoto Y, Ogura Y, Ogawa K, Uryuhara K, Yonekawa Y, Ueda M, Egawa H, Tanaka K. Impact of right lobe with middle hepatic vein graft in living-donor liver transplantation. Am J Transplant. 2005;5:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Scatton O, Belghiti J, Dondero F, Goere D, Sommacale D, Plasse M, Sauvanet A, Farges O, Vilgrain V, Durand F. Harvesting the middle hepatic vein with a right hepatectomy does not increase the risk for the donor. Liver Transpl. 2004;10:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Yamamoto H, Maetani Y, Kiuchi T, Ito T, Kaihara S, Egawa H, Itoh K, Kamiyama Y, Tanaka K. Background and clinical impact of tissue congestion in right-lobe living-donor liver grafts: a magnetic resonance imaging study. Transplantation. 2003;76:164-169. [PubMed] |
| 35. | Sotiropoulos GC, Radtke A, Molmenti EP, Schroeder T, Baba HA, Frilling A, Broelsch CE, Malagó M. Long-term follow-up after right hepatectomy for adult living donation and attitudes toward the procedure. Ann Surg. 2011;254:694-700; discussion 700-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Breitenstein S, Apestegui C, Petrowsky H, Clavien PA. “State of the art” in liver resection and living donor liver transplantation: a worldwide survey of 100 liver centers. World J Surg. 2009;33:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Pomfret EA, Pomposelli JJ, Gordon FD, Erbay N, Lyn Price L, Lewis WD, Jenkins RL. Liver regeneration and surgical outcome in donors of right-lobe liver grafts. Transplantation. 2003;76:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Hata S, Sugawara Y, Kishi Y, Niiya T, Kaneko J, Sano K, Imamura H, Kokudo N, Makuuchi M. Volume regeneration after right liver donation. Liver Transpl. 2004;10:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Yokoi H, Isaji S, Yamagiwa K, Tabata M, Sakurai H, Usui M, Mizuno S, Uemoto S. Donor outcome and liver regeneration after right-lobe graft donation. Transpl Int. 2005;18:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Schenk A, Hindennach M, Radtke A, Malagó M, Schroeder T, Peitgen HO. Formation of venous collaterals and regeneration in the donor remnant liver: volumetric analysis and three-dimensional visualization. Transplant Proc. 2009;41:2515-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, Lerut J, Materne R, Wang X, Encarnacion A. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 465] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 42. | Yigitler C, Farges O, Kianmanesh R, Regimbeau JM, Abdalla EK, Belghiti J. The small remnant liver after major liver resection: how common and how relevant? Liver Transpl. 2003;9:S18-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Nagai S, Fujimoto Y, Kamei H, Nakamura T, Kiuchi T. Mild hepatic macrovesicular steatosis may be a risk factor for hyperbilirubinaemia in living liver donors following right hepatectomy. Br J Surg. 2009;96:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Satou S, Sugawara Y, Tamura S, Kishi Y, Kaneko J, Matsui Y, Kokudo N, Makuuchi M. Three-dimensional computed tomography for planning donor hepatectomy. Transplant Proc. 2007;39:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |