Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5568
Peer-review started: November 19, 2014
First decision: December 11, 2014
Revised: January 2, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: May 14, 2015
Processing time: 180 Days and 10.3 Hours
AIM: To evaluate the efficacy of the 14-d moxifloxacin-based triple therapy for the second-line eradication of Helicobacter pylori (H. pylori) infection.
METHODS: Between 2011 and 2013, we conducted a retrospective review of the medical records of 160 patients who had experienced failure of their first-line proton pump inhibitor-based eradication therapy and subsequently received the moxifloxacin-based triple therapy as a second-line eradication treatment regimen. The patients who were treated with the moxifloxacin-based triple therapy (oral 20 mg rabeprazole b.i.d., 1000 mg amoxicillin b.i.d., and 400 mg moxifloxacin q.d.) for 7 d were assigned to the RAM-7 group (n = 79) while those who took them for 14 days were assigned to RAM-14 group (n = 81). The eradication rates for both groups were determined by intention-to-treat (ITT) and per-protocol (PP) analyses. ITT analysis compared the treatment groups as originally allocated while the PP analysis including only those patients who had completed the treatment as originally allocated. Successful eradication therapy for H. pylori infection was defined as the documentation of a negative 13C-urea breath test 4 wk after the end of the eradication treatment.
RESULTS: The overall ITT eradication rate was 76.2% (122/160). The final ITT eradication rates were 70.8% (56/79; 95%CI: 63.3%-77.1%) in the RAM-7 group and 81.4% (66/81; 95%CI: 74.6%-88.3%) in the RAM-14 group (P = 0.034). The overall PP eradication rate was 84.1% (122/145), and the final PP eradication rates were 77.7% (56/72; 95%CI: 70.2%-85.3%) in the RAM-7 group and 90.4% (66/73; 95%CI: 82.8%-98.1%) in the RAM-14 group (P = 0.017). The H. pylori-eradication rates in the RAM-14 group were significantly higher compared with that of the RAM-7 group according to both the ITT (P = 0.034) and the PP analyses (P = 0.017). Both groups exhibited good treatment compliance (RAM-7/RAM-14 group: 100%/100%). The adverse event rates were 19.4% (14/72) and 20.5% (15/73) in the RAM-7 and RAM-14 groups, respectively (P = 0.441). Adverse events occurred in 14 of the 72 patients (19.4) in the RAM-7 group and in 15 of the 73 patients (20.5) in the RAM-14 group. No statistically significant differences (P = 0.441) were observed.
CONCLUSION: The 14-d moxifloxacin-based triple therapy is a significantly more effective second-line eradication treatment as compared to the 7-d alternative for H. pylori infection in South Korea.
Core tip: This study aimed to evaluate the efficacy of the 14-d moxifloxacin-based triple therapy compared to the corresponding 7-d regimen for second-line Helicobacter pylori (H. pylori) eradication in South Korea. The H. pylori-eradication rates in the RAM-14 group were significantly higher compared to the RAM-7 group for both the intention-to-treat and per-protocol analysis. The high eradication rate, excellent compliance, and safety of the 14-d regimen suggest its potential suitability as an alternative to the standard bismuth-based quadruple therapy. The 14-d moxifloxacin-based triple therapy is a significantly more effective second-line eradication treatment than the 7-d alternative for H. pylori infection in Korean patients.
-
Citation: Hwang JJ, Lee DH, Lee AR, Yoon H, Shin CM, Park YS, Kim N. Efficacy of 14-d
vs 7-d moxifloxacin-based triple regimens for second-lineHelicobacter pylori eradication. World J Gastroenterol 2015; 21(18): 5568-5574 - URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5568.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5568
Helicobacter pylori (H. pylori) infection is the single most important factor causing chronic atrophic gastritis, peptic ulcer disease, gastric cancer as well as gastric mucosa associated lymphoid tissue lymphoma[1]. The eradication of H. pylori infection effectively reduces the incidence of peptic ulcer and gastric cancer, and prevents their recurrence[2]. The most important first-line treatment for the eradication of H. pylori is currently the standard triple therapy composed of a proton pump inhibitor (PPI), clarithromycin, and either amoxicillin or metronidazole[3,4]. Although many studies have reported excellent results with this therapy, eradication rates vary widely between 70% and 95%[5,6], with a tendency to decrease due to increasing antibiotic resistance[7,8]. For those patients who failed their first-line eradication therapy, second-line eradication therapy for persistent H. pylori infection is required.
Many alternative, second-line treatment regimens have been studied. Currently, most experts recommend a bismuth-based quadruple therapeutic regimen, consisting of a PPI, bismuth salt, metronidazole, and tetracycline, that is administered for 7-14 d[3]. However, a recent meta-analysis revealed a mean failure rate of nearly 25% and higher with this therapy[9,10].
In the Maastricht IV/Florence Consensus[3], a fluoroquinolone-based triple therapy such as moxifloxacin or levofloxacin was used as second-line treatment after failure of the standard triple and the bismuth-based quadruple therapies. Moxifloxacin is a second-generation fluoroquinolone widely used to treat respiratory and skin infections[11]. Unlike other fluoroquinolones, moxifloxacin has a low incidence of adverse events and fewer interactions with other drugs. Gastrointestinal disturbances such as diarrhea and nausea are the most common reported adverse events of moxifloxacin[12]. Recent studies have reported satisfactory eradication rates with moxifloxacin-based triple therapy for first-line H. pylori treatment[13,14]. This therapy has also shown excellent eradication rates as a second-line treatment regimen[15,16] with significantly better compliance and adverse event rates compared with the bismuth-based quadruple therapy. However, the eradication rate did not increase over the course of a prolonged, 7-10-d treatment period[16], due to increased resistance to moxifloxacin[17]. We hypothesized that a longer duration of treatment with moxifloxacin-based triple therapy might increase the efficacy of H. pylori eradication.
The aim of the present study was to compare the H. pylori-eradication, treatment compliance, and adverse event rates, between second-line 14-d moxifloxacin-based triple therapy and the 7-d alternative among Korean patients.
This study was conducted at Seoul National University Bundang Hospital between January 2011 and December 2013. The medical records of 160 patients who had experienced failure of first-line PPI-based eradication therapy and subsequently received moxifloxacin-based triple therapy as second-line eradication treatment for H. pylori infection were reviewed in this retrospective study. Eradication failure was defined on the basis of at least one of the following three tests: (1) a positive 13C-urea breath test (13C-UBT); (2) histological evidence of H. pylori by modified Giemsa staining in the lesser and greater curvature of the body and antrum; and (3) a positive rapid urease test (CLO test; Delta West, Bentley, Australia) by gastric mucosal biopsy from the lesser curvature of the body and antrum. None of the patients had previously received H. pylori-eradication therapy before the administration of first-line treatment. Patients were also excluded if they had received PPIs, H2 receptor antagonists or antibiotics in the previous 4 wk, or if they had used NSAIDs or steroids in the 2 wk prior to the 13C-UBT. The other exclusion criteria were as follows: (1) age below 18 years; (2) previous gastric surgery or endoscopic mucosal dissection for gastric cancer; (3) advanced gastric cancer; (4) severe concurrent disease (hepatic, renal, respiratory, or cardiovascular systems); (5) pregnancy; and (6) any condition probably associated with poor compliance (e.g., alcoholism or drug addiction). The study protocol was approved by the Ethics Committee at Seoul National University Bundang Hospital (IRB number: B-1406/256-105).
Patients received standard and orally administered triple-, bismuth-based quadruple-, or sequential therapy as first-line treatment for the eradication of H. pylori. The standard triple therapy included 1 g amoxicillin taken twice a day (b.i.d.), 500 mg clarithromycin b.i.d., and 20 mg rabeprazole (or 40 mg esomeprazole) b.i.d. for 7 d. Bismuth-based quadruple therapy consisting of 300 mg of tripotassium dicitrato bismuthate taken 4 times a day (q.i.d.), 500 mg of tetracycline q.i.d., 500 mg of metronidazole 3 times a day (t.i.d.), and 20 mg rabeprazole (or 40 mg esomeprazole) b.i.d. for 10 d. Sequential therapy was given for 2 wk, and included 1 g amoxicillin and 20 mg rabeprazole (or 40 mg esomeprazole) b.i.d. for the first week, followed by 500 mg clarithromycin, 500 mg metronidazole, and 20 mg rabeprazole (or 40 mg esomeprazole) b.i.d for the second week.
The patients were classified into two groups. Those who received moxifloxacin-based triple therapy (oral 20 mg rabeprazole b.i.d., 1000 mg amoxicillin b.i.d., and 400 mg moxifloxacin q.d.) for 7 d were assigned to the RAM-7 group while those who received moxifloxacin-based triple therapy for 14 d were assigned to the RAM-14 group. Treatment compliance was evaluated indirectly by remnant pill counting and directly through a discussion with a physician one week after completion of the treatment. Compliance was defined as good when drug intake was at least 85%. At the same time, all of the patients were asked questions about adverse events. Successful eradication therapy for H. pylori infection was defined as a negative 13C-UBT test 4 wk after the cessation of eradication treatment. Data that was recorded included demographics (age, gender distribution, smoking status, alcohol use), previous history of peptic ulcer, endoscopic findings, reasons for drop-outs, and the type of first-line regimen that was administered.
Before the 13C-UBT, patients were instructed to stop taking medications (i.e., bismuth, antibiotics for 4 wk; PPIs for 2 wk) that could affect the results, and fast for a minimum of 4 h. After washing the patient’s oral cavity through gargling, a pre-dose breath sample was obtained. Then, 100 mg of 13C-urea powder (UBiTkitTM; Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan) was dissolved in 100 mL of water and administered orally. Breath samplings were taken with special breath collection bags while patients were in the sitting position, both before drug administration (baseline) and 20 min after the powder medication. The samples were analyzed using an isotope-selective, non-dispersive infrared spectrometer (UBiT-IR 300®; Otsuka Pharmaceutical Co. Ltd, Tokyo, Japan).
The primary and secondary outcomes of the present study were the H. pylori-eradication rates and the treatment-related adverse events, respectively. The eradication rates were determined by intention-to-treat (ITT) and per-protocol (PP) analyses. ITT analysis compared the treatment groups including all of the patients as originally allocated while the PP analysis compared the treatment groups including only those patients who had completed the treatment as originally allocated. The mean ± SD were calculated for the quantitative variables. The student’s t test was used to evaluate the continuous variables, and the χ2 test and Fisher’s exact test were utilized to assess the non-continuous variables. Additionally, univariate and multivariate analyses were conducted to assess the effects of factors on the eradication rate. All of the statistical analyses were performed using the Predictive Analytics Software (PASW) 20.0 version for Windows (SPSS Inc., IBM, Chicago, IL, United States). A P-value of less than 0.05 was defined as statistically significant.
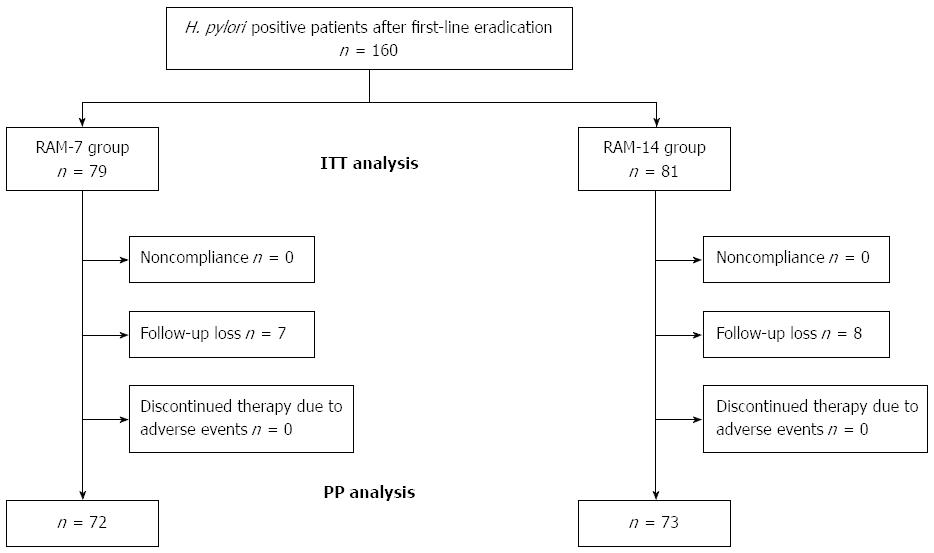
A schematic diagram of the study is provided in Figure 1. A total of 160 patients who had experienced failure of first-line eradication therapy for H. pylori were enrolled (mean age, 63 years; range: 24-86 years). Of the 160 patients, 145 (90.6) completed their allocated regimens. The remaining 15 (7% or 4.3% were from the RAM-7 group and 8% or 5.1% were from the RAM-14 group) patients (9.4) were excluded from the study because of loss to follow-up. No patient was excluded from either group for non-compliance (taking < 85% of the assigned tablets) or for treatment discontinuation due to adverse events. A total of 72 RAM-7 and 73 RAM-14 patients were included in the final PP analysis. The enrolled patients’ baseline demographic and clinical data are provided in Table 1. There were no statistical differences in age, gender distribution, smoking status, alcohol use, previous history of peptic ulcer, endoscopic findings, reasons for drop out, or the first-line regimen administered between the two groups (P > 0.05).
| RAM-7 | RAM-14 | P value | |
| Included in ITT analysis | 79 | 81 | |
| Male/female (n) | 36/43 | 35/46 | 0.741 |
| Age (yr), mean ± SD | 63.1 ± 10.6 | 63.5 ± 10.7 | 0.354 |
| Smoking | 6 (7.6) | 5 (6.1) | 0.785 |
| Alcohol use | 9 (11.4) | 11 (13.5) | 0.659 |
| Previous history of peptic ulcer | 2 (2.5) | 4 (4.9) | 0.348 |
| Endoscopic finding | 0.576 | ||
| Gastric ulcer | 1 (1.9) | 1 (1.2) | 0.775 |
| Duodenal ulcer | 3 (3.8) | 4 (4.9) | 0.182 |
| Gastric and duodenal ulcer | 0 (0.0) | 1 (1.2) | 0.411 |
| HPAG | 75 (94.9) | 75 (92.7) | 0.287 |
| Drop out | 7 (8.8) | 8 (9.8) | 0.125 |
| Noncompliance | 0 (0.0) | 0 (0.0) | - |
| Follow-up loss | 7 (8.8) | 8 (9.8) | 0.453 |
| Discontinued therapy | 0 (0.0) | 0 (0.0) | - |
| Due to adverse events | |||
| First-line regimen | 0.359 | ||
| RPZ/AMX/CLA | 21 (26.6) | 23 (28.3) | 0.176 |
| RPZ/BIS/MET/TEC | 29 (36.7) | 31 (38.2) | 0.207 |
| Sequential therapy | 29 (36.7) | 27 (33.5) | 0.478 |
Table 2 shows the rates of eradication of H. pylori infection according to the ITT and PP analyses. The overall ITT eradication rate was 76.2% (122/160). The final ITT eradication rates were 70.8% (56/79; 95%CI: 63.3%-77.1%) in the RAM-7 group and 81.4% (66/81; 95%CI: 74.6%-88.3%) in the RAM-14 group (P = 0.034, Table 2). The overall PP eradication rate was 84.1% (122/145), and the final PP eradication rates were 77.7% (56/72; 95%CI: 70.2%-85.3%) in the RAM-7 group and 90.4% (66/73; 95%CI: 82.8%-98.1%) in the RAM-14 group (P = 0.017). The H. pylori-eradication rates in the RAM-14 group were significantly higher than in the RAM-7 group according to both the ITT (P = 0.034) and the PP analyses (P = 0.017). The treatment compliance was 100% in both groups (Table 2).
| RAM-7 | RAM-14 | P value | |
| ITT analysis | |||
| Eradication rate, n (%) | 56 (70.8) | 66 (81.4) | 0.034 |
| 95%CI | 63.3%-77.1% | 74.6%-88.3% | |
| PP analysis | |||
| Eradication rate, n (%) | 56 (77.7) | 66 (90.4) | 0.017 |
| 95%CI | 70.2%-85.3% | 82.8%-98.1% | |
| Compliance | 100% | 100% | - |
Table 3 lists the adverse events that occurred in the two groups. Adverse events occurred in 14 of the 72 patients (19.4) in the RAM-7 group and in 15 of the 73 patients (20.5) in the RAM-14 group. No statistically significant differences (P = 0.441) were observed. The most common adverse events were nausea/vomiting (6/72, 8.3) and diarrhea (4/72, 5.5) in the RAM-7 group, and diarrhea (6/73, 8.2) and epigastric discomfort (5/73, 6.8) in the RAM-14 group. The differences between groups were not statistically significant (P > 0.05). Most of the adverse events were mild-to-moderate in intensity and none were serious enough to warrant discontinuation of treatment in either group.
| Adverse events | RAM-7 (n = 72) | RAM-14 (n = 73) | P value |
| Epigastric discomfort | 3 (4.1) | 5 (6.8) | 0.874 |
| Constipation | 0 (0.0) | 1 (1.4) | 0.411 |
| Diarrhea | 4 (5.5) | 6 (8.2) | 0.991 |
| Dizziness | 1 (1.4) | 1 (1.4) | 0.667 |
| Headache | 0 (0.0) | 1 (1.4) | 0.667 |
| Nausea or vomiting | 6 (8.3) | 1 (1.4) | 0.415 |
| Skin rash | 0 (0.0) | 0 (0.0) | - |
| Total | 14 (19.4) | 15 (20.5) | 0.441 |
Determination of the appropriate second-line eradication treatment for H. pylori infection remains uncertain. Many guidelines recommend a bismuth-based quadruple therapy (bismuth, PPI, metronidazole, and tetracycline) as second-line treatment after failure of first-line eradication therapy[3,5]. However, eradication failure rates of more than 20% have been found with this regimen in several countries (including South Korea) primarily because of bacterial resistance[7,8]. Additionally, poor patient compliance due to the occurrence of adverse events or complicated dosing schedules has also been implicated in treatment failures[16,18,19].
In order to address these problems, various alternative treatment options designed to prevent the development of bacterial resistance and promote treatment compliance have been reported[19-21]. Other regimens that have reported low rates of bacterial resistance to antibiotics and adverse events have also been investigated. Fluoroquinolone-based regimens, for example have been studied as first-line or salvage treatments. First-generation fluoroquinolones including pefloxacin and norfloxacin, have shown low eradication rates[22,23], while better rates have been recorded in second-generation fluoroquinolones such as levofloxacin and moxifloxacin. As first-line treatment, levofloxacin-based triple therapy revealed eradication rates of up to 90%[24,25], with higher rates reported in moxifloxacin-based triple therapy[13,14].
As first-line treatment, the H. pylori-eradication rate of moxifloxacin-based triple therapy was reported between 84.1% and 89%, which is higher than that of standard triple therapy[26,27]. As a second-line treatment, good efficacy with eradication rates as high as 90%, have been previously demonstrated[15]. Although bismuth-based quadruple therapy has generally been considered as the second-line treatment of choice[28], moxifloxacin-based therapy is often preferred because of poor compliance to the former regimen resulting from adverse events, complicated dosing schedules, and low eradication rates[18]. Other studies indicate that levofloxacin-based triple therapy administered for 10 d as a rescue treatment was more effective (compared to the 7-d regimen) than bismuth-based quadruple therapy[29,30]. In a separate study in Turkey, moxifloxacin-based triple therapy, has been evaluated as first-line treatment on a 14-d basis[31,32] with eradication rates determined at only 42%-53% on ITT and 47%-53.3% on PP analyses, respectively. These results might be related to the different regional and institutional usages of fluoroquinolones[33].
In this study, we compared the eradication rates of the 14-d with the 7-d regimens of the moxifloxacin-based triple therapy as second-line modalities for the treatment of H. pylori infection. We believe that these longer treatment durations would increase eradication efficacies without increasing or decreasing adverse-event or drug-compliance rates, respectively. The ITT and PP analyses revealed eradication rates of 70.8% and 77.7%, respectively, for the RAM-7 group and 81.4% and 90.4% respectively, for the RAM-14 group. Although statistically significant differences in eradication rates were reported, there were no statistically significant differences in adverse events or treatment compliance (P > 0.05) with the moxifloxacin-based therapy. Thus, a longer duration of moxifloxacin-based triple therapy (14 d) was found to be more effective as a salvage second-line eradication treatment compared to a shorter duration of therapy in patients whose first-line treatment had failed, without increasing or decreasing the adverse-event or drug-compliance rate, respectively.
The most common adverse events of moxifloxacin therapy are gastrointestinal disturbances such as diarrhea and nausea which were also observed in our present study. The total adverse-event rate for the 14-d moxifloxacin-based triple treatment was 20.5% (15/73), which was similar to that for the 7-d moxifloxacin-based triple treatment (19.4%, 14/72), although the difference was not statistically significant. In both groups, mild to moderate adverse events were reported. None was serious enough to require medication discontinuation or interfered with regular life. On the other hand, the medication discontinuation rate and the incidence of adverse events of 14-d bismuth-based quadruple therapy have been reported to be 7.5%-23% and 38.2%-65%[34,35], respectively, which were higher than the results obtained in our study. In other words, the adverse events of longer-duration moxifloxacin-based triple therapy (14 d) occur at a similar frequency to those of shorter-duration therapy (7 d) in the present study.
One limitation of our study is the lack of any analysis of antibiotic resistance relative to the eradication rates and treatment regimens. The efficacy and timing of antibiotic susceptibility testing after eradication therapy failure remain uncertain. Some researchers have argued that antibiotic susceptibility testing is unnecessary after first-line eradication therapy, because it is neither practical nor cost-effective for primary care practices[5,18]. For this reason, bacterial culture and antibiotic susceptibility testing were not performed in this study. Nevertheless, we believe such testing to be particularly necessary after two or more eradication failures.
In conclusion, 14-d moxifloxacin-based triple therapy is a more highly effective second-line eradication treatment than 7-d moxifloxacin-based triple therapy for H. pylori infection. The high eradication rate, excellent compliance, and safety of the 14-d regimen suggest its potential suitability as an alternative to the standard bismuth-based quadruple therapy. Further large prospective studies are required in order to determine the proper treatment duration and assess the efficacy and timing of antibiotic susceptibility testing after eradication therapy failure.
A recent meta-analysis reported a decreased eradication rate using standard triple therapy for Helicobacter pylori (H. pylori) infection due to increasing antibiotic resistance. For those patients whose first-line eradication therapy has failed, second-line eradication therapy for persistent H. pylori infection is required.
The potential role of moxifloxacin as an antibiotic agent for the eradication treatment in H. pylori infection has been suggested by a few animal and human studies.
This is a retrospective study was conducted to evaluate the efficacy of 14-d moxifloxacin-based triple therapy (as compared with 7-d moxifloxacin-based triple therapy) as a second-line eradication treatment of H. pylori infection. The high eradication rate, excellent compliance, and safety of the 14-d regimen suggest its potential suitability as an alternative to the standard bismuth-based quadruple therapy.
This retrospective study’s design and findings could be used to determine sample sizes for a larger, prospective study aiming to test the efficacy of moxifloxacin-based triple therapy as a second-line eradication treatment for H. pylori eradication.
H. pylori is found in the stomach and is associated with the development of gastritis, peptic ulcers, and stomach cancer. To prevent recurrence in patients with these diseases, it is necessary to eradicate H. pylori infection.
This retrospective study reports the results of 7 and 14 d moxifloxacin-based triple therapy for H. pylori eradication conducted with 160 patients who experienced failure of their first-line PPI-based therapy. The study is carefully designed, well described, and discussed according to data from the available literature. The information provided could serve as a basis for the development of potential alternative therapies for H. pylori eradication.
P- Reviewer: Slomiany BL, Tan HJ, Yokota S S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997;113:1983-1991. [PubMed] |
| 2. | Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110:1244-1252. [PubMed] |
| 3. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1589] [Article Influence: 122.2] [Reference Citation Analysis (5)] |
| 4. | Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [PubMed] |
| 5. | Lam SK, Talley NJ. Report of the 1997 Asia Pacific Consensus Conference on the management of Helicobacter pylori infection. J Gastroenterol Hepatol. 1998;13:1-12. [PubMed] |
| 6. | Laheij RJ, Rossum LG, Jansen JB, Straatman H, Verbeek AL. Evaluation of treatment regimens to cure Helicobacter pylori infection--a meta-analysis. Aliment Pharmacol Ther. 1999;13:857-864. [PubMed] |
| 7. | Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004;48:4843-4847. [PubMed] |
| 8. | Kim N, Kim JM, Kim CH, Park YS, Lee DH, Kim JS, Jung HC, Song IS. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol. 2006;40:683-687. [PubMed] |
| 9. | Hojo M, Miwa H, Nagahara A, Sato N. Pooled analysis on the efficacy of the second-line treatment regimens for Helicobacter pylori infection. Scand J Gastroenterol. 2001;36:690-700. [PubMed] |
| 10. | Lee JH, Cheon JH, Park MJ, Kim N, Lee DH, Kim JM, Kim JS, Jung HC, Song IS. [The trend of eradication rates of second-line quadruple therapy containing metronidazole for Helicobacter pylori infection: an analysis of recent eight years]. Korean J Gastroenterol. 2005;46:94-98. [PubMed] |
| 11. | Keating GM, Scott LJ. Moxifloxacin: a review of its use in the management of bacterial infections. Drugs. 2004;64:2347-2377. [PubMed] |
| 12. | Edlund C, Beyer G, Hiemer-Bau M, Ziege S, Lode H, Nord CE. Comparative effects of moxifloxacin and clarithromycin on the normal intestinal microflora. Scand J Infect Dis. 2000;32:81-85. [PubMed] |
| 13. | Di Caro S, Ojetti V, Zocco MA, Cremonini F, Bartolozzi F, Candelli M, Lupascu A, Nista EC, Cammarota G, Gasbarrini A. Mono, dual and triple moxifloxacin-based therapies for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2002;16:527-532. [PubMed] |
| 14. | Bago P, Vcev A, Tomic M, Rozankovic M, Marusić M, Bago J. High eradication rate of H. pylori with moxifloxacin-based treatment: a randomized controlled trial. Wien Klin Wochenschr. 2007;119:372-378. [PubMed] |
| 15. | Cheon JH, Kim N, Lee DH, Kim JM, Kim JS, Jung HC, Song IS. Efficacy of moxifloxacin-based triple therapy as second-line treatment for Helicobacter pylori infection. Helicobacter. 2006;11:46-51. [PubMed] |
| 16. | Kang JM, Kim N, Lee DH, Park YS, Kim YR, Kim JS, Jung HC, Song IS. Second-line treatment for Helicobacter pylori infection: 10-day moxifloxacin-based triple therapy versus 2-week quadruple therapy. Helicobacter. 2007;12:623-628. [PubMed] |
| 17. | Kim JM, Kim JS, Kim N, Jung HC, Song IS. Distribution of fluoroquinolone MICs in Helicobacter pylori strains from Korean patients. J Antimicrob Chemother. 2005;56:965-967. [PubMed] |
| 18. | Gisbert JP, Pajares JM. Review article: Helicobacter pylori “rescue” regimen when proton pump inhibitor-based triple therapies fail. Aliment Pharmacol Ther. 2002;16:1047-1057. [PubMed] |
| 19. | Wong WM, Gu Q, Lam SK, Fung FM, Lai KC, Hu WH, Yee YK, Chan CK, Xia HH, Yuen MF. Randomized controlled study of rabeprazole, levofloxacin and rifabutin triple therapy vs. quadruple therapy as second-line treatment for Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:553-560. [PubMed] |
| 20. | Perri F, Festa V, Merla A, Barberani F, Pilotto A, Andriulli A. Randomized study of different ‘second-line’ therapies for Helicobacter pylori infection after failure of the standard ‘Maastricht triple therapy’. Aliment Pharmacol Ther. 2003;18:815-820. [PubMed] |
| 21. | Gupta VK, Dhar A, Srinivasan S, Rattan A, Sharma MP. Eradication of H. pylori in a developing country: comparison of lansoprazole versus omeprazole with norfloxacin, in a dual-therapy study. Am J Gastroenterol. 1997;92:1140-1142. [PubMed] |
| 22. | Ahuja V, Dhar A, Bal C, Sharma MP. Lansoprazole and secnidazole with clarithromycin, amoxycillin or pefloxacin in the eradication of Helicobacter pylori in a developing country. Aliment Pharmacol Ther. 1998;12:551-555. [PubMed] |
| 23. | Cammarota G, Cianci R, Cannizzaro O, Cuoco L, Pirozzi G, Gasbarrini A, Armuzzi A, Zocco MA, Santarelli L, Arancio F. Efficacy of two one-week rabeprazole/levofloxacin-based triple therapies for Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:1339-1343. [PubMed] |
| 24. | Di Caro S, Zocco MA, Cremonini F, Candelli M, Nista EC, Bartolozzi F, Armuzzi A, Cammarota G, Santarelli L, Gasbarrini A. Levofloxacin based regimens for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol. 2002;14:1309-1312. [PubMed] |
| 25. | Wenzhen Y, Kehu Y, Bin M, Yumin L, Quanlin G, Donghai W, Lijuan Y. Moxifloxacin-based triple therapy versus clarithromycin-based triple therapy for first-line treatment of Helicobacter pylori infection: a meta-analysis of randomized controlled trials. Intern Med. 2009;48:2069-2076. [PubMed] |
| 26. | Nista EC, Candelli M, Zocco MA, Cazzato IA, Cremonini F, Ojetti V, Santoro M, Finizio R, Pignataro G, Cammarota G. Moxifloxacin-based strategies for first-line treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2005;21:1241-1247. [PubMed] |
| 27. | Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, Xiao SD, Lam SK, Goh KL, Chiba T. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 28. | Wu C, Chen X, Liu J, Li MY, Zhang ZQ, Wang ZQ. Moxifloxacin-containing triple therapy versus bismuth-containing quadruple therapy for second-line treatment of Helicobacter pylori infection: a meta-analysis. Helicobacter. 2011;16:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Gisbert JP, Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther. 2006;23:35-44. [PubMed] |
| 30. | Saad RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. Am J Gastroenterol. 2006;101:488-496. [PubMed] |
| 31. | Sezgin O, Altintaş E, Uçbilek E, Tombak A, Tellioğlu B. Low efficacy rate of moxifloxacin-containing Helicobacter pylori eradication treatment: in an observational study in a Turkish population. Helicobacter. 2007;12:518-522. [PubMed] |
| 32. | Kiliç ZM, Köksal AS, Cakal B, Nadir I, Ozin YO, Kuran S, Sahin B. Moxifloxacine plus amoxicillin and ranitidine bismuth citrate or esomeprazole triple therapies for Helicobacter pylori infection. Dig Dis Sci. 2008;53:3133-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | McMahon BJ, Hennessy TW, Bensler JM, Bruden DL, Parkinson AJ, Morris JM, Reasonover AL, Hurlburt DA, Bruce MG, Sacco F. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med. 2003;139:463-469. [PubMed] |
| 34. | Uygun A, Kadayifci A, Safali M, Ilgan S, Bagci S. The efficacy of bismuth containing quadruple therapy as a first-line treatment option for Helicobacter pylori. J Dig Dis. 2007;8:211-215. [PubMed] |
| 35. | Graham DY, Osato MS, Hoffman J, Opekun AR, Anderson SY, Kwon DH, El-Zimaity HM. Metronidazole containing quadruple therapy for infection with metronidazole resistant Helicobacter pylori: a prospective study. Aliment Pharmacol Ther. 2000;14:745-750. [PubMed] |