Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.5972
Peer-review started: December 21, 2014
First decision: January 8, 2015
Revised: January 21, 2015
Accepted: February 13, 2015
Article in press: February 13, 2015
Published online: May 21, 2015
Processing time: 150 Days and 20.3 Hours
AIM: To investigate remnant gastric cancer (RGC) at various times after gastrectomy, and lay a foundation for the management of RGC.
METHODS: Sixty-five patients with RGC > 2 years and < 10 years after gastrectomy (RGC I) and forty-nine with RGC > 10 years after gastrectomy (RGC II) who underwent curative surgery were enrolled in the study. The clinicopathologic factors, surgical outcomes, and prognosis were compared between RGC I and RGC II.
RESULTS: There was no significant difference in surgical outcomes between RGC I and RGC II. For patients reconstructed with Billroth II, significantly more patients were RGC II compared with RGC (71.9% vs 21.2%, P < 0.001), and more RGC II patients had anastomotic site locations compared to RGC I (31.0% vs 56.3%, P = 0.038). The five-year survival rates for the patients with RGC I and RGC II were 37.6% and 47.9%, respectively, but no significant difference was observed. Borrmann type and tumor stage were confirmed to be independent prognostic factors in both groups.
CONCLUSION: RGC II is located on the anastomotic site in higher frequency and more cases develop after Billroth II reconstruction than RGC I.
Core tip: This article is an important paper about clinicopathologic features of remnant gastric cancer (RGC) and the comparison of RGC with time interval of > 2 and ≤ 10 years (RGC I) after prior gastrectomy for gastric cancers. RGC after 10 years was easier to locate on the anastomotic site than RGC I. The predominant reconstruction type of the first operation is Billroth I for RGC I and Billroth II for RGC II. There may be different pathogeneses in different subgroups of RGC.
- Citation: Zhang DW, Dong B, Li Z, Dai DQ. Clinicopathologic features of remnant gastric cancer over time following distal gastrectomy. World J Gastroenterol 2015; 21(19): 5972-5978
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/5972.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.5972
Remnant gastric cancer (RGC) refers to a carcinoma detected in remnant stomach more than five years after primary surgery for a benign disease. Many studies have paid attention to this disease[1-4]. It has been reported to account for 2.4% to 5% of all gastric cancers[5,6]. In recent decades, due to improved outcomes of medical treatment for gastric or duodenum ulcer, the number of patients undergoing gastrectomy for benign disease has decreased by a wide margin. Hence, the incidence of RGC strictly according to the definition is on the downside.
On the contrary, the number of patients with RGC following gastrectomy for gastric cancer has progressively increased as a result of improved outcomes for patients with gastric cancer and the increasing proportion of patients diagnosed with early gastric cancer[7]. Recently, several reports have used RGC to define all cancers arising from the remnant stomach after partial gastrectomy, regardless of the initial disease[7-10]. Several studies evaluated the clinicopathologic characteristics and surgical outcomes of patients with RGC after gastric cancer and compared them with the RGC after benign disease[11-13], and observed no significant differences.
The preferred explanation for the pathogenesis of RGC is that Billroth II reconstruction produces a typical model of carcinogenesis. Gastroduodenal reflux and Helicobacter pylori colonization in the remnant stomach promote the development of RGC[14,15]. Because the gastric stump is constantly under carcinogenic influence, the time interval is one of the most important factors for the development of RGC. For the patients with RGC after benign disease, the average latency time is reported to be 20-27 years, and may go up to 40 years. Most authors have reported a steep increase in the risk of developing gastric stump cancer from the 20th year after the first gastrectomy[16-18]. Nevertheless, we consider that there maybe some differences in clinical pathology and prognosis between the RGC patients with a recurrence interval shorter than 10 years and those longer than 10 years.
In this study, we divided RGC following distal gastrectomy for gastric cancer into two subgroups: RGC I (2-10 years post-gastrectomy) and RGC II (> 10 years). The clinicopathologic features, type of operation, and the long-term survival results of the two subgroups were investigated and compared.
Sixty-five patients with RGC I and forty-nine with RGC II underwent treatment at the Department of Gastrointestinal surgery, cancer institute of China Medical University from January 1980 to December 2010. The patients whose cancers were detected in the distal stomach after proximal gastrectomy were excluded. To exclude the residual cancer in the initial surgery, the patients with time interval ≤ 2 years between the two cancers were excluded, though the proximal and distal resection margins were evaluated intraoperatively to confirm freedom from carcinoma at the initial surgery. Of them, ninety-five patients underwent surgical treatment and nineteen patients underwent non-surgical treatment for the distant metastasis or poor physical conditions. Seventy-four patients (RGC I: 42, RGC II: 32) underwent curative operation.
Clinicopathologic data were recorded based on the second English edition of the Japanese Classification System for Gastric Cancer, edited by the Japanese Gastric Cancer Association. The formalin-fixed specimens, containing the carcinoma lesions together with the surrounding gastric wall, were cut into multiple slices, principally parallel to the lesser curvature, at an interval of 5 mm. The hematoxylin and eosin-stained sections of tumor were initially examined independently by two pathologists and further confirmed by an additional expert pathologist for a final diagnosis. If there was disagreement in the diagnosis, the slides were rechecked by all three pathologists.
Clinicopathologic features, including age, sex, tumor location, tumor size, Borrmann type, histologic grade, Lauren grade, tumor stage, and type of operation, were investigated and compared between the two groups. The tumor locations of RGC were classified as anastomotic site, non-anastomotic site, and total stump. The total number of retrieved lymph nodes in all patients were > 15, so the tumors were staged according to the International Union Against Cancer (UICC, 7th edition) classification. Tumor histologic grade was classified into two groups: differentiated, which included papillary adenocarcinoma and moderately or well-differentiated adenocarcinoma, and undifferentiated, which included poorly or undifferentiated adenocarcinoma, mucinous carcinoma, and signet ring cell carcinoma. The type of the second resection included palliative resection, total gastrectomy, and extended gastrectomy. Extended gastrectomy included splenectomy, segmental T-colon resection, distal pancreatectomy, left lateral sectionectomy of liver, and diaphragm excision. For the patients with Billroth II reconstruction, the resection of segmental jejunum connected to the gastric stump was not recorded as extended gastrectomy.
All patients with RGC who underwent curative resection operations were followed-up. Patients were evaluated by chest radiograph, ultrasonography, abdominopelvic CT scan, serum tumor markers, and endoscopy to detect recurrence every 3 mo in the first two years. After the first two years, a telephone interview was conducted every 2 mo. Follow-up was complete for the entire study population to December 2013. At the end of follow-up, seven patients were lost. The rate of follow-up was 94.6%.
All the statistical analyses were performed by SPSS 16.0 statistical package (SPSS Inc., Chicago, IL, United States). Overall survival rates were determined using the Kaplan-Meier estimator method. The log-rank test was used to identify differences between the survivals of the two subgroups. In univariate analysis, two-tailed χ2 tests for categorical variables and 2 t tests for continuous variables were employed for statistical comparisons. In multivariate analysis, Cox’s proportional hazard model was used to identify independent factors correlated with prognosis. A P < 0.05 was considered statistically significant.
The statistical methods of this study were reviewed by Bo Qu, (professor and biostatistician), China Medical University.
There were 79 men and 35 women enrolled in the study with a mean age of 61 ± 12 years. The number of the patients with RGC according to the different periods and treatment type is shown in Table 1.
| Year | RGC I | RGC II | ||||
| Curative resection | PR or no resection | No operation | Curative operation | PR or no resection | No operation | |
| 1980-1990 | 6 | 6 | 5 | 3 | 3 | 4 |
| 1990-2000 | 12 | 3 | 3 | 11 | 2 | 2 |
| 2000-2010 | 22 | 4 | 2 | 18 | 3 | 3 |
| Total | 42 | 13 | 10 | 32 | 8 | 9 |
Of the 114 patients, 95 underwent surgical treatment. The types of surgery according to the different subgroups are listed in Table 2. A total of 74 patients (70.7%) received a curative resection. Because of peritoneal seeding, liver metastasis and serious adjacent organ invasion, 12 patients (RGC I: 8, RGC II: 4) underwent a palliative resection and 9 (RGC I: 5, RGC II: 4) patients underwent a non-resective operation such as bypass surgery or diagnostic laparotomy. In the RGC I group, 42 patients underwent curative resection.
| Parameters | RGC I | RGC II | P value |
| (n = 55) | (n = 40) | ||
| Type of surgery | 0.803 | ||
| No resection | 5 (9.1) | 4 (10.0) | |
| Bypass surgery | 2 | 3 | |
| Open biopsy only | 3 | 1 | |
| Palliative resection | 8 (14.5) | 4 (10.0) | |
| Curative resection | 42 (76.4) | 32 (80.0) | |
| Resection type | 0.189 | ||
| Resection of RG or gastrectomy | 30 | 26 | |
| Extended gastrectomy | 12 | 6 | |
| Splenectomy | 3 | 2 | |
| Whipple | 4 | 0 | |
| Segmental T-colon resection | 2 | 2 | |
| Distal pancreatectomy | 0 | 2 | |
| Left lateral sectionectomy of liver | 2 | 0 | |
| Diaphragm excision | 1 | 0 |
Clinicopathologic features of the 74 patients with curative resection are shown in Table 3. The second cancer was more frequently located on the anastomotic site in the patients with RGC II than that in the patients with RGC I (P = 0.038). Twenty-three patients with RGC II underwent Billroth II reconstruction in the first operation, which was significantly more than that in the patients with RGC I (P < 0.001). There were no significant differences in age, tumor size, Borrmann type, histologic grade, Lauren grade, or tumor stage between the two groups. The tumor stage of the initial cancer was only known for 33 patients with RGC I and 22 with RGC II because some clinical data were lost. There was a significant difference in the number of patients with stage III or IV initial cancers, observed in 60.6% of RGC I patients vs 22.7% of RGC II patients cases (P = 0.006).
| Parameters | RGC I | RGC II | P value |
| Sex | 0.805 | ||
| Male | 29 (69.0) | 21 (65.6) | |
| Female | 13 (31.0) | 11 (34.4) | |
| Age (yr), mean ± SD | 60.5 ± 11.6 | 62.3 ± 13.4 | 0.103 |
| Tumor size, cm | 0.301 | ||
| < 4.0 | 16 (38.1) | 15 (46.9) | |
| ≥ 4.0 | 26 (61.9) | 17 (53.1) | |
| Tumor location | 0.038 | ||
| Anastomotic site | 13 (31.0) | 18 (56.3) | |
| Non-anastomotic site | 19 (45.2) | 6 (18.7) | |
| Total stump | 10 (23.8) | 8 (25.0) | |
| Reconstruction of 1st operation | |||
| Billroth I | 31 (78.8) | 9 (28.1) | < 0.001 |
| Billroth II | 11 (21.2) | 23 (71.9) | |
| Borrmann type | 0.322 | ||
| I + II | 15 (35.7) | 14 (43.7) | |
| III + IV | 27 (64.3) | 18 (56.3) | |
| Histology grade | 0.421 | ||
| Differentiated | 19 (45.2) | 12 (37.5) | |
| Undifferentiated | 23 (54.8) | 20 (62.5) | |
| Lauren grade | 0.308 | ||
| Intestinal | 20 (47.6) | 18 (56.3) | |
| Diffuse | 22 (52.4) | 14 (43.7) | |
| Tumor stage | 0.106 | ||
| I + II | 15 (35.7) | 16 (50.0) | |
| III + IV | 27 (64.3) | 16 (50.0) | |
| 1st tumor stage | 0.006 | ||
| I + II | 13 (39.4) | 17 (77.3) | |
| III + IV | 20 (60.6) | 5 (22.7) |
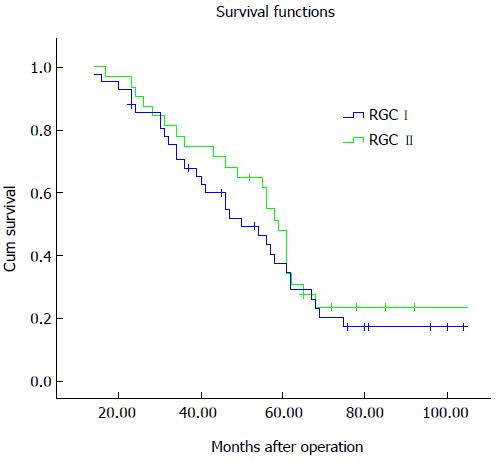
The overall five-year survival rate of patients with RGC was 43.6%. As shown in Figure 1, the five-year survival rates were 37.6% and 47.9% for the patients with RGC I and RGC II, respectively. Univariate analyses identified tumor size, Borrmann type, histologic grade, Lauren grade, and tumor stage as factors associated with prognosis (Table 4). In multivariate analysis, Borrmann type and tumor stage were confirmed as independent factors.
| Parameters | RGC I | RGC II | ||||
| 5-YSR | P value | 5-YSR | P value | |||
| (%) | UA | MA | (%) | UA | MA | |
| Sex | 0.574 | 0.179 | ||||
| Male | 31.9 | 51.0 | ||||
| Female | 51.9 | 43.6 | ||||
| Age, yr | 0.854 | 0.467 | ||||
| < 60 | 40.8 | 44.4 | ||||
| ≥ 60 | 34.2 | 49.2 | ||||
| Tumor size (cm) | 0.018 | 0.235 | 0.009 | 0.653 | ||
| < 4.0 | 59.8 | 60.0 | ||||
| ≥ 4.0 | 23.7 | 35.2 | ||||
| Reconstruction of 1st operation | 0.875 | 0.644 | ||||
| Billroth I | 34.4 | 44.4 | ||||
| Billroth II | 45.5 | 49.0 | ||||
| Borrmann type | 0.015 | 0.004 | 0.031 | 0.013 | ||
| I + II | 57.4 | 66.7 | ||||
| III + IV | 25.9 | 36.9 | ||||
| Histologic grade | 0.035 | 0.532 | 0.044 | 0.254 | ||
| Differentiated | 53.3 | 66.7 | ||||
| Undifferentiated | 24.3 | 36.9 | ||||
| Lauren grade | 0.026 | 0.108 | 0.032 | 0.321 | ||
| Intestinal | 56.9 | 55.6 | ||||
| Diffuse | 22.9 | 36.7 | ||||
| Tumor stage | 0.015 | < 0.001 | 0.009 | < 0.001 | ||
| I + II | 67.0 | 67.3 | ||||
| III + IV | 18.1 | 27.5 | ||||
Although the mortality of gastric cancer has substantially decline because of early diagnosis, radical surgery, and the development of adjuvant therapies, the deaths that do occur almost invariably follow tumor recurrence[19,20]. RGC following distal gastrectomy for gastric cancer is a frequent type of tumor recurrence. Operation is the most effective treatment for RGC. Some reports proposed different operative method for the curative resection of RGC[13,21]. We performed mainly total gastrectomy plus D2 lymphadenectomy and adjacent organ resection regardless of RGC I or RGC II. The reports concerning the rate of resectability for RGC have some discordant opinions. It had often been described as having low resectability rates (< 70%)[22,23]. It was also reported that the rate of curative resection was 60% in patients with RGC after benign disease, 78% in patients with RGC after gastric cancer, and 70% in total patients with RGC[24]. Our study reports higher rates, as the rate of curative resection was 76.4% for RGC I and 80.0% for RGC II, with an overall rate of 77.9%. The reason for this might be that some patients with distant metastasis or poor physical condition were recommended to undergo treatment in the department of medical oncology.
Some studies report a high rate (40%-70%) of combined organ resection, which was attributed it to the high incidence of adjacent organ invasion and the need for lymphadenectomy[25-27]. In the present study, although the combined resection of involved organs was more frequently selected in patients with RGC I than those in the patients with RGC II (25.8% vs 12.5%; P = 0.189), the rates are much lower than what has been reported. This might be induced by the different standard in the preoperational assessment. In this investigation, Whipple resection was conducted in four patients with RGC I, but not in patients with RGC II. This might account for the difference of the adjacent organs, which could be attributed to different reconstruction type.
According to a previous study, gastritis cystica polyposa, which was suspected to be of great relevance to cancer development in the remnant stomach, was detected more often on the anastomotic site, and more often in patients with Billroth II reconstruction than in those with Billroth I[24]. These suggest that long-term exposure of the gastric mucosa to duodenal contents is one of the major causes of RGC after distal gastrectomy and the anastomotic site, which is the most direct site for this disadvantage, and is understandably the most common location for the RGC. However, the time interval from the first resection to development of RGC varied to a great extent. The average latency time was reported to be 20-27 years, and up to 40 years. As the risk of developing gastric stump cancer increases after 20 years[16-18], the time interval appears to be one of the most important factors for the development of RGC. A reason for this is because the remnant stomach is constantly under carcinogenic influence. This is only applicable for RGC after benign disease, as the anastomotic site of Billroth II reconstruction is frequently involved by RGC 15 years or more after the first gastrectomy for benign disease[4,28]. In the present study, more than half of RGC following gastric cancer surgery developed less than 10 years after the first operation. The time interval did not seem to be enough for the development of RGC. Meanwhile, RGC I was more frequently located on the non-anastomotic site and more cases of them developed after Billroth I reconstruction. Thus, we infer that there might be some different pathogeneses for RGC I. It is well known that mucosal changes, such as atrophic gastritis and intestinal metaplasia, are often observed in tumor-adjacent tissues. Denervation during initial gastric cancer surgery might damage the defense mechanisms of the gastric mucosa, which facilitates the development of cancer from precancerous lesions[29]. We therefore also infer that preexisting mucosal changes in the remnant stomach, such as atrophic gastritis and intestinal metaplasia, rather than gastroduodenal reflux, are more relevant to the development of RGC I. On the other hand, RGC I was more common in patients with a more advantaged tumor stage of the first cancer. This may suggest that the development of RGC I is be due to residual carcinomas ignored at the initial operation. Although resection margins are deemed histologically free of tumor involvement at initial operation, tumor cells still may remain in the remnant stomach.
It has been reported that the incidence of synchronous multiple gastric cancers are approximately 4%-7% of surgically resected cases[30]. One study conducted a comprehensive evaluation of serial sections from the entire stomach and found an incidence of 13.2%-14.6%, suggesting a higher incidence of latent lesions[30]. According to another previous study[31], patients with multiple gastric cancers are more likely to develop secondary gastric cancers in the remnant stomach. All the above suggest that RGC II after Billroth II reconstruction might develop from a typical model of carcinogenesis for gastroduodenal reflux. However, preexisting mucosal changes or residual carcinomas ignored at initial treatment might be more responsible for RGC I . In patients whose re-oncogenesis locations were non-anastomotic sites and total stump, we did not observe other sites of re-oncogenesis via preoperative gastroscopy and intraoperative check, therefore, we surmise that the re-oncogenesis locations were associated with background mucosal change. Moreover, curative resection with a safe margin (all the margins have been proven postoperatively to be safe or called “negative margin”) was achieved in all 42 of these patients. Thus, more studies should be conducted to find the most possible reason for their early re-oncogenesis after prior gastrectomy.
In conclusion, there is no significant difference in surgical outcomes and prognosis between RGC I and RGC II. However, in clinicopathologic features, RGC II is more frequently located on the anastomotic site and more cases develop after Billroth II reconstruction. Therefore, we propose that gastroduodenal reflux might induce the development of RGC II. However, the development of RGC I could also be attributed to preexisting mucosal changes or residual carcinomas ignored at initial treatment for synchronous multiple gastric cancers.
We are grateful to all the previous study authors and the study participants. We thank Professor Bo Qu, who helps us with the statistical methods of this study.
The time interval between the primary cancer and the remnant gastric cancer (RGC) in patients is a key point in the definition of RGC. Several reports have used RGC to define all cancers arising from the remnant stomach after partial gastrectomy, regardless of the initial disease.
The preferred explanation for the pathogenesis of RGC is that Billroth II reconstruction produces a typical model of carcinogenesis. Gastroduodenal reflux and Helicobacter pylori colonization in the remnant stomach promote the development of RGC. Because the gastric stump is constantly under carcinogenic influence, the time interval is one of the most important factors for the development of RGC.
Most authors have reported a steep increase in the risk of developing gastric stump cancer from the 20th year after the first gastrectomy. However, the authors consider that there may be some differences in clinical pathology and prognosis between the RGC patients with a recurrence interval shorter than 10 years and those longer than 10 years. The authors found that RGC that occurs after > 10 years is more frequently located on the anastomotic site and more cases develop after Billroth II reconstruction. Therefore, the authors suggest that gastroduodenal reflux induces the development of these cases of RGC.
The development of RGC within 10 years after gastrectomy may result from preexisting mucosal changes or residual carcinomas ignored at initial treatment for synchronous multiple gastric cancers. This study may help to lay the foundation of the management of RGC.
RGC refers to a carcinoma detected in remnant stomach more than five years after primary surgery for a benign disease.
The study is done carefully. This article is an important paper concerning clinicopathologic features of RGC. Original point of this paper is the comparison of RGC with time interval of > 2 and ≤ 10 years after prior gastrectomy for gastric cancers.
P- Reviewer: Chen L, Umemura A S- Editor: Qi Y L- Editor: AmEditor E- Editor: Liu XM
| 1. | Takeno S, Hashimoto T, Maki K, Shibata R, Shiwaku H, Yamana I, Yamashita R, Yamashita Y. Gastric cancer arising from the remnant stomach after distal gastrectomy: a review. World J Gastroenterol. 2014;20:13734-13740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Sasaki K, Fujiwara Y, Kishi K, Motoori M, Yano M, Ohigashi H, Ohue M, Noura S, Maruhashi S, Takahashi H. Pathological findings of gastric mucosa in patients with gastric remnant cancer. Hepatogastroenterology. 2014;61:251-254. [PubMed] |
| 3. | Komatsu S, Ichikawa D, Okamoto K, Ikoma D, Tsujiura M, Nishimura Y, Murayama Y, Shiozaki A, Ikoma H, Kuriu Y. Progression of remnant gastric cancer is associated with duration of follow-up following distal gastrectomy. World J Gastroenterol. 2012;18:2832-2836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Thorban S, Böttcher K, Etter M, Roder JD, Busch R, Siewert JR. Prognostic factors in gastric stump carcinoma. Ann Surg. 2000;231:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Fischer AB. Twenty-five years after Billroth II gastrectomy for duodenal ulcer. World J Surg. 1984;8:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Nicholls JC. Stump cancer following gastric surgery. World J Surg. 1979;3:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Ohashi M, Katai H, Fukagawa T, Gotoda T, Sano T, Sasako M. Cancer of the gastric stump following distal gastrectomy for cancer. Br J Surg. 2007;94:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Irino T, Hiki N, Nunobe S, Ohashi M, Tanimura S, Sano T, Yamaguchi T. Subtotal gastrectomy with limited lymph node dissection is a feasible treatment option for patients with early gastric stump cancer. J Gastrointest Surg. 2014;18:1429-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Nozaki I, Hato S, Kobatake T, Ohta K, Kubo Y, Nishimura R, Kurita A. Incidence of metachronous gastric cancer in the remnant stomach after synchronous multiple cancer surgery. Gastric Cancer. 2014;17:61-66. [PubMed] |
| 10. | Inomata M, Shiraishi N, Adachi Y, Yasuda K, Aramaki M, Kitano S. Gastric remnant cancer compared with primary proximal gastric cancer. Hepatogastroenterology. 2003;50:587-591. [PubMed] |
| 11. | Ahn HS, Kim JW, Yoo MW, Park do J, Lee HJ, Lee KU, Yang HK. Clinicopathological features and surgical outcomes of patients with remnant gastric cancer after a distal gastrectomy. Ann Surg Oncol. 2008;15:1632-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Takeno S, Noguchi T, Kimura Y, Fujiwara S, Kubo N, Kawahara K. Early and late gastric cancer arising in the remnant stomach after distal gastrectomy. Eur J Surg Oncol. 2006;32:1191-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Isozaki H, Tanaka N, Fujii K, Nomura E, Tanigawa N. Surgical treatment for advanced carcinoma of the gastric remnant. Hepatogastroenterology. 1998;45:1896-1900. [PubMed] |
| 14. | Lorusso D, Linsalata M, Pezzolla F, Berloco P, Osella AR, Guerra V, Di Leo A, Demma I. Duodenogastric reflux and gastric mucosal polyamines in the non-operated stomach and in the gastric remnant after Billroth II gastric resection. A role in gastric carcinogenesis? Anticancer Res. 2000;20:2197-2201. [PubMed] |
| 15. | Seoane A, Bessa X, Alameda F, Munné A, Gallen M, Navarro S, O’Callaghan E, Panadès A, Andreu M, Bory F. Role of Helicobacter pylori in stomach cancer after partial gastrectomy for benign ulcer disease. Rev Esp Enferm Dig. 2005;97:778-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Lacaine F, Houry S, Huguier M. Stomach cancer after partial gastrectomy for benign ulcer disease. A critical analysis of epidemiological reports. Hepatogastroenterology. 1992;39:4-8. [PubMed] |
| 17. | Toftgaard C. Gastric cancer after peptic ulcer surgery. A historic prospective cohort investigation. Ann Surg. 1989;210:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Sinning C, Schaefer N, Standop J, Hirner A, Wolff M. Gastric stump carcinoma - epidemiology and current concepts in pathogenesis and treatment. Eur J Surg Oncol. 2007;33:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 19. | Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 554] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 20. | Varadhachary G, Ajani JA. Gastric cancer. Clin Adv Hematol Oncol. 2005;3:118-124. [PubMed] |
| 21. | Kunisaki C, Shimada H, Nomura M, Hosaka N, Akiyama H, Ookubo K, Moriwaki Y, Yamaoka H. Lymph node dissection in surgical treatment for remnant stomach cancer. Hepatogastroenterology. 2002;49:580-584. [PubMed] |
| 22. | Firat O, Guler A, Sozbilen M, Ersin S, Kaplan H. Gastric remnant cancer: an old problem with novel concerns. Langenbecks Arch Surg. 2009;394:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | An JY, Youn HG, Ha TK, Choi MG, Kim KM, Noh JH, Sohn TS, Kim S. Clinical significance of tumor location in remnant gastric cancers developed after partial gastrectomy for primary gastric cancer. J Gastrointest Surg. 2008;12:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Tanigawa N, Nomura E, Niki M, Shinohara H, Nishiguchi K, Okuzawa M, Toyoda M, Morita S. Clinical study to identify specific characteristics of cancer newly developed in the remnant stomach. Gastric Cancer. 2002;5:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Park DJ, Lee HJ, Kim HH, Yang HK, Lee KU, Choe KJ. Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg. 2005;92:1099-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Lehnert T, Rudek B, Buhl K, Golling M. Surgical therapy for loco-regional recurrence and distant metastasis of gastric cancer. Eur J Surg Oncol. 2002;28:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Chen L, Tian H, Chen J, He ZG, Tao SF, Lokesh G, Peng SY. Surgical management of gastric stump cancer: a report of 37 cases. J Zhejiang Univ Sci B. 2005;6:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Di Leo A, Pedrazzani C, Bencivenga M, Coniglio A, Rosa F, Morgani P, Marrelli D, Marchet A, Cozzaglio L, Giacopuzzi S. Gastric stump cancer after distal gastrectomy for benign disease: clinicopathological features and surgical outcomes. Ann Surg Oncol. 2014;21:2594-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Kaminishi M, Shimizu N, Yamaguchi H, Hashimoto M, Sakai S, Oohara T. Different carcinogenesis in the gastric remnant after gastrectomy for gastric cancer. Cancer. 1996;77:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Yamagiwa H, Yoshimura H, Matsuzaki O, Ishihara A. Pathological study of multiple gastric carcinoma. Acta Pathol Jpn. 1980;30:421-426. [PubMed] |
| 31. | Fujita T, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kinoshita T. Relationship between the histological type of initial lesions and the risk for the development of remnant gastric cancers after gastrectomy for synchronous multiple gastric cancers. World J Surg. 2010;34:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |