Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5751
Peer-review started: November 25, 2014
First decision: January 8, 2015
Revised: February 3, 2015
Accepted: March 12, 2015
Article in press: March 12, 2015
Published online: May 14, 2015
Processing time: 175 Days and 0.8 Hours
Children on exclusive jejunal feeding may be at risk of iron deficiency due to the feeds bypassing the duodenum, which is the primary site for iron absorption. We describe the biochemical and hematological features of six children on exclusive jejunal feeding who did not receive iron supplementation. At a mean (standard deviation) period of 11 (6.5) mo after commencing jejunal feeds, there was a significant reduction in both serum iron (18.5 g/L vs 9.8 g/L, P = 0.01) and transferrin saturation levels (23.1% vs 13.7%, P = 0.02), suggesting iron deficiency. However, there was no significant change in ferritin, hemoglobin and mean corpuscular volume levels post-commencement of jejunal feeds. This may be the result of small bowel adaptation in response to early iron deficiency. Larger and longer term prospective studies are required to investigate if children on jejunal feeds are at risk of developing iron deficiency.
Core tip: Children who are exclusively jejunally fed are prime candidates to develop iron deficiency. We found that serum iron and transferrin saturation levels were significantly reduced at a mean of 6.5 mo post commencement of exclusive jejunal feeds, suggesting that an early iron deficient state was induced. Ferritin levels, haemoglobin and mean corpuscular volumes however were stable during the same period, lending further evidence that the proximal jejunum may have the capacity to adapt to iron deficiency.
- Citation: Tan LZ, Adams SE, Kennedy A, Kepreotes H, Ooi CY. Are children on jejunal feeds at risk of iron deficiency? World J Gastroenterol 2015; 21(18): 5751-5754
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5751.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5751
Normal growth and development in childhood is dependent upon adequate nutritional and caloric intake. Certain paediatric populations are unable to safely feed orally or meet their nutritional and metabolic requirements through oral feeding for various reasons. Jejunal feeding is generally indicated in children with a high risk of aspiration and improves feed tolerance and caloric intake[1]. As jejunal feeds bypass the duodenum, children on exclusive jejunal feeding are theoretically at risk of developing iron deficiency. Thus we read with interest the recent study by Marambio et al[2], which reported the capacity of the proximal jejunum to adapt to an iron deficient state.
The duodenum is acknowledged as the primary site for iron absorption in the small bowel, an observation reported by Wheby[3] in 1970. Several mechanisms have been proposed. Foremost is that the low pH (from gastric acid) found in the duodenum favours the reduction of ferric (Fe3+) to soluble ferrous iron (Fe2+) which is then easily absorbed. As luminal pH becomes neutralized further down the intestine, iron is more likely to form insoluble ferric complexes which are less bioavailable[4]. Newer studies have reported that absorption of soluble iron is facilitated by the Divalent Metal Transporter 1 (DMT1), a transmembrane protein found mainly in duodenal enterocytes[4], and expression of DMT1 decreases along the digestive tract. Furthermore, some patients on jejunal feeds are non-ambulatory, due to their primary underlying disease, and thus may have their estimated energy requirements reduced, limiting their micronutrient intake[5,6]. Absorption of non-heme iron (90% of dietary iron) may also be reduced by proton pump inhibitors and antacids[5], which are commonly prescribed in these patients. Despite this, there is limited published knowledge on the impact of jejunal feeding upon iron levels in this vulnerable population.
We recently performed a retrospective chart review on patients less than 17 years of age who were commenced on exclusive jejunal feeds at the Sydney Children’s Hospital between January 2005 and October 2011. This study was approved by ethics. We excluded patients who were given supplemental iron. There were six patients (50% males) without iron supplementation who had iron studies [serum iron, ferritin, transferrin saturation (TSAT)], hemoglobin and mean corpuscular volume (MCV), performed prior to, and at least 4 wk after commencement of jejunal feeds. Patient characteristics are summarised in Table 1.
| Patient | Age at jejunal feeds commencement (yr) | Weight Z-scores | Primary diagnoses |
| 1 | 6.9 | 1.48 | Severe gastroesophageal reflux disease with oral aversion |
| 2 | 0.5 | -1.47 | Global developmental delay with severe gastroesophageal reflux disease |
| 3 | 14.1 | -0.10 | Gastroparesis and chronic nausea |
| 4 | 2.3 | -1.80 | Craniosynostosis, and developmental delay |
| 5 | 3.3 | -1.10 | Mitochondrial disease with, global developmental delay and severe gastroesophageal reflux disease |
| 6 | 16.3 | -4.00 | Severe gastroesophageal reflux disease and gastroparesis |
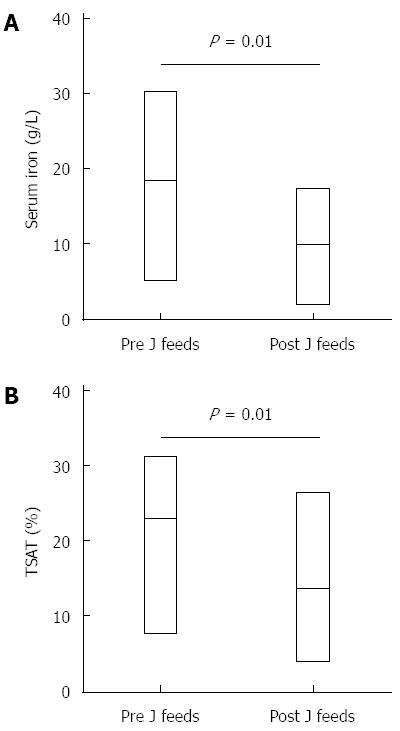
The mean ± SD age at time of jejunal feed commencement was 7.2 (6.5) years. All children were reviewed by a paediatric dietician and fed with commercially available age-appropriate formulas, which provided the recommended daily intake of iron. The mean pre- and post-jejunal feeding levels of serum iron, ferritin, TSAT, hemoglobin and MCV are summarized in Table 2. Investigations were performed at a mean of 11 (6.5) mo after commencing jejunal feeds. There was a significant reduction in serum iron and TSAT levels after commencing jejunal feeds (Figure 1). No significant difference was found in ferritin or hemoglobin levels. There was a reducing trend in MCV levels from pre- to post-commencement of feeds but this was not statistically significant.
| Pre J feeds | Post J feeds mean 11 ± 6.5 mo (range: 2-20 mo) | P value | |
| Serum Iron (g/L) | 18.5 ± 8.6 | 9.8 ± 5.7 (1.9-17.2) | 0.01a |
| (5.2-30.2) | |||
| Transferrin Saturation (%) | 23.1 ± 9.5 | 13.7 ± 8.6 (4.2-26.5) | 0.02a |
| (7.7-31.4) | |||
| Serum Ferritin (μg/L) | 39.8 ± 43.4 | 40.4 ± 43.4 (9.0-112.0) | 0.96 |
| (9.0-112.0) | |||
| Hemoglobin (g/L) | 119.3 ± 28.6 | 129.5± 12.9 (110-148) | 0.45 |
| (69-155) | |||
| MCV (fL) | 85.3 ± 9.2 | 83.8 ± 6.2 (78.3-95.4) | 0.60 |
| (74.1-96.7) | |||
| MCH (pg) | 29.2 ± 2.7 | 30.0 ± 2.4 (26.2-33.4) | 0.78 |
| (27.0-33.9) |
To our knowledge, this is the first case series in children on exclusive jejunal feeding evaluating for and demonstrating biochemical evidence of iron deficiency, with significant decline in serum iron and TSAT. This is of clinical relevance because TSAT (which measures the degree of plasma transferrin, calculated as the ratio of plasma iron to total iron binding capacity) is an indicator of circulating iron, and measures the availability of iron for the bone marrow. Despite levels being depressed by inflammation, a low TSAT is a useful screening measurement for iron deficiency[7,8]. The converse is also true; a normal or high TSAT is often used to exclude iron deficiency[7]. It was significantly lower post-jejunal feeds in this series.
However, similar to findings by Marambio et al[2], this series showed no significant change in ferritin levels. Marambio et al[2] reported that patients 6 mo post-Roux en Y gastric bypass surgery demonstrated no significant decrease in ferritin levels despite the nature of the surgery, in which the duodenum and part of the jejunum are excluded. The authors speculated that this was due to small intestinal adaptation and demonstrated an increased expression of DMT1 at the tips of the jejunal villi on biopsies using immunohistochemistry and western blot analysis[2]. Similarly, in a separate study, the expression of DMT1 was analysed in duodenal biopsy specimens by PCR and was found to correlate negatively with iron status[9].
In our study, there was also no significant difference in hemoglobin and MCV levels although this is less surprising given hemoglobin levels are usually preserved until a large amount of body iron is lost[7,8]. Equally, a reduction in MCV is a late parameter of iron deficiency. Our cohort did exhibit decreased MCV after the commencement of exclusive jejunal feeding but this did not reach statistical significance.
Iron is required for oxygen transport, DNA synthesis, mitochondrial function and protection of cells from oxidative damage. Studies have shown that early iron deficiency interferes with developing interneuronal connections, oligodendrocyte function and white matter myelination[10,11]. Children under the age of two years are particularly vulnerable as this is the period of rapid neurogenesis. Iron deficiency also affects sleep cycles[12], which may result in disturbances in cognitive, motor and emotional function. Iron deficiency states in children may also have significant long-term consequences. Despite correction with iron therapy, early insults appeared to have long lasting effects. A longitudinal 25 year study found that not only did early iron deficiency impact negatively on school achievement, memory and recall, attention and psycho-social behavior[13] it also had significant implications into young adulthood. Individuals with chronic iron deficiency in infancy completed less years of schooling and a higher proportion were not in further education or training and remained single at age 25 years[14].
This study was limited by a small sample size due to our strict exclusion criteria, which focused on a very select group of jejunal-fed patients who were monitored for iron deficiency and yet were not being supplemented with iron. Whether or not enteral iron supplementation would result in a different outcome, or if intestinal adaptation might occur over time to a sufficient extent to compensate for reduced iron absorption in the duodenum warrants further investigation. Future directions may include the use of more accurate and objective quantifiers such as soluble transferrin receptors, zinc protoporphyrin and reticulocyte hemoglobin. It is unclear whether the subjects in this study would have developed iron deficiency anaemia over a longer period of time without iron supplementation. A larger and longer term study is warranted but there may be ethical issues in withholding iron supplementation in already at-risk children.
In conclusion, children on exclusive jejunal feeding are vulnerable to micronutrient deficiencies including iron deficiency. In the short to medium term, the degree of iron deficiency appears mild in severity.
The authors thank Ms Felicity AC Fletcher, formerly of the School of Women’s and Children’s Health, Medicine, University of New South Wales, for her contribution in data acquisition.
P- Reviewer: Al-Haggar M, Classen CF S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Axelrod D, Kazmerski K, Iyer K. Pediatric enteral nutrition. JPEN J Parenter Enteral Nutr. 2006;30:S21-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Marambio A, Watkins G, Castro F, Riffo A, Zúñiga R, Jans J, Villanueva ME, Díaz G. Changes in iron transporter divalent metal transporter 1 in proximal jejunum after gastric bypass. World J Gastroenterol. 2014;20:6534-6540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Wheby MS. Site of iron absorption in man. Scand J Haematol. 1970;7:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Miret S, Simpson RJ, McKie AT. Physiology and molecular biology of dietary iron absorption. Annu Rev Nutr. 2003;23:283-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Muñoz M, Villar I, García-Erce JA. An update on iron physiology. World J Gastroenterol. 2009;15:4617-4626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 211] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 6. | Skelton JA, Havens PL, Werlin SL. Nutrient deficiencies in tube-fed children. Clin Pediatr (Phila). 2006;45:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol. 2005;18:319-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 254] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 8. | Burke RM, Leon JS, Suchdev PS. Identification, prevention and treatment of iron deficiency during the first 1000 days. Nutrients. 2014;6:4093-4114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G, Haile DJ, Vogel W, Weiss G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology. 2001;120:1412-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Beard JL. Why iron deficiency is important in infant development. J Nutr. 2008;138:2534-2536. [PubMed] |
| 11. | McCann JC, Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr. 2007;85:931-945. [PubMed] |
| 12. | Peirano PD, Algarín CR, Chamorro RA, Reyes SC, Durán SA, Garrido MI, Lozoff B. Sleep alterations and iron deficiency anemia in infancy. Sleep Med. 2010;11:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 526] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 14. | Lozoff B, Smith JB, Kaciroti N, Clark KM, Guevara S, Jimenez E. Functional significance of early-life iron deficiency: outcomes at 25 years. J Pediatr. 2013;163:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |