Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.5926
Peer-review started: November 2, 2014
First decision: December 2, 2014
Revised: January 10, 2015
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: May 21, 2015
Processing time: 198 Days and 20.1 Hours
AIM: To establish a scoring system to predict clinically relevant postoperative pancreatic fistula (CR-POPF) after pancreaticoduodenectomy (PD).
METHODS: The clinical records of 921 consecutive patients who underwent PD between 2008 and 2013 were reviewed retrospectively. Postoperative pancreatic fistula (POPF) was defined and classified by the international study group of pancreatic fistula (ISGPF). We used a logistic regression model to determine the independent risk factors of CR-POPF and developed a scoring system based on the regression coefficient of the logistic regression model. The optimal cut-off value to divide the risk strata was determined by the Youden index. The patients were divided into two groups (low risk and high risk). The independent sample t test was used to detect differences in the means of drain amylase on postoperative day (POD) 1, 2 and 3. The optimal cut-off level of the drain amylase to distinguish CR-POPF from non-clinical POPF in the two risk strata groups was determined using the receiver operating characteristic (ROC) curves.
RESULTS: Grade A POPF occurred in 106 (11.5%) patients, grade B occurred in 57 (6.2%) patients, and grade C occurred in 32 (3.5%) patients. A predictive scoring system for CR-POPF (0-6 points) was constructed using the following four factors: 1 point for each body mass index ≥ 28 [odds ratio (OR) = 3.86; 95% confidence interval (CI): 1.92-7.75, P = 0.00], soft gland texture (OR = 4.50; 95%CI, 2.53-7.98, P = 0.00), and the difference between the blood loss and transfusion in operation ≥ 800 mL (OR = 3.45; 95%CI, 1.92-7.75, P = 0.00); and from 0 points for a 5 mm or greater duct diameter to 3 points for a less than 2 mm duct (OR = 8.97; 95%CI: 3.70-21.77, P = 0.00). The ROC curve showed that the area under the curve of this score was 0.812. A score of 3 points was suggested to be the best cut-off value (Youden index = 0.485). In the low risk group, a drain amylase level ≥ 3600 U/L on POD3 could distinguish CR-POPF from non-clinical POPF (the sensitivity and specificity were 75% and 85%, respectively). In the high risk group, the best cut-off was a drain amylase level of 1600 (the sensitivity and specificity were 77 and 63%, respectively).
CONCLUSION: A 6-point scoring system accurately predicted the occurrence of CR-POPF. In addition, a drain amylase level on POD3 might be a predictor of this complication.
Core tip: Clinically relevant (CR) postoperative pancreatic fistula (POPF) after pancreaticoduodenectomy (PD) remains a challenge, even at high-volume centres. In our study, we established a novel predictive scoring system for CR-POPF after PD based on a large number of cases in a single centre and discovered that the drain amylase level on postoperative day 3 could distinguish CR-POPF from non-clinical POPF in the early period after PD according to the different risk strata of scores. This tool could help surgeons anticipate, identify and control CR-POPF proactively, with the aim of achieving better outcomes from this daunting postoperative complication.
- Citation: Chen JY, Feng J, Wang XQ, Cai SW, Dong JH, Chen YL. Risk scoring system and predictor for clinically relevant pancreatic fistula after pancreaticoduodenectomy. World J Gastroenterol 2015; 21(19): 5926-5933
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/5926.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.5926
Pancreaticoduodenectomy (PD) has been established as a standard surgical operation for malignant and benign diseases in the pancreatic head and periampulary regions[1,2]. With recent advances in surgical techniques and perioperative management, the mortality rate has decreased to less than 2% (in high-volume centres)[3-7]. However, the morbidity rate after PD remains high (30%-65%). In particular, postoperative pancreatic fistula (POPF) remains the most important cause of morbidity; this also contributes significantly to a prolonged hospitalisation course, increased health care costs, and mortality[1-8].
Although attempts have been made to decrease POPF rates by improving reconstruction techniques for the pancreatoenteric anastomosis[9-12], including the placement of pancreatic duct stents[13,14] or the use of somatostatin analogues[15], an effective strategy to prevent POPF has not yet been found. There has been a paradigm shift among pancreatic surgeons in the management of POPF, from a reactive “wait and see” approach that depends on treating fistulas when they become evident, to a proactive strategy that instead relies on early anticipation and timely prevention[8,16-18]. Recent studies have suggested that many factors influence POPF, such as gender, preoperative jaundice, operative time, pancreatic duct diameter and soft pancreatic parenchyma[2-8]. However, the predictive risk factors that can precisely distinguish clinically relevant POPF (CR-POPF) from transient pancreatic fistula in the early postoperative period remain unclear.
The aim of the present study was to construct a new and convenient scoring tool to predict CR-POPF and discover ways to distinguish CR-POPF from non-clinical POPF in the early period after PD. This was done using preoperative and surgical variables in a study group of 921 patients, according to the different risk strata of scores.
From January 2008 to December 2013, 921 consecutive patients underwent PD. Various patient factors were analysed at the Institution and Hospital of Hepatobiliary Surgery, PLA General Hospital, China. Informed consent for the surgical procedures was obtained from each patient. The local ethics committee approved this study.
The standard Whipple type operation was performed in 491 patients (53%), and the remaining 430 patients (47%) underwent a pylorus-preserving PD (PPPD). Pancreatic anastomosis after PD and PPPD was performed by duct-to-mucosa and end-to-side pancreaticojejunostomy in all patients. Biliary drainage was achieved by end-to-side hepaticojejunostomy. None of the patients received radiotherapy or chemotherapy perioperatively. All patients were managed in the intensive care unit for at least one day before transfer to the ward. Prophylactic octreotide was given subcutaneously and continued routinely for three days postoperatively.
POPF was defined and classified by the international study group of pancreatic fistula (ISGPF)[19]. Grade A POPF is a transient and asymptomatic fistula that does not need specific treatment. Grade B is symptomatic, clinically apparent, and requires diagnostic evaluation and specific medical treatment or prolonged drainage for longer than 3 wk. Grade C requires a major change in clinical management or deviation from the normal clinical pathway. Combined grade B + C is defined as CR-POPF. Biliary fistula[20] was defined as the presence of bile in the drainage fluid that persisted to postoperative day (POD) 4. Delayed gastric emptying was defined as any of the following: output from a nasogastric tube of > 500 mL per day that persisted beyond POD10, the failure to maintain oral intake by POD14 or reinsertion of a nasogastric tube[21].
Preoperative variables included patient demographics, past medical history, laboratory tests and preoperative biliary drainage by ERCP or PTBD. Intraoperative variables included pancreatic duct diameter, consistency of the pancreas, operation time, blood loss, blood transfusion, and the difference between the blood loss and transfusion. Postoperative variables included postoperative complications, amylase in the drainage fluid from POD1 to POD7, the day of starting oral feeding, the length of postoperative stay and hospital mortality. All pathological specimens were reviewed to confirm the diagnosis.
Statistical computations were performed using Statistical Package for the Social Sciences 16.0 for Windows (SPSS, Inc). For continuous variables, descriptive statistics were calculated and reported as the mean ± standard deviation (SD). Categorical variables were described using frequency distributions. The independent sample t test was used to detect differences in the means of continuous variables; the χ2 test was used in cases with low expected frequencies. A P value < 0.05 was considered to be significant. Variables with P < 0.1 were entered into a logistic regression model to determine independent risk factors of CR-POPF. We developed a scoring system using each independent risk factor, which was based on the regression coefficient of the logistic regression model. The points of this scoring system were further modified to develop a more utilitarian application. Receiver operating characteristic (ROC) curves and the corresponding area under the curve (AUC) were used to evaluate the performance of the prediction model. The optimal cut-off value to divide the risk strata was determined by the Youden index (sensitivity +, specificity - 1). The 921 patients were divided into two groups (low risk and high risk). The independent sample t test was used to detect differences in the means of drain amylase on POD1, 2, and 3. The optimal cut-off level of the drain amylase to distinguish CR-POPF from non-clinical POPF in the two risk strata groups was determined by the ROC curves.
Nine hundred and twenty one consecutive patients [591 (64%) men and 330 (36%) women] underwent PD; their mean age was 56 ± 12 years (range: 11-82 years). Preoperative biliary stenting was performed in 181 patients (19.7%), 491 patients (53%) underwent classic PD, and the remaining 430 patients (47%) underwent PPPD. Combined portal vein resection was performed in 31 patients (3.4%). Median operative time was 380 min (range: 135-1265 min) and the median operative blood loss was 400 ml (range: 100-5300 mL). Two hundred and twenty three patients (24.2%) received a blood transfusion; the median amount of blood received was 710 mL (range: 280-1700 mL). The mean difference between the blood loss and intra-operative transfusion was 400 mL (range: 50-2100 mL).
Postoperatively, the median hospital stay was 18 d (range: 3-72 d). Regarding postoperative complications, the overall morbidity was 294 (31.9%): 195 patients (21.2%) developed a POPF; 106 patients (11.5%) had an A-type fistula; 57 patients (6.2%) had a B-type fistula; and the remaining 32 patients (3.5%) had a C-type fistula. Other postoperative complications included delayed gastric emptying in 215 patients (23.3%), intra-abdominal infection in 42 patients (4.6%), wound infection in 44 patients (4.8%), biliary leakage in 33 patients (3.6%), pulmonary complication in 38 patients (4.1%) and postoperative haemorrhage in 54 patients (5.9%). The hospital mortality in this series was 29 patients (3.1%). Haemorrhage and secondary multiple organ failure was the main cause of death.
Univariate and multivariate analyses were used to determine the risk factors of CR-POPF. Table 1 shows the result of 19 parameters that were examined univariately as potential risk factors for the 89 patients with CR-POPF vs 832 with no CR-POPF. Body mass index (BMI) ≥ 28, alcohol use, pancreatic duct size < 3 mm, soft pancreatic parenchyma, ≥ 800 mL difference between the blood loss and intra-operative transfusion, and non-pancreatic diseases were associated with CR-POPF. However, on multivariate logistic regression analysis, only BMI ≥ 28, pancreatic duct < 3 mm, soft pancreatic parenchyma, and a difference ≥ 800 mL between the blood loss and intra-operative blood transfusion were significant factors. Further analysis reflected the effects of narrowing of the pancreatic duct diameter. A pancreatic duct diameter measuring 5 mm was considered a reasonable baseline, because this has been referred to as the normal diameter of the main pancreatic duct[8,22]. Table 2 shows that each 1-mm decrease in the diameter of the pancreatic duct from a baseline of 5 mm resulted in a more than 4-fold increase in the odds of developing CR-POPF (OR = 4.59, 95%CI: 2.47-8.53, P = 0.00).
| Parameters | Non B/C grade POPF group (n = 832) | B/C grade POPF group (n = 89) | P value |
| Age (n = 921) | |||
| < 65 yr | 640 (76.9) | 68 (76.4) | 0.91 |
| ≥ 65 yr | 192 (23.1) | 21 (23.6) | |
| Sex (n = 921) | |||
| Male | 529 (63.6) | 62 (69.7) | 0.26 |
| Female | 303 (36.4) | 27 (30.3) | |
| BMI (n = 921)3 | |||
| < 28 | 773 (92.9) | 71 (80.8) | 0.00 |
| ≥ 28 | 59 (7.1) | 18 (20.2) | |
| Personal history | |||
| Hypertension (n = 921) | |||
| Yes | 177 (21.3) | 16 (18.0) | 0.47 |
| No | 655 (78.7) | 73 (82.0) | |
| Diabetes mellitus (n = 921) | |||
| Yes | 95 (11.4) | 11 (12.4) | 0.79 |
| No | 737 (88.6) | 78 (87.6) | |
| Coronary artery disease (n = 921) | |||
| Yes | 81 (9.7) | 4 (4.5) | 0.54 |
| No | 781 (93.9) | 85 (95.5) | |
| Smoking (n = 921) | |||
| Yes | 199 (23.9) | 23 (25.8) | 0.69 |
| No | 633 (76.1) | 66 (74.2) | |
| Drinking (n = 921)3 | |||
| Yes | 174 (20.9) | 12 (13.5) | 0.09 |
| No | 658 (79.1) | 77 (86.5) | |
| Abdominal operation history (n = 921) | |||
| Yes | 111 (13.3) | 14 (15.7) | 0.53 |
| No | 721 (86.7) | 75 (84.3) | |
| Serum albumin (g/L, n = 893) | |||
| < 35 | 660 (81.8) | 13 (15.1) | 0.48 |
| ≥ 35 | 147 (18.2) | 73 (84.9) | |
| Serum total bilirubin (µmol/L, n = 905)1 | |||
| < 171 | 604 (73.9) | 61 (69.3) | 0.40 |
| ≥ 171 | 213 (26.1) | 27 (30.7) | |
| Type of resection (n = 921) | |||
| PD | 446 (53.6) | 45 (50.6) | 0.58 |
| PPPD | 386 (46.4) | 44 (49.4) | |
| Pancreatic duct (mm, n = 921)3 | |||
| < 3 | 250 (30.0) | 62 (69.7) | 0.00 |
| ≥ 3 | 582 (70.0) | 27 (30.3) | |
| Texture of remnant pancreas (n = 921)3 | |||
| Soft | 289 (34.7) | 68 (76.4) | 0.00 |
| Hard | 543 (65.3) | 21 (23.6) | |
| Operative time (min, n = 913) | |||
| < 360 | 346 (42.0) | 35 (39.3) | 0.65 |
| ≥ 360 | 478 (58.0) | 54 (60.7) | |
| Difference between the blood loss and transfusion in operation (mL, n = 920)3 | |||
| < 800 | 772 (92.9) | 75 (84.3) | 0.00 |
| ≥ 800 | 59 (7.1) | 14 (15.7) | |
| Reconstruction of blood vessels (n = 921) | |||
| Yes | 29 (3.5) | 2 (2.2) | 0.76 |
| No | 803 (96.5) | 87 (97.8) | |
| Pancreaticoduodenectomy extending to adjacent (n = 921)2 | |||
| Yes | 21 (2.5) | 0 (-) | 0.25 |
| No | 786 (94.5) | 89 (100.0) | |
| Pancreatic carcinoma | |||
| Yes | 219 (26.3) | 15 (16.9) | 0.05 |
| No | 613 (73.7) | 74 (83.1) | |
| P value | OR | 95%CI | |
| BMI (≥ 28) | 0.00 | 3.86 | 1.92-7.75 |
| Pancreatic duct (< 3 mm) | |||
| ≥ 5 mm | 1.00 | ||
| 3-5 mm | 0.00 | 4.59 | 2.47-8.53 |
| 2-3 mm | 0.00 | 7.91 | 4.07-15.39 |
| < 2 mm | 0.00 | 8.97 | 3.70-21.77 |
| Texture of remnant pancreas (soft) | 0.00 | 4.50 | 2.53-7.98 |
| Difference between the blood loss and transfusion in operation (≥ 800 mL) | 0.00 | 3.45 | 1.92-7.75 |
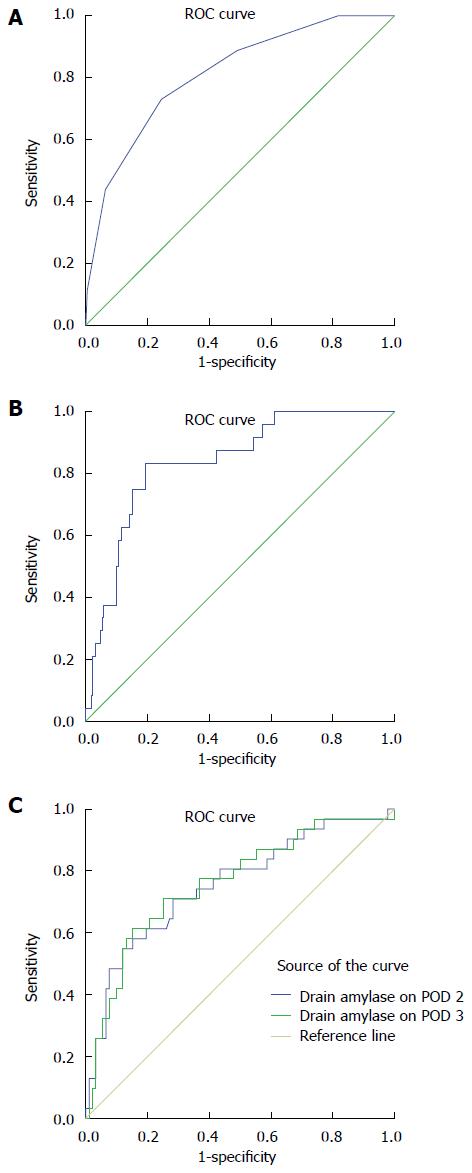
We developed a score model using each standardised variable, based on the regression coefficient of the logistic regression model. The equation for the scoring system was developed on the assumption that a patient receives 1 point each for BMI ≥ 28, soft gland texture, and the difference between the blood loss and intra-operative transfusion ≥ 800 mL, and from 0 points for a 5 mm or greater duct diameter to 3 points for less than a 2 mm duct (Table 3). The score values for individual patients ranged from 0 to 6. The ROC curve (Figure 1A) showed that the AUC of this score was 0.812 (95%CI: 0.766-0.858). A score of 3 points was suggested to be the best cut-off value to divide the risk strata because the Youden index was 0.485. Two risk strata were assigned according to the total score: low risk (0 to 2 points) and high risk (3 to 6 points).
| Risk factor | Points contributed |
| BMI (kg/m2) | |
| < 28 | 0 point |
| ≥ 28 | 1 point |
| Gland texture | |
| Firm | 0 point |
| Soft | 1 point |
| The difference between the blood loss and transfusion in operation | |
| < 800 mL | 0 point |
| ≥ 800 mL | 1 point |
| Pancreatic duct diameter | |
| ≥ 5 mm | 0 point |
| 3-5 mm | 1 point |
| 2-3 mm | 2 points |
| < 2 mm | 3 points |
These patients were divided into a low risk and a high risk group. The low risk group comprised 652 patients whose score was less than 3 points; the remaining 269 patients were classified into the high risk group. In the low risk group, there was no significant difference in the drain amylase level on POD1 and 2 between CR-POPF and non-clinical POPF. However, the mean drain amylase levels on POD3 were 26416.6 ± 16865.0 U/L in patients with CR-POPF compared with 2952.9 ± 606.0 U/L in those without complications (P = 0.000). Considering the sensitivity and specificity of the drain amylase on POD3, the AUC was 0.838 (Figure 1B). A drain amylase level ≥ 3600 U/L on POD3 was determined to be the best cut-off value for prediction of CR-POPF (the sensitivity and specificity of cut-off levels were 75% and 85%, respectively).
In the high risk group, there was no significant difference in the drain amylase level on POD1. The mean drain amylase level on POD2 was 22935 ± 8568 U/L in patients with CR-POPF compared with 6227 ± 2540 U/L in those without complications (P = 0.01), and on POD3, the mean levels were 13709 ± 2626 U/L vs 5122 ± 1290 U/L for these groups, respectively (P = 0.01). Regarding the sensitivity and specificity of the drain amylase on POD2 and 3, the AUCs were 0.756 and 0.761, respectively (Figure 1C). The drain amylase on POD 3 had a better performance. The drain amylase level ≥ 1600 U/L on POD 3 was the best cut-off for prediction of CR-POPF (the sensitivity and specificity of the cut-off levels were 77% and 63%, respectively).
CR-POPF remains the major cause of morbidity after PD. The ability to make a reliable individual prediction of the risk of CR-POPF may be a step towards more individualised surgical management of patients scheduled for PD[9]. However, the current widely used scoring systems are nonspecific and do not accurately predict CR-POPF, because they mostly focus on the physical status and operation tolerance of patients[8]. Given these drawbacks, we have developed a novel predictive scoring system for CR-POPF after PD using the following four independent perioperative parameters: (1) soft gland texture; (2) the narrowed pancreatic duct diameter; (3) BMI ≥ 28; and (4) the difference between the blood loss and intra-operative blood transfusion ≥ 800 mL.
The former two factors are associated with the presence of chronic pancreatitis and are a challenge for reconstruction. The soft gland texture and narrowed pancreatic duct diameter demonstrate that exocrine function is generally preserved, which is more susceptible to ischemia and injury[8], and results in the increased secretion of pancreatic juices[23-25]. These two factors may also increase the difficulty of performing a pancreaticojejunostomy[2,8]. A high BMI is associated with intra-abdominal obesity and fat tissue volume in the pancreas[24-28]. Finally, the difference between the blood loss and intra-operative blood transfusion is associated with rapid volume loss, which causes ischemia and tissue oedema, and may directly affect the healing of the pancreatic duct-to-mucosa anastomosis[8].
The risk factors of POPF have been proposed by recent studies, and some of these studies have also proposed a risk scoring system; the advantage of our risk assessment tool over other models[4,8,27] lies in three factors. First, our research is based on large single centre retrospective cases, where each surgeon performed more than 30 cases of PD, annually. Moreover, the form of pancreaticojejunostomy and perioperative management has a unified standard and thus can avoid the influence caused by the reconstruction techniques for the pancreatoenteric anastomosis. Second, the scoring is based on the independent perioperative factors (accurately determined in the operating room), without the need for information regarding postoperative parameters. Third, this system is different from the other risk assessment tools for POPF after PD[4,27] and provides a good early prediction of the occurrence of CR-POPF. Grade A POPF does not need specific clinical treatment; therefore, distinguishing CR-POPF from transient POPF in the early postoperative period is valuable in the clinical setting.
The role of a surgically placed prophylactic intra-abdominal drain after PD and its effect on the morbidity rate and optimal timing for drain removal, remain controversial[15,29]. However, there is a consensus that a prolonged period of drain placement may increase the rate of infection at the surgical site and may also increase the rate of POPF[17]. For this reason, although a high level of drain amylase indicates POPF, it is important to determine whether the POPF is grade A or grade B/C as soon as possible. In cases of grade A POPF, we could remove the drain, even if the drain amylase level was high. We considered whether CR-POPF could be distinguished from non-clinical POPF using only postoperative factors, such as drain amylase. However, previous studies have reported that measuring daily levels of amylase in drainage fluid may not reflect the severity of POPF, although the increase was significantly greater in cases of POPF than in those without POPF[3,30]. El Nakeeb et al[2] used 4000 U/L as a cut-off. A low drain amylase on POD1 excluded a CR-POPF. The sensitivity of this study was only 28.1%, but the specificity was 97.2%. Therefore, we divided patients into two groups according to the different risk strata of scores. We found that there was a relationship between CR-POPF and drain amylase level on POD3 in the two groups. Our study demonstrated that in the low risk group, a drain amylase level ≥ 3600 U/L on POD 3 was the best cut-off for the prediction of CR-POPF. The sensitivity and specificity for this cut-off level were 75% and 85%, respectively. Using 1600 U/L as a cut-off in the high risk group, a low drain amylase on POD3 excluded CR-POPF with a sensitivity and specificity of 77% and 63%, respectively.
Using the present scoring system and predictive drain amylase level, the following clinical advantages can be expected in the perioperative risk management of PD: (1) the selection of high risk patients for CR-POPF, with the surgeon planning the surgery accordingly; (2) the selection of patients who qualify for early removal of their drain; and (3) the selection of low-risk patients for PD and pancreatic reconstruction by junior trainees.
The current work is not a randomised controlled study and, therefore, is subject to certain limitations secondary to the retrospective nature of the data collection. First, gland texture was measured at the discretion of the operating surgeon and was classified as either firm or soft, rather than on a gradient as others have described[9]. Second, the surgical procedures, such as standard Whipple type operation, pylorus-preserving PD, or use of a pancreatic stent were not randomised, but depended on the surgeon’s preference. Therefore, further studies are necessary to evaluate prospectively the risk scoring system and the predictive drain amylase level for CR-POPF.
In conclusion, despite these limitations, this study has developed a novel predictive scoring system for CR-POPF after PD, with good discriminating ability. In addition, the drain amylase level on POD3 was useful to distinguish CR-POPF from non-clinical POPF in the early postoperative period following PD. The strength of this study lies in its ability to validate this scoring system in a high volume centre hospital. This tool may help surgeons anticipate, identify and control CR-POPF proactively, with the aim of achieving better outcomes from this complication.
Pancreaticoduodenectomy (PD) has been established as a standard surgical operation for malignant and benign diseases in the pancreatic head and periampullary regions. However, the morbidity rate after PD remains high (30%-65%). In particular, postoperative pancreatic fistula (POPF) remains the most important cause of morbidity. The ability to make a reliable individual prediction of the risk of clinically relevant- POPF (CR-POPF) may be a step towards more individualised surgical management of patients scheduled for PD.
There has been a paradigm shift among pancreatic surgeons in the management of POPF, from a reactive “wait and see” approach to a proactive strategy. Recent studies have suggested that many factors influence POPF, such as gender, preoperative jaundice, operative time, pancreatic duct diameter and soft pancreatic parenchyma. However, the predictive risk factors that can precisely distinguish CR-POPF from transient pancreatic fistula in the early postoperative period remain unclear.
In this study, a novel predictive scoring system for CR-POPF after PD was established. The ROC curve showed that the area under the curve of this score was 0.812 (95%CI: 0.766-0.858), which confirmed that this system provides a good early prediction of the occurrence of CR-POPF. Moreover, the authors discovered that the drain amylase level on postoperative day 3 could distinguish CR-POPF from non-clinical POPF in the early period after PD, according to the different risk strata of scores. In the low risk group, the sensitivity and specificity were 75% and 85%, and in the high risk group, the sensitivity and specificity were 77% and 63%, respectively.
This scoring system may help surgeons anticipate, identify, and control CR-POPF proactively, with the aim of achieving better outcomes from this complication. Further studies are necessary to evaluate prospectively the risk scoring system and the predictive drain amylase level for CR-POPF.
PD is a surgical procedure that includes resection of pancreatic head, stomachus pyloricus, duodenum, the lower part of common bile duct and regional lymph node, and then reconstruction, including pancreaticojejunostomy, hepaticojejunostomy and gastrojejunostomy. POPF: Any measurable volume of fluid on or after postoperative day 3 with an amylase content greater than three times the serum amylase activity.
This is an interesting article about some 900 patients that underwent PD. It is clever to construct a predictive scoring system to predict CR-POPF following PD. This scoring system may help surgeons anticipate, identify and control CR-POPF proactively and may be a step towards more individualised surgical management of patients scheduled for PD.
P- Reviewer: Hoetker MS, Pasricha PJ S- Editor: Yu J L- Editor: Stewart G E- Editor: Zhang DN
| 1. | Jimenez RE, Fernandez-del Castillo C, Rattner DW, Chang Y, Warshaw AL. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg. 2000;231:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | El Nakeeb A, Salah T, Sultan A, El Hemaly M, Askr W, Ezzat H, Hamdy E, Atef E, El Hanafy E, El-Geidie A. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience). World J Surg. 2013;37:1405-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Kawai M, Tani M, Hirono S, Ina S, Miyazawa M, Yamaue H. How do we predict the clinically relevant pancreatic fistula after pancreaticoduodenectomy?--an analysis in 244 consecutive patients. World J Surg. 2009;33:2670-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Yamamoto Y, Sakamoto Y, Nara S, Esaki M, Shimada K, Kosuge T. A preoperative predictive scoring system for postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg. 2011;35:2747-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Greenblatt DY, Kelly KJ, Rajamanickam V, Wan Y, Hanson T, Rettammel R, Winslow ER, Cho CS, Weber SM. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18:2126-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 6. | Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z’graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310-1314; discussion 1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 451] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 7. | Muscari F, Suc B, Kirzin S, Hay JM, Fourtanier G, Fingerhut A, Sastre B, Chipponi J, Fagniez PL, Radovanovic A. Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. Surgery. 2006;139:591-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 917] [Article Influence: 70.5] [Reference Citation Analysis (2)] |
| 9. | Ansorge C, Strömmer L, Andrén-Sandberg Å, Lundell L, Herrington MK, Segersvärd R. Structured intraoperative assessment of pancreatic gland characteristics in predicting complications after pancreaticoduodenectomy. Br J Surg. 2012;99:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Maggiori L, Sauvanet A, Nagarajan G, Dokmak S, Aussilhou B, Belghiti J. Binding versus conventional pancreaticojejunostomy after pancreaticoduodenectomy: a case-matched study. J Gastrointest Surg. 2010;14:1395-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Ji W, Shao Z, Zheng K, Wang J, Song B, Ma H, Tang L, Shi L, Wang Y, Li X. Pancreaticojejunostomy with double-layer continuous suturing is associated with a lower risk of pancreatic fistula after pancreaticoduodenectomy: a comparative study. Int J Surg. 2015;13:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Lei P, Fang J, Huang Y, Zheng Z, Wei B, Wei H. Pancreaticogastrostomy or pancreaticojejunostomy? Methods of digestive continuity reconstruction after pancreaticodudenectomy: a meta-analysis of randomized controlled trials. Int J Surg. 2014;12:1444-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Sachs TE, Pratt WB, Kent TS, Callery MP, Vollmer CM. The pancreaticojejunal anastomotic stent: friend or foe? Surgery. 2013;153:651-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Motoi F, Egawa S, Rikiyama T, Katayose Y, Unno M. Randomized clinical trial of external stent drainage of the pancreatic duct to reduce postoperative pancreatic fistula after pancreaticojejunostomy. Br J Surg. 2012;99:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | Anderson RJ, Dunki-Jacobs E, Callender GG, Burnett N, Scoggins CR, McMasters KM, Martin RC. Clinical evaluation of somatostatin use in pancreatic resections: Clinical efficacy or limited benefit? Surgery. 2013;154:755-760; discussion 760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Callery MP, Pratt WB, Vollmer CM. Prevention and management of pancreatic fistula. J Gastrointest Surg. 2009;13:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, Talamini G, Pederzoli P. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg. 2010;252:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 385] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 18. | Rebibo L, Yzet C, Yzet T, Delcenserie R, Bartoli E, Regimbeau JM. Management of persistent pancreatic fistula after pancreatoduodenectomy. Am Surg. 2014;80:E281-E286. [PubMed] |
| 19. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3512] [Article Influence: 175.6] [Reference Citation Analysis (34)] |
| 20. | Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1407] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 21. | Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM. Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg. 2007;245:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 293] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Yekebas EF, Bogoevski D, Honarpisheh H, Cataldegirmen G, Habermann CR, Seewald S, Link BC, Kaifi JT, Wolfram L, Mann O. Long-term follow-up in small duct chronic pancreatitis: A plea for extended drainage by “V-shaped excision” of the anterior aspect of the pancreas. Ann Surg. 2006;244:940-946; discussion 946-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Suzuki Y, Fujino Y, Tanioka Y, Hiraoka K, Takada M, Ajiki T, Takeyama Y, Ku Y, Kuroda Y. Selection of pancreaticojejunostomy techniques according to pancreatic texture and duct size. Arch Surg. 2002;137:1044-1047; discussion 1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Lee SE, Jang JY, Lim CS, Kang MJ, Kim SH, Kim MA, Kim SW. Measurement of pancreatic fat by magnetic resonance imaging: predicting the occurrence of pancreatic fistula after pancreatoduodenectomy. Ann Surg. 2010;251:932-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Casadei R, Ricci C, Taffurelli G, D’Ambra M, Pacilio CA, Ingaldi C, Minni F. Are there preoperative factors related to a “soft pancreas” and are they predictive of pancreatic fistulas after pancreatic resection? Surg Today. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Armellini F, Zamboni M, Robbi R, Todesco T, Rigo L, Bergamo-Andreis IA, Bosello O. Total and intra-abdominal fat measurements by ultrasound and computerized tomography. Int J Obes Relat Metab Disord. 1993;17:209-214. [PubMed] |
| 27. | Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, Lévy P, Sauvanet A, Ruszniewski P, Belghiti J. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 285] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 28. | Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, Nakeeb A, Zyromski NJ, Lillemoe KD. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007;246:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 29. | Kawai M, Tani M, Terasawa H, Ina S, Hirono S, Nishioka R, Miyazawa M, Uchiyama K, Yamaue H. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006;244:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 364] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 30. | Molinari E, Bassi C, Salvia R, Butturini G, Crippa S, Talamini G, Falconi M, Pederzoli P. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg. 2007;246:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |