Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.5778
Peer-review started: October 18, 2014
First decision: December 2, 2014
Revised: February 19, 2015
Accepted: April 17, 2015
Article in press: April 17, 2015
Published online: May 21, 2015
Processing time: 216 Days and 18.6 Hours
The incidence of gastric cancer (GC) fell dramatically over the last 50 years, but according to IARC-Globocan 2008, it is the third most frequent cause of cancer-related deaths with a case fatality GC ratio higher than other common malignancies. Surgical resection is the primary curative treatment for GC though the overall 5-year survival rate remains poor (approximately 20%-25%). To improve the outcome of resectable gastric cancer, different treatment strategies have been evaluated such as adjuvant or perioperative chemotherapy. In resected gastric cancer, the addition of radiotherapy to chemotherapy does not appear to provide any additional benefit. Moreover, in metastatic patients, chemotherapy is the mainstay of palliative therapy with a median overall survival of 8-10 mo and objective response rates of merely 20%-40%. Therefore, the potential for making key beneficial progress is to investigate the GC molecular biology to realize innovative therapeutic strategies, such as specific immunotherapy. In this review, we provide a panoramic view of the different immune-based strategies used for gastric cancer treatment and the results obtained in the most significant clinical trials. In detail, firstly we describe the therapeutic approaches that utilize the monoclonal antibodies while in the second part we analyze the cell-based immunotherapies.
Core tip: The overall 5-year survival rate of gastric cancer after surgery resection remains poor (approximately 20%-25%) also adopting different treatment strategies, such as adjuvant chemotherapy, adjuvant chemo-radiotherapy and perioperative chemotherapy. Several data support the idea that anti-gastric cancer (GC) specific immunotherapy could be an interesting therapeutic strategy. In this review, we provide a panoramic view of the various immune-based approaches adopted and the results obtained in the most significant clinical trials with GC patients.
- Citation: Niccolai E, Taddei A, Prisco D, Amedei A. Gastric cancer and the epoch of immunotherapy approaches. World J Gastroenterol 2015; 21(19): 5778-5793
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/5778.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.5778
The incidence of gastric cancer (GC) fell dramatically over the last 50 years, but according to IARC-Globocan 2008, GC is still the third most frequent cause of cancer-related deaths after lung and liver cancer in male, and after breast and lung cancer in female[1,2]. Interestingly, there is a marked geographical variation in GC incidence, with the highest rate in Japan, China and Eastern Europe and the lowest in North America, India, Philippines and Australia. Histologically, there are three subtypes of gastric adenocarcinoma: intestinal, diffuse and indeterminate (mixed type). Of those, intestinal subtype influences the changes in the epidemiological incidence[3].
Worldwide, the well known epidemiological observation in gastric cancer includes: (1) If migrants from high risk areas move to low risk areas (China to North America), the incidence rate shows a remarkable reduction reaching to almost equal rates as in low risk countries[4]; and (2) preventable by lifestyle modification such as reduced salt intake and increased vegetable and fruit consumption, together with avoidance of smoking and countermeasures against Helicobacter pylori (H. pylori) infection, reduce the risk of gastric cancer[5].
Case fatality GC ratio is higher than other common malignancies, such as colon, breast and prostate cancer[6]. Cancer excision is the principal remedy for GC though the 5-year survival rate is not high (approximately 20%-25%). To improve the outcome of resectable gastric cancer, different treatment strategies have been evaluated such as adjuvant chemotherapy, adjuvant chemo-radiotherapy, and perioperative chemotherapy.
The US Intergroup 0116 trial reported the benefit of postoperative-chemo-radiotherapy using 5-fluorouracil (5-FU)/leucovorin in a US population. In this study, only 10 % of patients received D2 resection. In Korean patients after D2 resection, the ARTIST trial failed to show any benefit from adding radiotherapy to adjuvant chemotherapy in terms of 3-year disease-free survival.
The MAGIC trial compared perioperative chemotherapy with surgery alone and reported a prolonged 5-year overall survival in the perioperative chemotherapy arm. In resectable gastric cancer, the benefit of adjuvant chemotherapy compared with surgery alone has been clearly demonstrated. After D2 dissection, S-1 adjuvant chemotherapy improved the overall survival (ACTS-GC trial) and capecitabine/oxaliplatin combination chemotherapy improved 3-year disease-free survival (CLASSIC trial). To date, for resectable gastric cancer, the use of chemotherapy in addition to surgery has proved to be beneficial in decreasing the rate of recurrence and improving overall survival. The optimal sequence of chemotherapy and surgery, as well as the development of new optimal chemotherapeutic agents, are future goals for research. In D2-resected gastric cancer, the addition of radiotherapy to chemotherapy does not appear to provide any additional benefit[7-11].
First-line chemotherapy raises the overall survival (OS) of patients with advanced GC and the association of two drugs (generally fluorouracil and cisplatin) was more effective than monotherapy[12]. Moreover, the addition of a third drug (e.g., anthracycline or docetaxel) to a platinum-fluoropyrimidine association further increases the OS[13,14]. Lastly, both capecitabine and oxaliplatin had similar results to fluorouracil and cisplatin, respectively, about the OS and progression-free survival (PFS)[14,15].
In metastatic GC patients, chemotherapy is the column of palliative treatments with a median OS of 8-10 mo and objective response rates (ORRs) of merely 20%-40%[16].
Therefore, the potential for making key beneficial progress is to investigate the molecular biology of tumors to realize innovative therapeutic strategies, such as specific immunotherapy[17,18].
The behavior of immune response is centered on a task partition involving the innate (especially macrophages, dendritic and NK cells) and specific immune response (T and B lymphocytes), that often cooperate to obtain an efficacious anti-cancer response. Up to now, various strategies (vaccines, T cells infusion or cytokines) have been proved to be able to stimulate the immune system, exploiting essentially two principal mechanisms: (1) strengthening the anti-tumor response (by raising the amount of effective cells and/or cytokines/chemokines); or (2) increasing the immunogenicity and/or susceptibility of cancer cells.
However, the neoplastic cells are able to develop various strategies to evade immune surveillance: decrease of tumor antigen or MHC expression, modulation of Fas-L, secretion of inhibitory cytokines [interleukin (IL)-10 and/or TGF-β] and generation of regulatory cells such as Tregs (regulatory cells) and myeloid-derived suppressor cells (MDSC)[19,20].
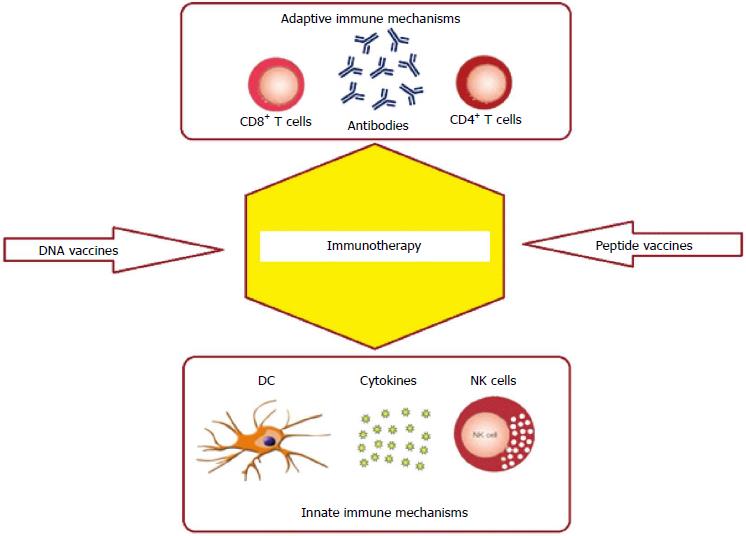
In other words, the prerequisite for an effective anti-tumor immune-based treatment is the stimulation of a successful cancer-specific immune response, able to crack the immunological tolerance of tumor cells. The aim of this review is to provide a panoramic view of the different immune-based strategies used for GC management (Figure 1) and discuss the data of the most significant clinical trials. In detail, firstly we describe the therapeutic approaches that utilize the monoclonal antibodies while in the second part we analyze the cell-based anti-GC treatments.
The typical paradigm of drug development, especially for targeted therapies, has primarily been in the metastatic setting, followed by introduction into chemoradiation, and finally, subsequent evaluation in a randomized trial.
In this paragraph, we will focus on three of the most widely studied therapies with monoclonal antibodies (moAb) in gastric cancer: anti-epidermal growth factor receptor (anti-EGFR), anti-HER2 and anti-vascular endothelial growth factor (anti-VEGF); as they have been evaluated by this paradigm. The second part will focus on pathways and drug targets currently under evaluation for gastric cancer.
EGFR (ERBB1) is an element of the ERBB trans-membrane growth factor receptor family, that promotes and modulates, via a receptor-associated tyrosine kinase (TK), various cell processes such as apoptosis or proliferation[21].
The EGFR hyperexpression shows a relationship with augmented invasion and more unfavorable prognosis of patients with esophago-gastric cancers (EGC)[22-25]. In addition, the anti-EGFR MoAb therapy is ineffective in colorectal cancer (CRC) patients that have K-ras mutations[26-28].
Lately, Janmaat et al[29] showed mutated K-ras in 8.7% patients with EGC; but the prognostic role of K-ras status in the anti-EGFR therapy is practically indefinite.
Existing anti-EGFR treatments in EGC patients consist of oral TK inhibitors (TKIs; erlotinib, gefitinib) and moAb (cetuximab, panitumimab and matuzumab).
Cetuximab obstructs the lignad junction with the EGFR[30], promotes EGFR internalization[31] and also, can start the immune-mediated cytotoxicity[32,33].
Due to the better ORR and time-to-progression (TTP) for the cetuximab/irinotecan association compared with the irinotecan monotherapy[34] , the FDA (Food and Drug Administration) has been approved the cetuximab use in irinotecan-refractory CRC.
In addition, the FDA has been authorized the Panitumumab therapy of chemo-refractory EGFR-positive CRC, because a recent study showed an amelioration in ORR and PFS over best current treatment[35]. Besides, a phase I study reported a stable disease (SD), for 7 mo, in one refractory EGC patient, treated with panitumumab[36].
Lastly, a recent study showed that one patient, with esophageal cancer (EC), cured with Matuzumab (the last anti-EGFR moAb) had a durable six-month PR[37]. Also, the combination of matuzumab with the ECX regimen (epirubicin/cisplatin/capecitabine) registered encouraging results as first-line therapy in patients with EGFR+ gastric cancer. The ORR in 20 evaluable patients was 65% with a median TTP of 5.2 mo[38].
Metastatic results: Numerous phase II studies have been performed with cetuximab in combination with chemotherapy in advanced EGC. One of the first trials[39] evaluated cetuximab with FOLFIRI in thirty-eight patients with untreated advanced gastric or GE junction adenocarcinoma. Cetuximab was given with an initial loading dose of 400 mg/m2 followed by weekly doses of 250 mg/m2. The overall response rate was 44.1%, with a median survival of 16 mo. In another randomized phase II study, cetuximab was added to 3 chemotherapy regimens: ECF (epirubicin, cisplatin, 5-FU), IC (irinotecan/cisplatin), and FOLFOX[40]. The response rates were 58%, 38%, and 51% in the 3 arms, respectively.
The role of anti-EGFR therapy in advanced EGC was tested in a phase III study evaluating the efficacy of panitumumab with combination chemotherapy in the REAL 3 study[41]. Patients with inoperable/metastatic esophageal, gastric, or GE junction cancer were randomized to receive EOC (epirubicin, oxaliplatin, capecitabine) with or without panitumumab. An early planned interim analysis showed that the panitumumab arm was statistically inferior after 553 (76%) patients were enrolled. Median survival was 11.3 mo in the chemotherapy-alone arm vs 8.8 mo for chemotherapy plus panitumumab [hazard ratio (HR) = 1.37, P = 0.013). Although patients with rash in the panitumumab arm did better than those without rash, the subgroup of patients with rash still had a numerically worse median survival than the entire chemotherapy-alone group.
Chemoradiation results: Chemoradiation with cetuximab has been extensively studied in the phase II setting. One clinical study evaluated 60 patients treated with cetuximab, paclitaxel, and cisplatin in combination with radiation therapy. A pathologic complete response rate of 27% was seen with this regimen[42]. In the Swiss Group for Clinical Cancer Research phase Ib/II trial (SAKK 75/06), 28 patients with adenocarcinoma or squamous cell carcinoma were treated with induction cisplatin, docetaxel, and cetuximab followed by radiation therapy to 45 Gy along with concurrent cisplatin and cetuximab. A pathologic complete response (pCR) rate of 32% was seen with this regimen. Neither of these studies demonstrated excess risk with the addition of cetuximab[43].
In contrast, ECOG 2205 evaluated a neoadjuvant regimen of cetuximab in combination with infusional 5-FU, oxaliplatin, and radiation therapy. The study was closed after an excessive number of early deaths. Four of 18 patients died postoperatively of the acute respiratory death syndrome (ARDS) despite compliance with strict radiation lung dosimetry guidelines. This high rate of ARDS, not seen in other studies of 5-FU with oxaliplatin and radiation, raised the possibility that cetuximab may have added to the risk of postoperative pulmonary complications[44].
Evolution of chemoradiation: Radiation Therapy Oncology Group (RTOG) 0436 is a randomized phase III trial of cisplatin, paclitaxel, and radiation therapy to 50.4 Gy with or without cetuximab in inoperable esophageal cancer. In the spring of 2012, the study underwent a planned interim analysis to document superiority of the cetuximab arm as measured by clinical complete response rate. The study failed to meet this end point and closed to further patient enrollment.
The SCOPE1 study from the United Kingdom is a similarly designed 2-arm randomized phase II/III study comparing cisplatin/capecitabine/radiation with or without cetuximab[45]. This study will also undergo a planned analysis after the phase II portion to document a freedom from treatment failure rate exceeding 75% at 24 wk in the cetuximab containing arm.
Given the negative results of the REAL 3 trial, and RTOG 0436 closing enrollment to adenocarcinoma due to a lack of efficacy with cetuximab, it is unlikely that anti-EGFR strategies will be further developed in the United States in unselected patients.
HER-2/neu (ERBB2) is part of the ERBB TK receptor family. The ligand of these receptors leads to homo/ hetero-dimerization of the receptors and with their formation displaying a distinct hierarchy. In this system, HER-2/neu has a key role because each receptor with a specific ligand promotes the association with Her-2/neu. This predilection is more influenced by Her-2/neu hyperexpression, as seen in numerous types of human tumor cells[46].
About EGCs, HER-2/New hyperexpression has been shown in esophageal cancer and GE junction carcinoma[47,48]. HER-2/neu hyperexpression has been connected with increased invasion and poor response to neo-adjuvant chemotherapy[49] or overall reduced survival[50].
The anti-HER2/neu moAb treatment that has been tested in EGC patients is Trastuzumab, that exercises its role by different ways: blocking HER-2 receptor dimerization, favoring the receptor demolition and promoting the cytotoxicity[51]. Currently, it has been used in association with chemotherapy for HER-2/neu+ and node+ breast cancer[52-56].
Metastatic results: The proof of the therapeutic benefit of HER2-directed therapy in gastric and GE cancer comes from the trastuzumab for gastric cancer trial, a large randomized trial of trastuzumab added to standard chemotherapy in HER2+ advanced gastric cancer[57]. In this study, patients with HER2+ gastric or GE cancer were randomized to either trastuzumab and chemotherapy or chemotherapy alone.
Chemotherapy consisted of (5-FU or capecitabine in combination with cisplatin given every 3 wk for 6 cycles. Trastuzumab was continued until disease progression. HER2 positivity was defined as 3+ staining by immunohistochemistry (IHC). Tumors with IHC 2+ staining had to be confirmed by the evidence of amplification by fluorescence in situ hybridization.
Tumors from 3807 patients were centrally tested for HER2 status, of which 22.1% were HER2+. These 594 patients were randomized to 1 of the 2 treatment groups, with well-balanced clinical characteristics. A planned interim analysis was performed after 75% of the events had occurred, and the independent data monitoring committee recommended release of the data because the prespecified boundary had been exceeded, with a median follow-up of 17.1 mo. Median survival was improved with the addition of trastuzumab to chemotherapy from 11.1 to 13.5 mo (P = 0.0048; HR = 0.74; 95%CI: 0.60-0.91). The overall response rate was also improved from 34.5% to 47.3% with the addition of trastuzumab (P = 0.0017). The toxicity was similar in both arms.
Specifically, there was no difference in congestive heart failure. Asymptomatic decreases in left ventricular ejection fraction were similar in both arms (4.6% with trastuzumab, 1.1% without). Based on this study, trastuzumab was approved in the setting of HER2+ advanced gastric and GE cancer.
Chemoradiation results: Recently it has been performed a pilot study of trastuzumab added to chemoradiation in patients with locally advanced esophageal carcinoma[58].
Patients were required to have HER2 positivity (HER2 2+/3+ expression). Chemoradiation was delivered with a dose of 50.4 Gy along with concurrent weekly cisplatin (25 mg/m2) and paclitaxel (50 mg/m2). In cohort 1, 3 patients received a 2-mg/kg bolus dose in week 1 followed by a weekly dose of 1 mg/kg. In cohort 2, 3 patients received a 3-mg/kg bolus dose in week 1 followed by a weekly dose of 1.5 mg/kg. In the third cohort, 13 patients received a 4-mg/kg bolus dose in week 1 followed by a weekly dose of 2 mg/kg. Maintenance trastuzumab was given for 1 year at a dose of 6 mg/kg every 3 wk. Despite the advanced disease in many patients, such as celiac adenopathy (37%) or retroperitoneal (37%), a striking 3-year overall survival of 47% was observed, although a lot of patients did not undergo surgery owing to extensive adenopathy or medical morbidities. Additionally, there were no observed increases in adverse events from the addition of concurrent or maintenance trastuzumab. Because surgery was not required, there was no meaningful pCR data.
Trastuzumab emtansine, or T-DM1, is an antibody- drug conjugate linking trastuzumab to a highly potent anti-microtubule agent. Preclinical data on human GC cells and xenografted tumors suggested that T-DM1 is more effective than trastuzumab. Recently, a phase III study evaluating T-DM1 vs lapatinib and capecitabine in HER2+ trastuzumab-refractory breast cancer demonstrated an improvement in median survival favoring T-DM1 (not reached vs 23.3 mo; HR = 0.621; 95%CI: 0.475-0.813; P = 0.0005)[59]. Additionally, T-DM1 had a higher response rate (43.6% vs 30.8%) and duration of response (12.6 mo vs 6.5 mo). Furthermore, T-DM1 showed antitumor effects even in xenografted tumors that had developed resistance to trastuzumab. Based on this evidence, an international phase II/III trial in second-line advanced EGC will open randomizing between T-DM1 vs a taxane (weekly paclitaxel or q3w docetaxel).
Evolution of chemoradiation: Based on the positive results observed in the metastatic setting with the addition of trastuzumab as well as the encouraging safety and efficacy data from the Brown group, the RTOG has initiated a randomized trial, RTOG 1010, studying the addition of trastuzumab to chemoradiation. In this study, patients with operable locally advanced adenocarcinoma of the esophagus and GE junction are centrally screened for HER2 positivity. If the tumor is found to be HER2+, patients are randomized to concurrent and maintenance trastuzumab in addition to chemoradiation. Chemoradiation consists of a dose of 50.4 Gy along with weekly carboplatin (AUC 2) and paclitaxel (50 mg/m2). The results of this ongoing trial will inform the future use of trastuzumab in localized HER2-overexpressing esophageal and GE junction cancer[60].
The action of most powerful angiogenic factor, VEGF, is started by linking to various high-affinity trans-membrane receptors, most remarkably VEGFR types 1 and 2[61].
VEGF is over-expressed in different cancers[62] and besides, in esophageal and gastric cancer the hyperexpression correlates with cancer stage, bad prognosis and reduced survival[63-70]. Also, the moAb bevacizumab is an anti-VEGF monoclonal antibody, that associated with the chemotherapy increases the ORR and TTP in patients with CRC[71] NSCLC[72] and breast cancer[73]. It seems that bevacizumab have a double anti-cancer effect: as anti-angiogenic factor and also increasing chemotherapy drug delivery, favoring the decrease of interstitial fluid pressures[74,75].
Metastatic results: Multiple phase II studies evaluated bevacizumab in combination with a variety of chemotherapy regimens in esophagogastric cancer. In a phase II study[76], the addition of bevacizumab to cisplatin and irinotecan showed a response rate of 65% and a median survival of 12.3 mo. In another phase II study, Shah et al[77] evaluated bevacizumab in combination with docetaxel, cisplatin, and 5-FU. This regimen yielded a response rate of 67% and an impressive median survival of 16.8 mo. Similarly, a high response rate of 68% was observed when bevacizumab was combined with docetaxel, cisplatin, and irinotecan[78].
With these higher obtained response rates, a randomized phase III trial was performed evaluating the efficacy and safety of bevacizumab in combination with chemotherapy. The Avastin in Gastric Cancer (AVAGAST) trial randomized patients with inoperable locally advanced or metastatic gastric or GE junction adenocarcinoma with no previous therapy to bevacizumab or placebo in combination with capecitabine (or 5-FU) and cisplatin. 774 patients were randomized, with 95% of patients having metastatic disease[79]. The median survival was 10.1 mo for chemotherapy alone vs 12.1 mo for chemotherapy plus bevacizumab (HR = 0.87; P = 0.1002). Although this result did not reach statistical significance, there was an improvement in progression-free survival from 5.3 to 6.7 mo (HR = 0.80; P = 0.0037), and the overall response rate increased from 29.5% to 38% (P = 0.0121). However, despite this negative trial, some of the trends in the secondary endpoints have led to further evaluation of bevacizumab in the metastatic setting.
Chemoradiation results: An interesting study[80] demonstrated that bevacizumab could change tumor physiology of rectal cancer and theoretically potentiate the effects of radiation therapy. In localized esophageal cancer, a similar approach was used in a phase II trial evaluating bevacizumab with erlotinib in a neoadjuvant chemoradiation study[81]. Patients with stage I-III esophageal or GE junction cancer were enrolled. Ninety-five percent of patients had adenocarcinoma, and 93% of patients had stage II or III disease. Bevacizumab was added to a regimen consisting of carboplatin (AUC 5, days 1 and 22), paclitaxel (200 mg/m2, days 1 and 22), and continuous infusion of 5-FU (225 mg/m2 per day, from day 1 to 35) in combination with radiation therapy to 45 Gy. Of sixty patients enrolled, a pathologic complete response rate of 30% was observed.
Another phase II study, reported results of preoperative chemoradiation with cisplatin, irinotecan, and bevacizumab. Patients with Siewert I/II adenocarcinoma of the esophagus received induction chemotherapy with cisplatin, irinotecan, and bevacizumab. This was followed by concurrent chemotherapy with cisplatin, irinotecan, and bevacizumab in combination with radiation therapy to 50.4 Gy. Surgery was followed by adjuvant bevacizumab. A pathologic complete response was seen in 4 of 33 patients (12%). Progression-free survival and overall survival were 14 and 30 mo, respectively[82].
Evolution of chemoradiation: The negative primary result of the AVAGAST study has mitigated some of the enthusiasm for bevacizumab in the context of chemoradiation for esophageal cancer. Given the lack of improvement in the pathologic complete response rate in the phase II study discussed earlier in the text compared with historical control groups, further development of bevacizumab with chemoradiation for esophageal cancer is currently not being pursued in a phase III study[60].
The cell surface receptor c-MET [mesenchymal-epithelial transition factor (MET)] and its ligand hepatocyte growth factor (HGF) are potential therapeutic targets in esophagogastric cancer. Physiological MET tyrosine kinase activation is mediated by binding of HGF, leading to signal transduction down multiple downstream pathways, including those involving Ras, PI3K, mTOR, STAT3, and NF-κB[83,84]. Additionally, the HGF/MET axis can stimulate tumor endothelial cells, thereby altering the tumor microenvironment and promoting angiogenesis[85,86]. Physiological MET signaling can be altered by ligand/receptor overexpression or gene amplification as well as MET gene mutations[85]. Specifically, MET gene amplification is a driver in some esophagogastric cancers[87-90].
Additionally, MET gene mutations have been documented in hereditary and sporadic renal carcinoma, esophagogastric cancer, hepatocellular cancer, head and neck cancer, ovarian carcinoma, small-cell lung cancer, and glioma[85,91,92]. Strategies to inhibit the HGF/MET axis include blocking both the ligand and the receptor.
Rilotumumab is a human moAb to HGF. In a randomized phase II study, patients with newly diagnosed GC were randomized to receive 1 of 2 doses of rilotumumab (15 mg/kg or 7.5 mg/kg) in combination with ECX (epirubicin, cisplatin, capecitabine) chemotherapy or chemotherapy alone[93]. Tumors that were IHC+ in > 50% of cells were defined as MET high. In the MET-high subgroup, representing approximately half of the patients, the 2 rilotumumab arms had a median survival superior in the chemotherapy-alone arm (11.1 mo vs 5.7 mo; HR = 0.29; 95%CI: 0.11-0.76; P = 0.012). In contrast, the MET-low patients in the 2 rilotumumab-containing arms had a trend toward a worse survival than the MET-low patients in the chemotherapy-alone arm (HR = 1.84; 95%CI: 0.78-4.34). In the chemotherapy- alone arm, patients with MET-high tumors had a worse overall survival (HR = 3.22; 95%CI: 1.08-9.63) than those with MET-low tumors. This study suggested that expression, as opposed to amplification, may be a reasonable biomarker. In this study, MET expression was both predictive (good) for anti-HGF antibody therapy and prognostic (poor). Based on these data, a phase III study has been planned in the first-line setting for EGC patients with MET-high tumors.
The central anti-cancer role of T cells has been highlighted by the documented cancer incidence in immunodeficient disorders[94] and by evidence that the intra-tumoral T cell infiltration is associated with better patient survival[95].
Currently, there isn’t FDA-approved adoptive T-cell therapy, but the growing new acquisitions on the cancer nature and lymphocyte role do hope that, shortly, the adoptive T-cell therapy can become a clinical cancer practice. Topical information obtained from adoptive transfer in lymphodepleted hosts[96], the immunosuppressive capacity of Tregs[97] and the utilize of better culture systems[98] have not yet been tested in clinical studies.
Essentially exist two different therapeutic protocols of T cell-based anti-cancer treatment: (1) cytotoxic T lymphocytes (CTL); (2) tumor infiltrating lymphocytes (TIL) (Figure 1).
Cytotoxic T lymphocytes: Improved CTL cell culture technology has permitted the first clinical tests for adoptive transfer of CTLs and this technique[99,100] seems to result in substantial activity in melanoma patients: 40% of patients showed an anti-tumor immune responses[101]. Similar results were obtained by Yee et al[102] in an independent trial in which engraftment of the CTLs was detectable up to two weeks after T-cell transfer in all patients.
Survivin has been demonstrated to be an excellent target for immunotherapy in various cancer types and recent data suggest a role also in gastric cancer[103]. In this study, elevated efficiency was obtained upon inducing survivin-derived peptide-specific CTL from mononuclear cells isolated from blood of healthy donors. The induced CTLs showed specific lysis against tumor cells in vitro, and vs primary cell cultures isolated from GC patients. These data suggest that survivin epitope peptide could be a promising vaccine candidate for GC immunotherapy.
Instead, another recent study[104] examined the possibility of using cancer-specific immunotherapy based upon mitotic centromere-associated kinesin (MCAK), a new cancer antigen. To evaluate the feasibility of developing cancer immunotherapy using MCAK peptides, Kawamoto et al[104] studied HLA-A10201 and 12402 as targets for CTLs.
The CTLs killed HLA-A-10201/12402 colon and gastric cancer MCAK+ cells, as well as the peptide-pulsed target cells, in an HLA-l restricted manner. These results prospect the opportunity of designing peptide-based immunotherapeutic treatments for patients with MCAK+ gastric cancer.
Of late, Kim et al[105] demonstrated the anti-gastric cancer power of cytokine-induced killer (CIK) cells (essentially T CD80+ cells), that were isolated from the human peripheral blood mononuclear cells (PBMC), cultured in medium with IL-2 and anti-CD3 antibody. The CIK cells were able to destroy, in vitro, the MKN74 cells (a human gastric cancer cell line) and to inhibit the MKN74 tumor growth in nude mouse model. These results suggest the potential use of CIK cells as adoptive GC immunotherapy patients, as described in different studies[106,107]. In fact, the CTLs from GC patients are capable to attack the autologous cancer cells, recognizing specific tumor-associated antigens[107,108], such as MG7-antigen, that shows a big potential for starting immune responses to gastric cancer[109,110]. In addition, the use of HLA-A-restricted allogeneic GC cells to stimulate tumor-specific CTLs could be a different immunotherapeutic approach for GC patients[111].
Of note, different studies suggest that the association of CIK cells with chemotherapy can be functional in advanced GC patients[112,113]. In fact, the patients cured with the combined therapy showed a significant decrease of serum levels of the cancer markers and a marked improvement of life quality, in comparison to patients treated only with chemotherapy.
In summary, preclinical/clinical evidence supports the idea that CIK cell immunotherapy can be a successful anti-GC treatment, but it is still unclear what is the injection via which guarantees the best distribution of effector cells.
In a mouse model of gastric cancer Du et al[114] observed the distribution of CIK cells injected by three different via of infusion: peritumoral (pt), intravenous (iv) and intraperitoneal (ip).
They demonstrated that the pt injection produced a considerable tumor infiltration of CIK cells for 48 h and induced the most tumor inhibition in comparison to the ip or iv infusion, that caused a very small CIK intratumoral accumulation and a short in vivo inhibition of tumor growth only following injection. In conclusion, the pt injection of CIK can be an effective and minimally invasive approach of adoptive cellular immunotherapy for GC patients.
Adoptive transfer therapy with TILs: The use of TILs as adoptive transfer therapy is a “not immediate” therapeutic approach because it requires about six weeks before the T cells would be ready for infusion. In fact, the protocol necessitates firstly the T cell isolation from neoplastic tissue, after the in vitro expansion and finally the selection of tumor-specific T cells. In addition, only 30%-40% of the biopsies yield acceptable T-cell populations and the whole process[115]. So, the adoptive transfer of TILs has been promising in preclinical models[116] but not in clinical trials[117,118], except for the melanoma patients for easy surgical availability of the tumor tissue. However, should technical limitations of current tissue culture approaches are overcome; new data indicate that the presence of TILs positively correlates with patient survival in ovarian and colorectal cancer[95,119] and have a important role in pancreatic cancer[120] , thus prompting the enforcement of this protocol for other usually encountered epithelial cancers.
In the past, we have demonstrated, in GC patients, the functional role of TILs reactive vs different peptides of GC-associated antigens[121]. We have documented a peptide-specific T-cell response in 17 out of 20 enrolled patients and the majority of specific TILs had an effective role showing a T helper 1 (Th1) cytokine profile with high cytotoxic activity. In other words, in most of GC patients, a specific type-1 T-cell response to GC antigens was detectable and would have the potential of killing the cancer cells. But, in order to get “in vivo” tumor cell destroying, the the quantity and quality of tumor-specific T cells almost certainly need to be enhanced by vaccination with the appropriate cancer antigens/peptides or by injection of the autologous cancer-specific T cells, previously expanded in vitro.
It is remarkable to note that not always the lymphocytic infiltrate has an anti-cancer role and often the TILs can promote the expansion of tumor cells. Recently, we have investigated the functional profile of HP0175-specific TILs in GC patients, infected with H. pylori. The TILs cells were able to produce IL-17 and IL-21 in response to HP0175 but showed poor cytolytic activity and high helper activity for monocyte MMP-2, MMP-9 and VEGF production. In a nutshell, these data suggest that HP0175 drives gastric Th17 response and promoting pro-inflammatory low cytotoxic TIL response, so providing a link between H. pylori and gastric cancer[122].
In addition, different studies highlight that most of GC TILs show a Treg profile. Recently, Shen et al[123] demonstrated that that CD4+ and CD8+ TILs were not associated with the OS of GC patients and that in the tumor sites, higher Tregs/CD8+ ratio was an independent factor for worse OS (P = 0.037). The 1-year, 2-year and 3-year OS rates were 90%, 77.5% and 70% for the group with intratumoral high Tregs/CD8+ ratio, compared with 100%, 94.3% and 90.5% for the group with intratumoral low ratio. So, intratumoral high Tregs/CTLs ratio was a prognostic factor for GC patients. Accordingly, an independent study showed that a higher Tregs/Th cell ratio is associated with an unfavorable prognosis and loco-regional recurrence pattern in gastric cancer[124].
It can be inferred that a combination of deletion of Tregs and stimulation of effector T cells may be a successful immunotherapy to prolong survival of GC patients.
Antigen presentation by dendritic cells (DCs) is essential to start the cellular immune responses required for tumor immunotherapy[125,126] (Figure 1). In addition, in mouse models ex vivo generated DCs can provoke antigen-specific T-cell responses[127], supporting the use of DC-based anticancer vaccines in clinical studies[128].
In GC patients the number of DCs correlates with the clinical stage and prognosis: patients with abundant DCs infiltration showed a better 5-year survival rates than patients with smaller amount of DCs[129,130]. Moreover, it has been documented that the use of adjuvant immunotherapy enhances the survival in resected GC patients with small tumor DCs infiltration[131].
Of the 325 trials reported in ClinincalTrials. Gov on DC therapy, six studies involve GC patients (Table 1)[132-134] but only three have been terminated (Table 1) and only two have published their results.
| Ref. | Title | Sponsor/collaborator | Status | Duration |
| Kono et al[132], Clin Cancer Res 2002 | Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer | Yamanashi Medical University Japanese Clinical Oncology Fund and from the Public Trust Haraguchi Memorial Cancer Research Fund | Completed published | NA |
| Sadanaga et al[133], Clin Cancer Res 2001 | Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas | Medical Institute of Bioregulation, Kyushu University Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (A) (08557074) | Completed published | Study started: January 1997 Completed: August 2000 |
| http://www.clinicaltrials.gov/ | A phase I study of active immunotherapy with carcinoembryonic antigen RNA-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen | Duke Cancer Institute NCI (NCT00004604) | Unknow1 | Study first received: May 2, 2000 Last updated: December 13, 2011 |
| http://www.clinicaltrials.gov/ | A pilot study of active immunotherapy with HER2/neu intracellular domain protein-pulsed, autologous, cultured dendritic cells in patients with no evidence of disease after standard treatment for HER2/neu expressing malignancies | Duke Cancer Institute NCI (NCT00005956) | Unknow1 | Study first received: July 5, 2000 Last updated: December 13, 2011 |
| http://www.clinicaltrials.gov/ | A phase I study of active Immunotherapy with autologous dendritic cells infected with CEA-6D expressing fowlpox -tricom in patients with advanced or metastatic malignancies expressing CEA | Duke Cancer Institute NCI (NCT00027534) | Completed | Study first received: December 7, 2001 Last updated: December 13, 2011 |
| http://www.clinicaltrials.gov/ | A Phase I clinical trial of mTOR inhibition with rapamycin for enhancing intranodal dendritic cell vaccine induced anti-tumor immunity in patients with NY-ESO-1 expressing solid tumors | Roswell Park Cancer Institute (NCT01522820) | Not yet recruiting | Study first received: January 25, 2012 Last updated: February 3, 2012 |
Kono et al[132] reported a phase-I clinical study of GC patients treated with DCs pulsed with HER-2/neuro-peptides. After the vaccination, one (out of 9 patients HER-2/neu+) showed decreased levels of CEA and CA19-9 while two registered a significative cancer regression (> 50%). Of note, the vaccine protocol did not register considerable side effects.
Recently Sadanaga et al[133] published the results of a phase-I trial, where twelve patients, with advanced gastrointestinal carcinoma, were immunized with DC pulsed with MAGE-3 peptides without significant side effects. After vaccination, in four patients was registered the presence of peptide-specific CTL while in seven was observed the serum decrease of cancer markers. In addition, small cancer regressions were highlighted in three patients.
Finally, Galetto et al[135] described that memory T cell specific to GC antigens could be activated by cancer-loaded autologous DCs, isolated from blood mononuclear cells and activated by stimulation with apoptotic autologous tumor cells.
Nevertheless, the clinical application of DC vaccines has been limited for the the short lifespan of DCs, and one of the factors threatening DC survival is antigen-specific CD8+ that acquire cytolytic activities after activation by DCs[136].
In recent times, Kim et al[98] ameliorated the efficiency of a DC vaccine with a small interfering RNA (siRNA) targeting phosphatase and tensin homolog (PTEN), that has a key role as a negative regulator in the signal transduction of the PI3K/AKT pathway[137].
The PTEN downregulation in DCs resulted in AKT-dependent maturation, which generated a considerable surface hyperexpression of costimulatory molecules and the chemokine receptor, CCR7, leading to an increased T cell activation in vitro and a migration to a draining lymph node in vivo, respectively. In addition, the PTEN siRNA transfected DCs (DC/siPTEN) showed an augmented survival and most remarkably, DC/siPTEN generated a major number of cancer-specific Tc cells and a stronger anticancer response in vaccinated mice compared to the controls.
In short, these data suggest that manipulation of the PI3K/AKT pathway with the siRNA system could improve the efficacy of a DC-based tumor vaccine, such as in GC treatment.
Immunosuppressive factors, such as IL-10, secreted by DCs (or other regulatory cells) downregulates the Dcs functionality by specific surface receptors (e.g., IL-10R). Recent data showed that the targeting IL-10 receptor with siRNA, can increase the effectiveness of DC-based vaccine, suggesting a potential clinical use of siRNA[138].
In addition, very interesting are the results of He et al[139] concerning the opportunity of increasing the anticancer immunity through GM-CSF gene-modified DCs. After GM-CSF gene modification, DCs are able to secrete elevated levels of GM-CSF and have a major propensity to be maturated. In this way, the DCs increase their ability of activating the proliferation of T cells. In addition, in vitro the dendritic cells with GM-CSF gene modified can stimulate specific CTL to kill the cancer cells.
Finally, in comparison with the vaccination alone, DCs vaccination and the preventive removal of Tregs substantially enhances the activation of tumor-specific T-cell responses[140].
The NK cells are able to arrest the metastatic spreading of human cancers[141] and also, the intra-cancer infiltration of NK cells is associated with a better prognosis of GC patients[142]. The key function of NK cells in anti-tumor response gives us the possibility to counteract the cancer progression by manipulating the NK cell “arms”. However, some obstacles make it difficult to the therapeutic use of NK cell-based treatments: (1) unfinished characterization of the specific function of the different NK cell subpopulations; (2) little knowledge of the mechanisms involved in NK functionality; (3) the exiguous amount of blood NK cells; and (4) the troubles about a massive production in good manufacturing practices (GMP) of NK cells[143].
About gastric cancer, a recent study evaluated the NK number in 72 patients with gastric adenocarcinoma and its correlation with patient survival. The conclusions are that patients with high concentration of NK cells showed a higher survival rate when compared to the low concentration, especially in the advanced stage[144].
Moreover, very interesting data have been obtained by Saito et al[145] which demonstrated that the frequency of apoptotic NK cells in GC patients was significantly higher than in normal controls. Moreover, their frequency was related to the GC progression. Fas+ NK cells were significantly more common in GC patients compared with normal controls and Fas expression was closely related to the frequency of NK cell apoptosis. Also, the frequency of tumor-infiltrating NK cell apoptosis was significantly higher than that of circulating NK cell apoptosis. Furthermore, apoptotic circulating NK cells significantly decreased after surgery compared to before surgery.
Finally, Voskens et al[146] showed that numerous cytotoxic NK cells can be obtained from cancer patients co-culturing autologous PBMC with K562 cells. Of note, the ex vivo development increased the cytotoxic activity of NK cells vs the autologous derived, suggesting a future clinical application, as cell-based immunotherapy, for autologous expanded NK cells (as alone as and in association with specific monoclonal antibodies).
Very interesting are the recent results about lupeol, a triterpene that has curative action vs various diseases. Recently, Wu et al[147] showed that lupeol is able to favor the proliferation of NK cells, increasing also their killing action vs the GC cells. Moreover, lupeol inhibit the proliferation of different GC cell lines. These data suggest that lupeol could serve as a potential agent against gastric cancer alone or with adoptive transfer of NK cells.
We have used this review to provide a panoramic view about the current immunotherapeutic anti-GC approaches, some of which have been used in clinical trials with fairly good results about the tumor regression and patient survival. But, the role of immunotherapy for gastric cancer continues to evolve. As the current development suggests, gastric cancer therapy has suffered from a relative lack of gastric-specific biological exploration. The most common developmental path to date has been limited to the study of immune-based therapies that have demonstrated efficacy in other somewhat similar diseases, and then have been tested in gastric cancer.
Moreover, an additional great challenge in the field is to develop randomized clinical trials validating the medical benefits to justify the logistics and especially the costs of these personalized cell treatments.
Usually the clinical trials enroll patients with advanced GC, this factor could determine an unfavorable result, because the anti-cancer battle of current immune response is already a lot compromised. For this purpose, it would be strategic to recruit early-stage GC patients, that being in the early stages of tumor development, may better react to the immunotherapy strategies.
Finally, to set up successful anti-GC immunotherapy approaches, it is necessary to understand the “fine” immune escape mechanisms, adopted by the cells of gastric cancer.
Past and recent studies have supplied new insights about the thick crosstalk between tumor and immune cells. Comprehending this operative dialogue and the hierarchic grade of the various cancer-immune evasion mechanisms at distinct steps of neoplastic evolution, will guide the development of innovative curative strategies aiming to demolish the “tumor fortress”.
So, it will be remarkable to evaluate the pathways of the various components that modulate the growth and mobility of Tregs, MDSCs and tolerogenic DCs within cancer-draining lymph nodes and the cancer surroundings.
Another important target for the future anti-GC immunotherapy treatment could be the immune checkpoints[148] that are inhibitory pathways hardwired into the immune system. The immune checkpoints play critical roles for physiological homeostasis because they are essential for maintaining self-tolerance and modulating the duration and amplitude of physiological immune responses in order to minimize collateral tissue damage. But, these checkpoints may also allow immune escape in cancer.
Checkpoint pathways are regulated by ligand/receptor interactions. For example, programmed death-1 receptor (PD-1) and CTL-associated antigen 4 (CTLA-4) are inhibitory molecules whose presence on lymphocytes signifies a blunted immune response. PD-1 negatively regulates T cell responses and downregulation and eventually apoptosis is initiated following binding of a PD-1 ligand with PD-1. PD-1 ligands, PD-L1 or PD-L2, are frequently expressed on tumor cells and can thus thwart the immune response. One approach to overcome this inhibition of the immune response has been to target immune checkpoints with blocking moAb. For example, PD-1 moAb binds to the PD-1 receptors on T cells and inhibits their binding to the ligands on tumor cells thus preventing the tumors from down regulating the cytotoxic lymphocyte response. This approach has been successful clinically in advanced melanoma[149,150]. and phase I clinical trials of anti-PD-L1 moAb are under investigation for gastric cancer[148].
It is realistic to declare that, in the future effective anti-GC immunotherapy strategies must include combined approaches, which should use both systemic radio/chemotherapy and transplantation, to diminish the burden or to remove immune suppressive cells, and tailored immunotherapies customized to each single patient.
P- Reviewer: Guo ZS S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 783] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 2. | Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 324] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 3. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 4. | Haenszel W, Kurihara M, Segi M, Lee RK. Stomach cancer among Japanese in Hawaii. J Natl Cancer Inst. 1972;49:969-988. [PubMed] |
| 5. | Terry P, Nyrén O, Yuen J. Protective effect of fruits and vegetables on stomach cancer in a cohort of Swedish twins. Int J Cancer. 1998;76:35-37. [PubMed] |
| 6. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 7. | Schuhmacher C, Reim D, Novotny A. Neoadjuvant treatment for gastric cancer. J Gastric Cancer. 2013;13:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4080] [Article Influence: 313.8] [Reference Citation Analysis (0)] |
| 9. | Kofoed SC, Muhic A, Baeksgaard L, Jendresen M, Gustafsen J, Holm J, Bardram L, Brandt B, Brenø J, Svendsen LB. Survival after adjuvant chemoradiotherapy or surgery alone in resectable adenocarcinoma at the gastro-esophageal junction. Scand J Surg. 2012;101:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Oh DY, Bang YJ. Adjuvant and neoadjuvant therapy for gastric cancer. Curr Treat Options Oncol. 2013;14:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45:931-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 12. | Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;CD004064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 350] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 13. | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991-4997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1458] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 14. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1693] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 15. | Okines AF, Norman AR, McCloud P, Kang YK, Cunningham D. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol. 2009;20:1529-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2219] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 17. | Elkord E, Hawkins RE, Stern PL. Immunotherapy for gastrointestinal cancer: current status and strategies for improving efficacy. Expert Opin Biol Ther. 2008;8:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Niccolai E, Prisco D, D’Elios MM, Amedei A. What is recent in pancreatic cancer immunotherapy? Biomed Res Int. 2013;492372. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6952] [Article Influence: 316.0] [Reference Citation Analysis (0)] |
| 20. | Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1466] [Cited by in RCA: 1561] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 21. | Yarden Y, Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1329] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 22. | Itakura Y, Sasano H, Shiga C, Furukawa Y, Shiga K, Mori S, Nagura H. Epidermal growth factor receptor overexpression in esophageal carcinoma. An immunohistochemical study correlated with clinicopathologic findings and DNA amplification. Cancer. 1994;74:795-804. [PubMed] |
| 23. | Kitagawa Y, Ueda M, Ando N, Ozawa S, Shimizu N, Kitajima M. Further evidence for prognostic significance of epidermal growth factor receptor gene amplification in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 1996;2:909-914. [PubMed] |
| 24. | Gibault L, Metges JP, Conan-Charlet V, Lozac’h P, Robaszkiewicz M, Bessaguet C, Lagarde N, Volant A. Diffuse EGFR staining is associated with reduced overall survival in locally advanced oesophageal squamous cell cancer. Br J Cancer. 2005;93:107-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Wilkinson NW, Black JD, Roukhadze E, Driscoll D, Smiley S, Hoshi H, Geradts J, Javle M, Brattain M. Epidermal growth factor receptor expression correlates with histologic grade in resected esophageal adenocarcinoma. J Gastrointest Surg. 2004;8:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, McAllister PK, Morton RF, Schilsky RL. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 895] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 27. | Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2504] [Cited by in RCA: 2403] [Article Influence: 141.4] [Reference Citation Analysis (0)] |
| 28. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2901] [Cited by in RCA: 3126] [Article Influence: 195.4] [Reference Citation Analysis (1)] |
| 29. | Janmaat ML, Gallegos-Ruiz MI, Rodriguez JA, Meijer GA, Vervenne WL, Richel DJ, Van Groeningen C, Giaccone G. Predictive factors for outcome in a phase II study of gefitinib in second-line treatment of advanced esophageal cancer patients. J Clin Oncol. 2006;24:1612-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311-1318. [PubMed] |
| 31. | Baselga J, Norton L, Masui H, Pandiella A, Coplan K, Miller WH, Mendelsohn J. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst. 1993;85:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 279] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 32. | Kawaguchi Y, Kono K, Mimura K, Sugai H, Akaike H, Fujii H. Cetuximab induce antibody-dependent cellular cytotoxicity against EGFR-expressing esophageal squamous cell carcinoma. Int J Cancer. 2007;120:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6:714-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 554] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 34. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3767] [Cited by in RCA: 3708] [Article Influence: 176.6] [Reference Citation Analysis (1)] |
| 35. | Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1472] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 36. | Foon KA, Yang XD, Weiner LM, Belldegrun AS, Figlin RA, Crawford J, Rowinsky EK, Dutcher JP, Vogelzang NJ, Gollub J. Preclinical and clinical evaluations of ABX-EGF, a fully human anti-epidermal growth factor receptor antibody. Int J Radiat Oncol Biol Phys. 2004;58:984-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 37. | Vanhoefer U, Tewes M, Rojo F, Dirsch O, Schleucher N, Rosen O, Tillner J, Kovar A, Braun AH, Trarbach T. Phase I study of the humanized antiepidermal growth factor receptor monoclonal antibody EMD72000 in patients with advanced solid tumors that express the epidermal growth factor receptor. J Clin Oncol. 2004;22:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 38. | Rao S, Starling N, Cunningham D, Benson M, Wotherspoon A, Lüpfert C, Kurek R, Oates J, Baselga J, Hill A. Phase I study of epirubicin, cisplatin and capecitabine plus matuzumab in previously untreated patients with advanced oesophagogastric cancer. Br J Cancer. 2008;99:868-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Pinto C, Di Fabio F, Siena S, Cascinu S, Rojas Llimpe FL, Ceccarelli C, Mutri V, Giannetta L, Giaquinta S, Funaioli C. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study). Ann Oncol. 2007;18:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 40. | Enzinger PC, Burtness B, Hollis D, Niedzwiecki D, Ilson D, Benson AB, Mayer RJ, Goldberg RM. CALGB 80403/ECOG 1206: A randomized phase II study of three standard chemotherapy regimens (ECF, IC, FOLFOX) plus cetuximab in metastatic esophageal and GE junction cancer. J Clin Oncol. 2010;28 Suppl 15:4006. |
| 41. | Okines AF, Ashley SE, Cunningham D, Oates J, Turner A, Webb J, Saffery C, Chua YJ, Chau I. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for advanced esophagogastric cancer: dose-finding study for the prospective multicenter, randomized, phase II/III REAL-3 trial. J Clin Oncol. 2010;28:3945-3950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 42. | Safran H, Suntharalingam M, Dipetrillo T, Ng T, Doyle LA, Krasna M, Plette A, Evans D, Wanebo H, Akerman P. Cetuximab with concurrent chemoradiation for esophagogastric cancer: assessment of toxicity. Int J Radiat Oncol Biol Phys. 2008;70:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Ruhstaller T, Pless M, Dietrich D, Kranzbuehler H, von Moos R, Moosmann P, Montemurro M, Schneider PM, Rauch D, Gautschi O. Cetuximab in combination with chemoradiotherapy before surgery in patients with resectable, locally advanced esophageal carcinoma: a prospective, multicenter phase IB/II Trial (SAKK 75/06). J Clin Oncol. 2011;29:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Kleinberg LR, Catalano PJ, Gibson MK, Staley CA, Montgomery EA, Song W, Mulcahy MF, Leichman LP, Benson AB. ECOG 2205: A phase II study to measure response rate and toxicity of neo-adjuvant chemoradiotherapy (CRT) (IMRT permitted) with oxaliplatin and infusional 5-fluorouracil plus cetuximab in patients with operable adenocarcinoma of the esophagus: High risk of post-op adult respiratory distress syndrome. Int J Radiat Oncol Biol Phys. 2011;78:S72. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Hurt CN, Nixon LS, Griffiths GO, Al-Mokhtar R, Gollins S, Staffurth JN, Phillips CJ, Blazeby JM, Crosby TD. SCOPE1: a randomised phase II/III multicentre clinical trial of definitive chemoradiation, with or without cetuximab, in carcinoma of the oesophagus. BMC Cancer. 2011;11:466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Casalini P, Iorio MV, Galmozzi E, Ménard S. Role of HER receptors family in development and differentiation. J Cell Physiol. 2004;200:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 47. | al-Kasspooles M, Moore JH, Orringer MB, Beer DG. Amplification and over-expression of the EGFR and erbB-2 genes in human esophageal adenocarcinomas. Int J Cancer. 1993;54:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 165] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 49. | Brien TP, Odze RD, Sheehan CE, McKenna BJ, Ross JS. HER-2/neu gene amplification by FISH predicts poor survival in Barrett’s esophagus-associated adenocarcinoma. Hum Pathol. 2000;31:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1925] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 51. | Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3742] [Cited by in RCA: 3681] [Article Influence: 184.1] [Reference Citation Analysis (0)] |
| 52. | Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4010] [Cited by in RCA: 3923] [Article Influence: 196.2] [Reference Citation Analysis (0)] |
| 53. | Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1060] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 54. | Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639-2648. [PubMed] |
| 55. | Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 905] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 56. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8204] [Cited by in RCA: 8139] [Article Influence: 339.1] [Reference Citation Analysis (0)] |
| 57. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5326] [Article Influence: 355.1] [Reference Citation Analysis (3)] |
| 58. | Safran H, Dipetrillo T, Akerman P, Ng T, Evans D, Steinhoff M, Benton D, Purviance J, Goldstein L, Tantravahi U. Phase I/II study of trastuzumab, paclitaxel, cisplatin and radiation for locally advanced, HER2 overexpressing, esophageal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2007;67:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Pegram MD, Blackwell K, Miles D, Bianchi GV, Krop IE, Welslau M, Baselga J, Oh D, Dieras V, Guardino E. Primary results from EMILIA, a phase III study of trastuzumab emtansine (T-DM1) vs capecitabine and lapatinib in HER2-positive locally advanced or metastatic breast cancer previously treated with trastuzumab and a taxane. J Clin Oncol. 2012;30 suppl 27:abstract 98. |
| 60. | Hong TS, Wo JY, Kwak EL. Targeted therapies with chemoradiation in esophageal cancer: development and future directions. Semin Radiat Oncol. 2013;23:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer. 2006;42:3127-3139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 62. | Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 902] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 63. | Inoue K, Ozeki Y, Suganuma T, Sugiura Y, Tanaka S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer. 1997;79:206-213. [PubMed] |
| 64. | Kitadai Y, Haruma K, Tokutomi T, Tanaka S, Sumii K, Carvalho M, Kuwabara M, Yoshida K, Hirai T, Kajiyama G. Significance of vessel count and vascular endothelial growth factor in human esophageal carcinomas. Clin Cancer Res. 1998;4:2195-2200. [PubMed] |
| 65. | Kleespies A, Guba M, Jauch KW, Bruns CJ. Vascular endothelial growth factor in esophageal cancer. J Surg Oncol. 2004;87:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 66. | Shih CH, Ozawa S, Ando N, Ueda M, Kitajima M. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2000;6:1161-1168. [PubMed] |
| 67. | Imdahl A, Bognar G, Schulte-Mönting J, Schöffel U, Farthmann EH, Ihling C. Predictive factors for response to neoadjuvant therapy in patients with oesophageal cancer. Eur J Cardiothorac Surg. 2002;21:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Shimada H, Hoshino T, Okazumi S, Matsubara H, Funami Y, Nabeya Y, Hayashi H, Takeda A, Shiratori T, Uno T. Expression of angiogenic factors predicts response to chemoradiotherapy and prognosis of oesophageal squamous cell carcinoma. Br J Cancer. 2002;86:552-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858-863. [PubMed] |
| 70. | Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Yanoma S, Noguchi Y. Plasma concentrations of VEGF and bFGF in patients with gastric carcinoma. Cancer Lett. 2000;153:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7733] [Article Influence: 368.2] [Reference Citation Analysis (1)] |
| 72. | Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4457] [Cited by in RCA: 4447] [Article Influence: 234.1] [Reference Citation Analysis (0)] |
| 73. | Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2393] [Cited by in RCA: 2302] [Article Influence: 127.9] [Reference Citation Analysis (0)] |
| 74. | Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1573] [Cited by in RCA: 1573] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 75. | Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1443] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 76. | Shah MA, Ramanathan RK, Ilson DH, Levnor A, D’Adamo D, O’Reilly E, Tse A, Trocola R, Schwartz L, Capanu M. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201-5206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 302] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 77. | Shah MA, Jhawer M, Ilson DH, Lefkowitz RA, Robinson E, Capanu M, Kelsen DP. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol. 2011;29:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 78. | Enzinger PC, Ryan DP, Regan E, Lehman N, Abrams TA, Hezel AF, Fidias P, Sequist LV, Blaszkowsky LS, Fuchs CS. Phase II trial of docetaxel, cisplatin, irinotecan and bevacizumab in patients with metastatic esophagogastric cancer (abstract 97). San Francisco, CA: Gastrointestinal Cancers Symposium 2008; Jan 25. |
| 79. | Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968-3976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 887] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 80. | Willett CG, Boucher Y, Duda DG, di Tomaso E, Munn LL, Tong RT, Kozin SV, Petit L, Jain RK, Chung DC. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136-8139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 320] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 81. | Bendell JC, Meluch A, Peyton J, Rubin M, Waterhouse D, Webb C, Burris HA, Hainsworth JD. A phase II trial of preoperative concurrent chemotherapy/radiation therapy plus bevacizumab/erlotinib in the treatment of localized esophageal cancer. Clin Adv Hematol Oncol. 2012;10:430-437. [PubMed] |
| 82. | Ilson D, Goodman KA, Janjiigian YY, Shah MA, Kelsen DP, Rizk NP, Rusch VW, Wu AJ, Campbell J, Capanu M, Bains MS, Memorial Sloan-Kettering Cancer Center, New York, NY, Gastrointestinal Oncology Service, Memorial Sloan-Kettering Cancer Center, New York, NY Phase II trial of bevacizumab, irinotecan, cisplatin, and radiation as preoperative therapy in esophageal adenocarcinoma. J Clin Oncol. 2012;20 suppl 4:abstract 67. |
| 83. | Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 969] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 84. | Eder JP, Vande Woude GF, Boerner SA, LoRusso PM. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15:2207-2214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 420] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 85. | Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1992] [Cited by in RCA: 2088] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 86. | Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, Schwall R, Ferrara N, Gerritsen ME. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol. 2001;158:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 302] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 87. | Smolen GA, Sordella R, Muir B, Mohapatra G, Barmettler A, Archibald H, Kim WJ, Okimoto RA, Bell DW, Sgroi DC. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci USA. 2006;103:2316-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 427] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 88. | Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3442] [Cited by in RCA: 3668] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 89. | Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, Toschi L, Rogers A, Mok T, Sequist L. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 855] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 90. | Ou SH, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW, Camidge DR, Solomon BJ, Maki RG, Bang YJ. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6:942-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 355] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 91. | Ma PC, Tretiakova MS, MacKinnon AC, Ramnath N, Johnson C, Dietrich S, Seiwert T, Christensen JG, Jagadeeswaran R, Krausz T. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes Cancer. 2008;47:1025-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 256] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 92. | Ma PC, Tretiakova MS, Nallasura V, Jagadeeswaran R, Husain AN, Salgia R. Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: implications for tumour invasion. Br J Cancer. 2007;97:368-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 93. | Oliner KS, Tang R, Anderson A, Lan Y, Iveson T, Donehower RC, Jiang Y, Dubey S, Loh E, Amgen Inc. Thousand Oaks, CA; Southampton General Hospital, Southampton, United Kingdom; Johns Hopkins Cancer Center, Baltimore, MD; Amgen Inc., South San Francisco, CA Evaluation of MET pathway biomarkers in a phase II study of rilotumumab (R, AMG 102) or placebo in combination with epirubicin, cisplatin, and capecitabine (ECX) in patients with locally advanced or metastatic gastric or esophagogastric junction cancer. J Clin Oncol. 2012;30:4005. |
| 94. | Zeier M, Hartschuh W, Wiesel M, Lehnert T, Ritz E. Malignancy after renal transplantation. Am J Kidney Dis. 2002;39:E5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 4910] [Article Influence: 258.4] [Reference Citation Analysis (0)] |
| 96. | Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2258] [Cited by in RCA: 2109] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 97. | Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766-4772. [PubMed] |
| 98. | Kim JH, Kang TH, Noh KH, Kim SH, Lee YH, Kim KW, Bae HC, Ahn YH, Choi EY, Kim JS. Enhancement of DC vaccine potency by activating the PI3K/AKT pathway with a small interfering RNA targeting PTEN. Immunol Lett. 2010;134:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 100. | Eberts D, Fatho M, Lennerz V, Schmidt C, Van Der Bruggen P, Woelfel C, Woelfel T, Melanoma-associated Mhc Class I associated oligopeptides and the uses thereof; WO/2007/025760, 2007. . |
| 101. | Argonex Pharmaceuticals. Cytotoxic T Lymphocyte-stimulation peptides for prevention, treatment, and diagnosis of melanoma. WO/2001/032193. San Francisco, CA: Gastrointestinal Cancers Symposium 2001; . |
| 102. | Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168-16173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 908] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 103. | Gang Y, Zhang X, He Y, Zheng J, Wu K, Ding J, Fan D. Efficient induction of specific cytotoxic T lymphocytes against gastric adenocarcinoma by a survivin peptide. Biochem Cell Biol. 2012;90:701-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 104. | Kawamoto M, Tanaka F, Mimori K, Inoue H, Kamohara Y, Mori M. Identification of HLA-A*0201/-A*2402-restricted CTL epitope-peptides derived from a novel cancer/testis antigen, MCAK, and induction of a specific antitumor immune response. Oncol Rep. 2011;25:469-476. [PubMed] |
| 105. | Kim YJ, Lim J, Kang JS, Kim HM, Lee HK, Ryu HS, Kim JY, Hong JT, Kim Y, Han SB. Adoptive immunotherapy of human gastric cancer with ex vivo expanded T cells. Arch Pharm Res. 2010;33:1789-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Sangiolo D. Cytokine induced killer cells as promising immunotherapy for solid tumors. J Cancer. 2011;2:363-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 107. | Hoshino T, Seki N, Kikuchi M, Kuramoto T, Iwamoto O, Kodama I, Koufuji K, Takeda J, Itoh K. HLA class-I-restricted and tumor-specific CTL in tumor-infiltrating lymphocytes of patients with gastric cancer. Int J Cancer. 1997;70:631-638. [PubMed] |
| 108. | Kono K, Rongcun Y, Charo J, Ichihara F, Celis E, Sette A, Appella E, Sekikawa T, Matsumoto Y, Kiessling R. Identification of HER2/neu-derived peptide epitopes recognized by gastric cancer-specific cytotoxic T lymphocytes. Int J Cancer. 1998;78:202-208. [PubMed] |
| 109. | Guo DL, Dong M, Wang L, Sun LP, Yuan Y. Expression of gastric cancer-associated MG7 antigen in gastric cancer, precancerous lesions and H. pylori -associated gastric diseases. World J Gastroenterol. 2002;8:1009-1013. [PubMed] |
| 110. | Wu K, Nie Y, Guo C, Chen Y, Ding J, Fan D. Molecular basis of therapeutic approaches to gastric cancer. J Gastroenterol Hepatol. 2009;24:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 111. | Nie Y, Wu K, Yang J, Tian F, Li L, Chen B, Fan D. Induction of T lymphocytes specific to human gastric cancer using HLA-A matched allogeneic gastric tumor cells. J Immunother. 2003;26:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 112. | Jiang J, Xu N, Wu C, Deng H, Lu M, Li M, Xu B, Wu J, Wang R, Xu J. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res. 2006;26:2237-2242. [PubMed] |
| 113. | Wu C, Jiang J, Shi L, Xu N. Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer. Anticancer Res. 2008;28:3997-4002. [PubMed] |
| 114. | Du X, Jin R, Ning N, Li L, Wang Q, Liang W, Liu J, Xu Y. In vivo distribution and antitumor effect of infused immune cells in a gastric cancer model. Oncol Rep. 2012;28:1743-1749. [PubMed] |
| 115. | Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 557] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 116. | Alexander RB, Rosenberg SA. Long-term survival of adoptively transferred tumor-infiltrating lymphocytes in mice. J Immunol. 1990;145:1615-1620. [PubMed] |
| 117. | Kono K, Takahashi A, Ichihara F, Amemiya H, Iizuka H, Fujii H, Sekikawa T, Matsumoto Y. Prognostic significance of adoptive immunotherapy with tumor-associated lymphocytes in patients with advanced gastric cancer: a randomized trial. Clin Cancer Res. 2002;8:1767-1771. [PubMed] |
| 118. | Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 699] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 119. | Tomsová M, Melichar B, Sedláková I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 120. | Amedei A, Niccolai E, Benagiano M, Della Bella C, Cianchi F, Bechi P, Taddei A, Bencini L, Farsi M, Cappello P. Ex vivo analysis of pancreatic cancer-infiltrating T lymphocytes reveals that ENO-specific Tregs accumulate in tumor tissue and inhibit Th1/Th17 effector cell functions. Cancer Immunol Immunother. 2013;62:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 121. | Amedei A, Niccolai E, Della Bella C, Cianchi F, Trallori G, Benagiano M, Bencini L, Bernini M, Farsi M, Moretti R. Characterization of tumor antigen peptide-specific T cells isolated from the neoplastic tissue of patients with gastric adenocarcinoma. Cancer Immunol Immunother. 2009;58:1819-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 122. | Amedei A, Munari F, Bella CD, Niccolai E, Benagiano M, Bencini L, Cianchi F, Farsi M, Emmi G, Zanotti G. Helicobacter pylori secreted peptidyl prolyl cis, trans-isomerase drives Th17 inflammation in gastric adenocarcinoma. Intern Emerg Med. 2014;9:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 123. | Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136:1585-1595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 124. | Kim HI, Kim H, Cho HW, Kim SY, Song KJ, Hyung WJ, Park CG, Kim CB. The ratio of intra-tumoral regulatory T cells (Foxp3+)/helper T cells (CD4+) is a prognostic factor and associated with recurrence pattern in gastric cardia cancer. J Surg Oncol. 2011;104:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 125. | Caux C, Ait-Yahia S, Chemin K, de Bouteiller O, Dieu-Nosjean MC, Homey B, Massacrier C, Vanbervliet B, Zlotnik A, Vicari A. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22:345-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 217] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 126. | Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 735] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 127. | Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996;183:283-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 547] [Cited by in RCA: 537] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 128. | Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 451] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 129. | Ishigami S, Natsugoe S, Uenosono Y, Hata Y, Nakajo A, Miyazono F, Matsumoto M, Hokita S, Aikou T. Infiltration of antitumor immunocytes into the sentinel node in gastric cancer. J Gastrointest Surg. 2003;7:735-739. [PubMed] |
| 130. | Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Xiangming C, Iwashige H, Aridome K, Hokita S, Aikou T. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Lett. 2000;159:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 131. | Tsujitani S, Kakeji Y, Orita H, Watanabe A, Kohnoe S, Baba H, Anai H, Maehara Y, Sugimachi K. Postoperative adjuvant immunochemotherapy and infiltration of dendritic cells for patients with advanced gastric cancer. Anticancer Res. 1992;12:645-648. [PubMed] |
| 132. | Kono K, Takahashi A, Sugai H, Fujii H, Choudhury AR, Kiessling R, Matsumoto Y. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002;8:3394-3400. [PubMed] |
| 133. | Sadanaga N, Nagashima H, Mashino K, Tahara K, Yamaguchi H, Ohta M, Fujie T, Tanaka F, Inoue H, Takesako K. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin Cancer Res. 2001;7:2277-2284. [PubMed] |
| 134. | Available from: http://www.clinicaltrials.gov/. |
| 135. | Galetto A, Contarini M, Sapino A, Cassoni P, Consalvo E, Forno S, Pezzi C, Barnaba V, Mussa A, Matera L. Ex vivo host response to gastrointestinal cancer cells presented by autologous dendritic cells. J Surg Res. 2001;100:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 136. | Kim TW, Hung CF, Ling M, Juang J, He L, Hardwick JM, Kumar S, Wu TC. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest. 2003;112:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 137. | Leslie NR, Batty IH, Maccario H, Davidson L, Downes CP. Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene. 2008;27:5464-5476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 138. | Kim JH, Kang TH, Noh KH, Bae HC, Ahn YH, Lee YH, Choi EY, Chun KH, Lee SJ, Kim TW. Blocking the immunosuppressive axis with small interfering RNA targeting interleukin (IL)-10 receptor enhances dendritic cell-based vaccine potency. Clin Exp Immunol. 2011;165:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 139. | He SB, Sun K, Wang L, Li DC, Zhang YY. [GM-CSF gene-modified dendritic cell vaccine enhances antitumor immunity in vitro]. Zhonghua Zhongliu Zazhi. 2010;32:410-414. [PubMed] |
| 140. | Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623-3633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 726] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 141. | Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci USA. 2000;97:2731-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 346] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 142. | Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320-2328. [PubMed] |
| 143. | Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 144. | Rosso D, Rigueiro MP, Kassab P, Ilias EJ, Castro OA, Novo NF, Lourenço LG. [Correlation of natural killer cells with the prognosis of gastric adenocarcinoma]. Arq Bras Cir Dig. 2012;25:114-117. [PubMed] |
| 145. | Saito H, Takaya S, Osaki T, Ikeguchi M. Increased apoptosis and elevated Fas expression in circulating natural killer cells in gastric cancer patients. Gastric Cancer. 2013;16:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 146. | Voskens CJ, Watanabe R, Rollins S, Campana D, Hasumi K, Mann DL. Ex-vivo expanded human NK cells express activating receptors that mediate cytotoxicity of allogeneic and autologous cancer cell lines by direct recognition and antibody directed cellular cytotoxicity. J Exp Clin Cancer Res. 2010;29:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 147. | Wu XT, Liu JQ, Lu XT, Chen FX, Zhou ZH, Wang T, Zhu SP, Fei SJ. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int Immunopharmacol. 2013;16:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 148. | Matsueda S, Graham DY. Immunotherapy in gastric cancer. World J Gastroenterol. 2014;20:1657-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 149. | Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3331] [Cited by in RCA: 3409] [Article Influence: 243.5] [Reference Citation Analysis (0)] |
| 150. | Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |