Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.5941
Peer-review started: December 24, 2014
First decision: January 8, 2014
Revised: January 16, 2015
Accepted: February 13, 2015
Article in press: February 13, 2015
Published online: May 21, 2015
Processing time: 152 Days and 1.1 Hours
AIM: To compare safety and therapeutic efficacy of laparoscopic radiofrequency (RF) ablation vs computed tomography (CT)-guided RF ablation for large hepatic hemangiomas abutting the diaphragm.
METHODS: We retrospectively reviewed our sequential experience of treating 51 large hepatic hemangiomas abutting the diaphragm in 51 patients by CT-guided or laparoscopic RF ablation due to either the presence of symptoms and/or the enlargement of hemangioma. Altogether, 24 hemangiomas were ablated via a CT-guided percutaneous approach (CT-guided ablation group), and 27 hemangiomas were treated via a laparoscopic approach (laparoscopic ablation group).
RESULTS: The mean diameter of the 51 hemangiomas was 9.6 ± 1.8 cm (range, 6.0-12.0 cm). There was no difference in the diameter of hemangiomas between the two groups (P > 0.05). RF ablation was performed successfully in all patients. There was no difference in ablation times between groups (P > 0.05). There were 23 thoracic complications in 17 patients: 15 (62.5%, 15/24) in the CT-guided ablation group and 2 (7.4%, 2/27) in the laparoscopic ablation group (P < 0.05). According to the Dindo-Clavien classification, two complications (pleural effusion and diaphragmatic rupture grade III) were major in two patients. All others were minor (grade I). Both major complications occurred in the CT-guided ablation group. The minor complications were treated successfully with conservative measures, and the two major complications underwent treatment by chest tube drainage and thoracoscopic surgery, respectively. Complete ablation was achieved in 91.7% (22/24) and 96.3% (26/27) in the CT-guided and the laparoscopic ablation groups, respectively (P > 0.05).
CONCLUSION: Laparoscopic RF ablation therapy should be used as the first-line treatment option for large hepatic hemangiomas abutting the diaphragm. It avoids thermal injury to the diaphragm and reduces thoracic complications.
Core tip: Radiofrequency (RF) ablation is an accepted non-surgical treatment for hepatic hemangiomas. If a tumor is located in the hepatic dome which abuts the diaphragm, complete tumor ablation without injury to the diaphragm or lung is challenging under percutaneous approach. The study preliminarily proved that laparoscopic RF ablation therapy should be used as the first-line treatment option for hepatic hemangiomas abutting the diaphragm, which can avoid thermal injury to the diaphragm effectively and reduce the thoracic complications obviously.
-
Citation: Gao J, Kong J, Ding XM, Ke S, Niu HG, Xin ZH, Ning CM, Guo SG, Li XL, Zhang L, Dong YH, Sun WB. Laparoscopic
vs computerized tomography-guided radiofrequency ablation for large hepatic hemangiomas abutting the diaphragm. World J Gastroenterol 2015; 21(19): 5941-5949 - URL: https://www.wjgnet.com/1007-9327/full/v21/i19/5941.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.5941
Hepatic hemangiomas are the most common benign tumors affecting the liver. They occur in the general population at an incidence ranging from 0.4% to 20%. In most cases, they are discovered incidentally on abdominal imaging studies[1,2]. As most hepatic hemangiomas are < 5 cm in diameter and asymptomatic, medical or surgical intervention is not necessary. Treatment is required, however, if the tumor is growing and manifests abdominal symptoms or if rupture has occurred (or may occur in the future)[2-5]. Although surgical resection is the most effective treatment for symptomatic, enlarging hepatic hemangiomas, it is a highly invasive procedure associated with morbidity and mortality rates of up to 27% and 3%, respectively[6-9]. Alternatively, minimally invasive procedures of transcatheter arterial embolization or radiation therapy may be used, but these treatments are not curative[10-13].
Radiofrequency (RF) ablation is an effective, minimally invasive, safe treatment for hepatic hemangiomas > 5 cm[14-20]. On the basis of the accumulated experience with treating hepatic hemangiomas by RF ablation and the development of RF equipment for this procedure, this therapy has also been performed successfully for those larger than > 10 cm[21-23].
The RF technique can be performed percutaneously, laparoscopically, or as an open procedure. Each approach has theoretical and proven advantages and disadvantages, which can lead to confusion and inefficiencies in the referral process for the patients. The percutaneous approach can be performed under general or local anesthesia as an outpatient procedure, which has the advantage of not requiring a surgical procedure. However, lesions located in the dome of the liver abutting the diaphragm or close to the stomach or colon are not always accessible by a percutaneous approach because of the risk of injuring adjacent organs. The laparoscopic approach has the advantage of being minimally invasive while providing access for intraoperative ultrasonography (US) examination of the liver for better lesion detection and more accurate targeting, although it requires a high level of skill. The laparoscopic approach is also indicated when the tumor is adherent to structures that may be damaged by thermal ablation such as the colon, stomach, or duodenum[24].
If a tumor is located in the hepatic dome, which abuts the diaphragm, complete tumor ablation without injury to the diaphragm or lung is challenging using the percutaneous approach[25-29]. In contrast, using the laparoscopic approach, establishment of pneumoperitoneum causes elevation of the diaphragm, which increases the operative space and avoids injuring the diaphragm. It also facilitates needle placement. We therefore hypothesized that the risk of thoracic complications would be reduced during RF ablation for large hepatic hemangiomas abutting the diaphragm under the laparoscopic approach.
To our knowledge, there are no published comparative controlled studies evaluating the protective effect on the diaphragm or therapeutic efficacy of various approaches to RF ablation for large hepatic hemangiomas abutting the diaphragm. This study aimed to evaluate the protective effect on the diaphragm and the therapeutic efficacy of a laparoscopic vs a computed tomography (CT)-guided percutaneous approach to RF ablation for large hepatic hemangiomas.
We retrospectively reviewed the records of consecutive patients with hepatic hemangiomas abutting the diaphragm whom we had treated by CT-guided or laparoscopic RF ablation from October 2011 to May 2014. The following hospitals in China participated in the study: Beijing Chaoyang Hospital Affiliated to Capital Medical University, Beijing, China; Affiliated Hospital of Chifeng University, Neimenggu, China; Chaoyang Central Hospital, Liaoning, China; Shanxi Provincial People’s Hospital, Shanxi, China; Fenyang Hospital, Shanxi, China; and Zhanhua People’s Hospital, Shandong, China.
From October 2011 to May 2014, a total of 9978 patients were diagnosed with hepatic hemangioma in the outpatient clinics of the six hospitals. Among them, 1025 patients suffered from large hepatic hemangiomas (≥ 5 cm). All were initially managed by clinical observation. Over time, 187 of these patients were considered candidates for active treatment because of the following indications[20]: (1) the patient complained of persistent abdominal pain or discomfort related to the hemangioma. Upper gastrointestinal endoscopy and colonoscopy were performed to rule out any potential gastrointestinal diseases that might be causing these symptoms; and (2) the lesion increased in size by > 1 cm, which was confirmed on regular follow-up imaging studies during a 2-year observation period. On the basis of the accumulated experience of treating large hepatic hemangiomas by RF ablation[20,22], we treated these patients with hepatic hemangiomas using RF ablation as the first-line treatment.
Of the 187 patients, 51 patients with 51 hepatic hemangiomas abutting the diaphragm were included in the study. We defined tumors abutting the diaphragm when they were located near the diaphragm (< 5 mm) on axial CT scans[27,28]. All tumors in this study were mainly located in liver segment 4, 5, 7, or 8.
The staff team consisted of hepatobiliary surgeons, anesthesiologists, and radiologists. For the fact that intraoperative US guidance was available in Beijing Chao-yang Hospital, the patients in this hospital were treated under laparoscopic approach. Whereas, intraoperative US guidance was not available in the other 5 hospitals, so the patients there were treated under CT-guided percutaneous approach. The same experienced operator (Sun WB) performed all of the procedures, using internally cooled cluster electrodes, Cool-tip ACTC 2025 or ACTC 1525 electrodes, and an RF generator (Covidien Healthcare, Dublin, Ireland). Out of 51 hemangiomas, 27 were treated by laparoscopic RF ablation (laparoscopic ablation group), and 24 were treated by CT-guided percutaneous RF ablation (CT-guided ablation group).
Local review boards approved the study. Written informed consent was obtained from each patient before the treatment.
For laparoscopic RF ablation, after induction of general anesthesia, patients were placed in a supine position. Two 10-mm trocars were placed in the abdomen, and initial laparoscopic exploration of the peritoneal cavity was performed. Under US guidance, the RF probe was introduced into the peritoneal cavity through the subcostal abdominal wall with laparoscopic visualization and deployed into the tumor. The ablation strategies were described in our previously published article[22]. The RF process was monitored by intraoperative US. The ablated lesion became hyperechoic because of outgassing from heated tissues.
For the CT-guided RF procedure, a 35F or 37F left-side double-lumen endobronchial tube was intubated under general anesthesia. The tube position was checked and confirmed by auscultation or by fiberoptic bronchoscopy. The right lung was permitted to collapse, with selective left lung ventilation. The skin entrance point of the RF probe was chosen in the CT scanning plane containing the tumor. With CT monitoring, the RF probe was inserted through the chest wall and then through the empty pleural space and the diaphragm to the liver, finally reaching the targeted tumor. After the position of the probe was confirmed to be appropriate by CT, RF procedures were performed in a manner similar to that used for the laparoscopic procedure.
One day after ablation, all patients were evaluated on lung CT scans, which were repeated on the following day if necessary. All patients were followed by enhanced CT or magnetic resonance imaging (MRI) 1 mo after ablation. Complete ablation was defined as no nodular or irregular enhancement adjacent to the ablated zone, as shown on enhanced CT or MRI scans. Incomplete ablation was defined as irregular, peripheral-enhanced foci in the ablated zone. In the case of complete ablation, subsequent CT or MRI examinations were repeated at 6-mo intervals. In the case of incomplete ablation, repeat RF ablation was not performed unless the residual tumor had progressed during follow-up at 6-mo intervals.
Primary endpoints of the study were technical success, safety (no thoracic complications related to RF ablation), mean hospital stay, and confirmed complete ablation. Secondary endpoints were alleviation of symptoms, change in the size of the ablation zone, recurrence of the residual tumor, and quality of life. The endpoints of the study were defined at 6 mo after the RF ablation treatment.
Values are expressed as mean ± SD. Continuous variables were compared between groups using Student’s t-test and analysis of variance. Differences in the categorical data were analyzed by use of the χ2 test or Fisher’s exact test. Two-tailed P values < 0.05 were deemed significant. Statistical analyses were performed using SPSS version 15.0 for Windows (SPSS, Chicago, IL, United States). The statistical methods of this study were reviewed by Chunmin Ning and Shigang Guo from Department of General Surgery, Chaoyang Central Hospital
Of the 51 patients, 21 (41.2%) were male and 30 (58.8%) female. The mean diameter of the 51 hemangiomas was 9.6 ± 1.8 cm (range, 6.0-12.0 cm). The patients’ demographic characteristics are given in Table 1.
| Group | CT-guided ablation(n = 24) | Laparoscopic ablation(n = 27) | P value |
| Age (yr), mean (SD) | 50.0 (14.5) | 49.5 (8.27) | 0.748 |
| Gender (male: female) | 10:14 | 11:16 | 0.947 |
| Co-morbidities | |||
| Gallbladder stones | 0 (0) | 3 (11.1) | 0.238 |
| Type 2 diabetes mellitus | 2 (8.3) | 3 (10.3) | 1.000 |
| History of open cholecystectomy | 1 (4.2) | 0 (0) | 0.471 |
| Chronic hepatitis B | 1 (4.2) | 0 (0) | 0.471 |
| History of previous liver surgery | 0 (0) | 1 (3.7) | 1.000 |
| Hepatic cysts | 0 (0) | 1 (3.7) | 1.000 |
| Reasons for radiofrequency ablation | |||
| Abdominal discomfort only | 3 (12.5) | 3 (11.1) | 1.000 |
| Enlargement of hemangioma only | 9 (37.5) | 11 (40.7) | 0.813 |
| Abdominal discomfort and enlargement | 12 (50.0) | 13 (48.2) | 0.895 |
| Maximal size of hemangioma, (cm), mean (SD) | 9.6 (2.5) | 9.4 (1.8) | 0.686 |
| Min | 6.0 | 6.5 | |
| Max | 11.5 | 12.0 |
Outcome data for the RF ablation treatments are given in Table 2. Ablation treatment was conducted according to predefined protocols. There were no technical failures. There was no difference in ablation times between the two groups (P > 0.05) (Table 2).
| Group | CT-guided ablation(n = 24) | Laparoscopic ablation(n = 27) | P value |
| Technical success rate | 24 (100) | 27 (100) | |
| Complete ablation | 22 (91.7) | 26 (96.3) | 0.595 |
| Time of ablation per lesion (min), mean (SD) | 94.2 (20.4) | 95.4 (18.3) | 0.875 |
| Diameter of ablated zone 1 mo after ablation (cm), mean (SD) | 6.4 (1.3) | 4.4 (1.4) | 0.457 |
| Diameter of ablated zone 6 mo after ablation (cm), mean (SD) | 5.5 (1.4) | 3.7 (1.5) | 0.387 |
In the laparoscopic ablation group, laparoscopic cholecystectomy or deroofing was performed during the ablation procedure in patients with gallstones (n = 3) or hepatic cysts (n = 1).
There were 23 thoracic complications in 17 patients, including 15 (62.5%, 15/24) in the CT-guided ablation group and 2 (7.4%, 2/27) in the laparoscopic ablation group (P < 0.05) (Table 3). According to the Dindo-Clavien classification[30], two complications (pleural effusion and diaphragmatic rupture, grade III) were major in two patients. All others were minor (grade I).
| Group | CT-guided ablation(n = 24) | Laparoscopic ablation(n = 27) | P value |
| Total No. of patients with complication | 15 (62.5) | 2 (7.4) | 0.000 |
| Incidence of complication | |||
| Right shoulder pain | 11 (45.8) | 2 (7.4) | 0.003 |
| Transient lung injury | 3 (12.5) | 0 (0) | 0.097 |
| Plural effusion | 5 (20.8) | 0 (0) | 0.018 |
| Diaphragmatic perforation | 1 (4.2) | 0 (0) | 0.471 |
| Hemothorax | 1 (4.2) | 0 (0) | 0.471 |
| Hospital stay, mean (SD) | 9.3 (6.5) | 3.7 (0.9) | 0.032 |
Eleven patients who underwent CT-guided ablation and two who underwent laparoscopic ablation had right shoulder pain after ablation. All of these patients required a pethidine injection for relief. The mean duration of the postprocedural pain was 2.5 d in the CT-guided ablation group and 1 d in the laparoscopic ablation group.
Three cases of transient lung injury without respiratory symptoms were detected on CT scans in the CT-guided ablation group 1 d after ablation. The injuries appeared as an abnormal round shape of the lung parenchymal density on the CT scans. These lesions resolved spontaneously without specific treatment as detected on follow-up CT scans.
Five patients in the CT-guided ablation group developed pleural effusion. Four of the five patients recovered after conservative treatment. The other patient underwent drainage via a chest tube (grade III complication).
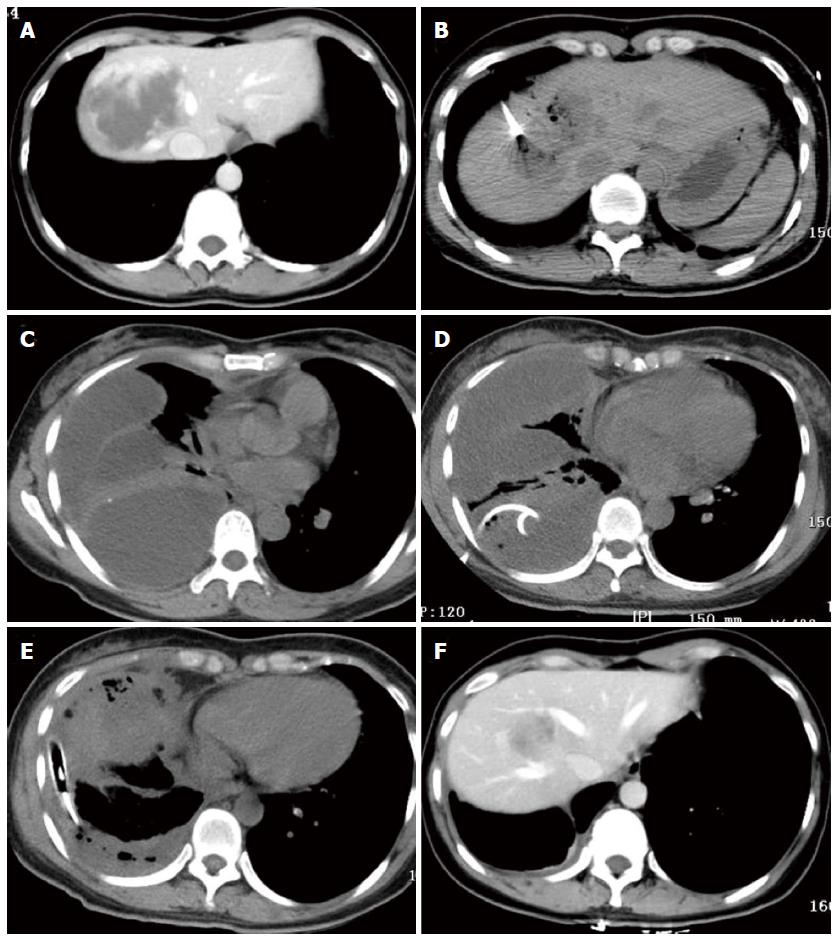
One patient with a 7.5-cm hemangioma mainly in segment 8 in the CT-guided ablation group developed a diaphragmatic rupture after ablation (Figure 1A and B). She was clinically normal for the first 2 d after ablation but then developed right shoulder pain and a high fever (39.0 °C). CT scans of the chest (Figure 1C) showed a multiloculated pleural effusion occupying the right hemithorax with collapse of the right lung. An attempt at drainage via a chest tube yielded only 100 mL of bloody fluid (Figure 1D). She subsequently underwent thoracoscopic surgery, which showed multiple, fluid-filled loculations mostly around the right lower lobe with significant adhesions to the diaphragm and along the major and minor fissures. The loculations were dissected along with decortication of a thick pleural rind, after which a diaphragmatic defect of 1.0 cm diameter was found. The diaphragm was not repaired because of the tissue edema surrounding the defect. Two chest tubes were inserted into the pleural space and were removed 1 wk later (Figure 1E). The patient was discharged 23 d after ablation. Six months after ablation, CT scans in the arterial phase showed that the hemangioma had been completely ablated without formation of a diaphragmatic hernia (Figure 1F).
The hospital stay was significantly shorter in the laparoscopic ablation group than in the CT-guided ablation group (3.7 ± 0.9 d vs 9.3 ± 6.5 d, P < 0.05). This difference was directly related to thoracic complications in the CT-guided ablation group.
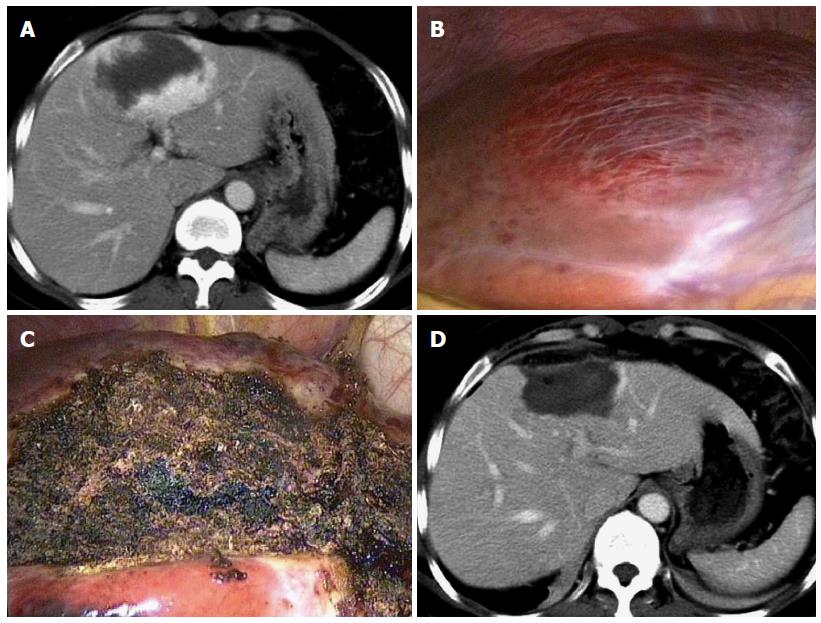
Complete ablation was achieved in 91.7% (22/24) and 96.3% (26/27) of the CT-guided and laparoscopic ablation groups, respectively (P > 0.05) (Table 3 and Figure 2). Three hemangiomas were incompletely ablated, showing subtle enhancement on the peripheral rim of the ablated tumors on follow-up CT or MRI.
At 1 mo, the mean diameter of the ablation zone was reduced from 9.6 ± 2.5 cm to 6.4 ± 1.3 cm in the CT-guided ablation group and from 9.4 ± 1.8 cm to 4.4 ± 1.4 cm in the laparoscopic ablation group - an insignificant difference between the groups (P > 0.05). At 6 mo after ablation, the mean diameter of the ablation zone had decreased further in both groups (to 5.5 ± 1.4 cm in the CT-guided ablation group and to 3.7 ± 1.5 cm in the laparoscopic ablation group), also an insignificant difference.
After RF ablation, there was no perioperative mortality or delayed complications, such as local tumor progression, destructive biliary damages, or liver abscess. The three residual lesions shrunk somewhat during the follow-up period and necessitated no further treatment. Of the 31 patients who had pretreatment symptoms related to their hemangiomas, 28 had complete resolution of symptoms and three experienced symptom amelioration without further therapy after ablation. At the 6-mo follow-up, no patient had developed new symptoms attributable to the hemangiomas. The subjective health status and quality of life were rated as good to excellent in 100% of patients at follow-up[3]. After RF ablation of the hemangiomas, all patients were able to perform full-time or part-time work.
The study aimed to compare the safety and therapeutic efficacy for patients who underwent CT-guided percutaneous or laparoscopic RF ablation for large hepatic hemangiomas abutting the diaphragm. Our data suggest that the laparoscopic approach for RF ablation of large hepatic hemangiomas abutting the diaphragm was successful. It was associated with fewer thoracic complications than the CT-guided percutaneous approach (7.4% vs 62.5%). We also found that the hospital stay was significantly shorter for patients treated with the laparoscopic approach because of their lower incidence of complications. A high frequency of complete ablation of these tumors was attained with both treatment approaches. The immediate and sustained reduction in the size of the tumors was also similar in the two groups.
The hepatic tumors situated in the hepatic dome area abut the diaphragm. Thus, collateral thermal damage to the diaphragm easily occurs during RF treatment under the percutaneous approach. Several thoracic complications have been reported, including diaphragmatic perforation, right shoulder pain, pleural effusion, and other problems following RF ablation of subcapsular malignant tumors abutting the diaphragm[25-29].
The diaphragm is innervated by phrenic nerves arising from nerve roots C3, C4, and C5, which represent the same dermatome as that in shoulder skin. Thus, diaphragmatic irritation is referred to the shoulder and can cause right shoulder pain. Hence, right shoulder pain (referred pain) was reportedly the representative indicator of diaphragmatic thermal injury[25-29]. Diaphragmatic perforation and herniation were reported as major complications of RF ablation for hepatic tumors abutting the diaphragm in nine cases[29]. Kang et al[27] retrospectively assessed 80 patients who underwent percutaneous RF ablation for a single nodular (< 4 cm) hepatocellular carcinoma. They divided the patients into two groups based on whether the index tumor was abutting the diaphragm: group A (abutting; n = 31) vs group B (nonabutting; n = 49). They found that the percutaneous RF ablation of hepatocellular carcinoma abutting the diaphragm seemed safe without major complications, although it was associated with right shoulder pain (26%) and transient lung injury (23%). It was less effective, however, with regard to technical success (84% vs 98%) and local tumor progression (29% vs 6%). The operator’s concern about thermal injury to the adjacent diaphragm may lead to incomplete ablation.
In theory, it is more difficult and risky for percutaneous RF ablation of large hepatic hemangiomas abutting the diaphragm than hepatic malignant tumors abutting the diaphragm. This is because the hemangiomas to be treated are usually larger than the malignant tumors and have wider contact area with the diaphragm. Theoretically, this difficulty and the risk can be maximally solved with a preference for the laparoscopic approach. Laparoscopic RF ablation is increasingly being used for malignant tumors because of its several advantages[31]. It allows accurate identification of patients with extrahepatic disease (i.e., peritoneal metastases) who would otherwise be undetectable by CT. Moreover, intraoperative ultrasonography was used routinely in conjunction with the laparoscopic approach to increase the ability to determine real-time RF electrode placement and evaluate the efficacy of ablation[20,31]. For the large hepatic hemangiomas abutting the diaphragm, establishment of pneumoperitoneum causes elevation of the diaphragm, which increases the operative space, thereby avoiding diaphragmatic injury and facilitating needle placement during laparoscopic RF ablation. Also, a single well-done ablation of a hepatic hemangioma can lead to obvious collapse of tumor tissue around the ablation zone, increasing the operative space further, making the following ablation easier.
During this era of evidence-based medical practice, determining the best approach for each patient is increasingly important. To our best knowledge, the present study is the first comparative controlled study to evaluate the protective effect on the diaphragm or therapeutic efficacy of different approaches of RF ablation for large hepatic hemangiomas abutting the diaphragm. The study shows that the incidence of thoracic complications was significantly lower in patients undergoing laparoscopic ablation than in those subjected to CT-guided ablation. Most of the thoracic complications were trivial, although two were of major significance. In the current study, a high frequency of complete ablation of these tumors was attained with both treatment approaches. Interestingly, the remnant tumor tissues were situated within the liver in the laparoscopic ablation group, whereas in the CT-guided ablation group they were on the surface of the lesions. We therefore suggest that the laparoscopic approach of RF ablation should be the first-line treatment for large hepatic hemangiomas abutting the diaphragm. If a second ablation session is needed[22], the repeat RF ablation would be performed percutaneously.
The major limitations of our study include its retrospective nature, the nonrandomized selection of patients, the short follow-up period, and the relatively small number of patients evaluated. We believe it is important that all of our RF procedures were performed by a single surgeon, thus minimizing the chance of bias that might have occurred had multiple surgeons been involved. Also, the patients in our study were managed from a surgical perspective and by a team of surgeons and making the results less applicable for nonsurgical institutions.
In conclusion, laparoscopic RF ablation therapy should be used as the first-line treatment for large hepatic hemangiomas abutting the diaphragm. Its use avoids thermal injury to the diaphragm and reduces the occurrence of thoracic complications.
Radiofrequency (RF) ablation is an accepted non-surgical treatment for hepatic hemangiomas. If a tumor is located in the hepatic dome which abuts the diaphragm, complete tumor ablation without injury to the diaphragm or lung is challenging under percutaneous approach. In contrast, using the laparoscopic approach, establishment of pneumoperitoneum causes elevation of the diaphragm, which increases the operative space and avoids injuring the diaphragm. In theory, it is more difficult and risky for percutaneous RF ablation of large hepatic hemangiomas abutting the diaphragm than hepatic malignant tumors abutting the diaphragm. This is because the hemangiomas to be treated are usually larger than the malignant tumors and have wider contact area with the diaphragm.
The present study is the first comparative controlled study to evaluate the protective effect on the diaphragm or therapeutic efficacy of different approaches of RF ablation for large hepatic hemangiomas abutting the diaphragm.
The study shows that the incidence of thoracic complications was significantly lower in patients undergoing laparoscopic ablation than in those subjected to computerized tomography (CT)-guided ablation.
The study results provide evidence that laparoscopic RF ablation therapy should be used as the first-line treatment for large hepatic hemangiomas abutting the diaphragm. Its use avoids thermal injury to the diaphragm and reduces the occurrence of thoracic complications.
The tumor abutting the diaphragm was defined as the lesion that was located near the diaphragm (< 5 mm) on axial CT scans.
This is an excellent retrospective study in which the authors used data of 6 institutions to evaluate the protective effect on the diaphragm or therapeutic efficacy of various approaches to RF ablation for large hepatic hemangiomas abutting the diaphragm. The results are interesting and suggest that laparoscopic RF ablation therapy should be used as the first-line treatment option for hepatic hemagiomas abutting the diaphragm, which can avoid thermal injury to the diaphragm effectively and reduce the thoracic complications obviously.
P- Reviewer: Sicklick JK, Sozener U, Uchida H S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Yamashita S, Okita K, Harada K, Hirano A, Kimura T, Kato A, Okita K. Giant cavernous hepatic hemangioma shrunk by use of sorafenib. Clin J Gastroenterol. 2013;6:55-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Hasan HY, Hinshaw JL, Borman EJ, Gegios A, Leverson G, Winslow ER. Assessing normal growth of hepatic hemangiomas during long-term follow-up. JAMA Surg. 2014;149:1266-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 3. | Schnelldorfer T, Ware AL, Smoot R, Schleck CD, Harmsen WS, Nagorney DM. Management of giant hemangioma of the liver: resection versus observation. J Am Coll Surg. 2010;211:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Gupta S, Agarwal V, Acharya AN. Spontaneous rupture of a giant hepatic hemangioma-report of a case. Indian J Surg. 2012;74:434-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Beatty JS, Mitchell JW, Holsten SB, Ferdinand CH. Traumatic rupture of a previously undiagnosed giant hepatic hemangioma. Am Surg. 2013;79:e314-e315. [PubMed] |
| 6. | Hanazaki K, Kajikawa S, Matsushita A, Monma T, Hiraguri M, Koide N, Nimura Y, Adachi W, Amano J. Giant cavernous hemangioma of the liver: is tumor size a risk factor for hepatectomy? J Hepatobiliary Pancreat Surg. 1999;6:410-413. [PubMed] |
| 7. | Lerner SM, Hiatt JR, Salamandra J, Chen PW, Farmer DG, Ghobrial RM, Busuttil RW. Giant cavernous liver hemangiomas: effect of operative approach on outcome. Arch Surg. 2004;139:818-821; discussion 821-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Terkivatan T, de Wilt JH, de Man RA, van Rijn RR, Zondervan PE, Tilanus HW, IJzermans JN. Indications and long-term outcome of treatment for benign hepatic tumors: a critical appraisal. Arch Surg. 2001;136:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Yoon SS, Charny CK, Fong Y, Jarnagin WR, Schwartz LH, Blumgart LH, DeMatteo RP. Diagnosis, management, and outcomes of 115 patients with hepatic hemangioma. J Am Coll Surg. 2003;197:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Hoekstra LT, Bieze M, Erdogan D, Roelofs JJ, Beuers UH, van Gulik TM. Management of giant liver hemangiomas: an update. Expert Rev Gastroenterol Hepatol. 2013;7:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Giavroglou C, Economou H, Ioannidis I. Arterial embolization of giant hepatic hemangiomas. Cardiovasc Intervent Radiol. 2003;26:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Huang XQ, Huang ZQ, Duan WD, Zhou NX, Feng YQ. Severe biliary complications after hepatic artery embolization. World J Gastroenterol. 2002;8:119-123. [PubMed] |
| 13. | Park WC, Rhillips R. The role of radiation therapy in the management of hemangiomas of the liver. JAMA. 1970;212:1496-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Cui Y, Zhou LY, Dong MK, Wang P, Ji M, Li XO, Chen CW, Liu ZP, Xu YJ, Zhang HW. Ultrasonography guided percutaneous radiofrequency ablation for hepatic cavernous hemangioma. World J Gastroenterol. 2003;9:2132-2134. [PubMed] |
| 15. | Zagoria RJ, Roth TJ, Levine EA, Kavanagh PV. Radiofrequency ablation of a symptomatic hepatic cavernous hemangioma. AJR Am J Roentgenol. 2004;182:210-212. [PubMed] |
| 16. | Fan RF, Chai FL, He GX, Wei LX, Li RZ, Wan WX, Bai MD, Zhu WK, Cao ML, Li HM. Laparoscopic radiofrequency ablation of hepatic cavernous hemangioma. A preliminary experience with 27 patients. Surg Endosc. 2006;20:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Tak WY, Park SY, Jeon SW, Cho CM, Kweon YO, Kim SK, Choi YH, Chung JM. Ultrasonography-guided percutaneous radiofrequency ablation for treatment of a huge symptomatic hepatic cavernous hemangioma. J Clin Gastroenterol. 2006;40:167-170. [PubMed] |
| 18. | Hinshaw JL, Laeseke PJ, Weber SM, Lee FT. Multiple-electrode radiofrequency ablation of symptomatic hepatic cavernous hemangioma. AJR Am J Roentgenol. 2007;189:W146-W149. [PubMed] |
| 19. | Park SY, Tak WY, Jung MK, Jeon SW, Cho CM, Kweon YO, Kim KC. Symptomatic-enlarging hepatic hemangiomas are effectively treated by percutaneous ultrasonography-guided radiofrequency ablation. J Hepatol. 2011;54:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Gao J, Ke S, Ding XM, Zhou YM, Qian XJ, Sun WB. Radiofrequency ablation for large hepatic hemangiomas: initial experience and lessons. Surgery. 2013;153:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Zou H, Yan J, Wu YX, Ou X, Li XW, Xia F, Ma KS, Bie P. [The new technology of enhanced radiofrequency ablation is safe and effective for treating giant hepatic hemangioma]. Zhonghua Ganzangbing Zazhi. 2012;20:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Gao J, Ding X, Ke S, Xin Z, Ning C, Sha Q, Sun W. Radiofrequency ablation in the treatment of large hepatic hemangiomas: a comparison of multitined and internally cooled electrodes. J Clin Gastroenterol. 2014;48:540-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | van Tilborg AA, Nielsen K, Scheffer HJ, van den Tol P, van Waesberghe JH, Sietses C, Meijerink MR. Bipolar radiofrequency ablation for symptomatic giant (& gt; 10 cm) hepatic cavernous haemangiomas: initial clinical experience. Clin Radiol. 2013;68:e9-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Cassera MA, Potter KW, Ujiki MB, Swanström LL, Hansen PD. Computed tomography (CT)-guided versus laparoscopic radiofrequency ablation: a single-institution comparison of morbidity rates and hospital costs. Surg Endosc. 2011;25:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Head HW, Dodd GD, Dalrymple NC, Prasad SR, El-Merhi FM, Freckleton MW, Hubbard LG. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology. 2007;243:877-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Wang ZY, Sun WB, Li MY, Zhang XX, Ding XM. Percutaneous extrapulmonary radiofrequency ablation for tumors in the hepatic dome. Hepatogastroenterology. 2008;55:1164-1166. [PubMed] |
| 27. | Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, Lim HK. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10:34-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Kang TW, Rhim H, Lee MW, Kim YS, Choi D, Lee WJ, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: comparison of effects of thermal protection and therapeutic efficacy. AJR Am J Roentgenol. 2011;196:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Zhou M, He H, Cai H, Chen H, Hu Y, Shu Z, Deng Y. Diaphragmatic perforation with colonic herniation due to hepatic radiofrequency ablation: A case report and review of the literature. Oncol Lett. 2013;6:1719-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24805] [Article Influence: 1181.2] [Reference Citation Analysis (0)] |
| 31. | Herbold T, Wahba R, Bangard C, Demir M, Drebber U, Stippel DL. The laparoscopic approach for radiofrequency ablation of hepatocellular carcinoma--indication, technique and results. Langenbecks Arch Surg. 2013;398:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |