Published online May 7, 2015. doi: 10.3748/wjg.v21.i17.5311
Peer-review started: November 3, 2014
First decision: December 26, 2014
Revised: January 22, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: May 7, 2015
Processing time: 191 Days and 12.3 Hours
AIM: To compare laparoscopic pancreaticoduodenectomy (TLPD) during the initial learning curve with open pancreaticoduodenectomy in terms of outcome and costs.
METHODS: This is a retrospective review of the consecutive patients who underwent TLPD between December 2009 and April 2014 at our institution. The experiences of the initial 15 consecutive TLPD cases, considered as the initial learning curve of each surgeon, were compared with the same number of consecutive laparotomy cases with the same spectrum of diseases in terms of outcome and costs. Laparoscopic patients with conversion to open surgery were excluded. Preoperative demographic and comorbidity data were obtained. Postoperative data on intestinal movement, pain score, mortality, complications, and costs were obtained for analysis. Complications related to surgery included pneumonia, intra-abdominal abscess, postpancreatectomy hemorrhage, biliary leak, pancreatic fistula, delayed gastric emptying, and multiple organ dysfunction syndrome. The total costs consisted of cost of surgery, anesthesia, and admission examination.
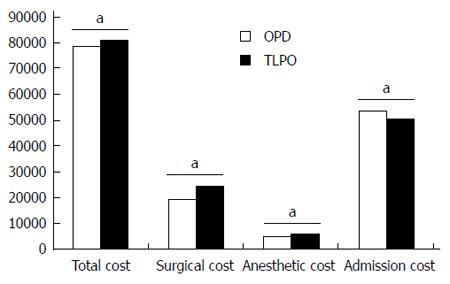
RESULTS: A total of 60 patients, including 30 consecutive laparoscopic cases and 30 consecutive open cases, were enrolled for review. Demographic and comorbidity characteristics of the two groups were similar. TLPD required a significantly longer operative time (513.17 ± 56.13 min vs 371.67 ± 85.53 min, P < 0.001). The TLPD group had significantly fewer mean numbers of days until bowel sounds returned (2.03 ± 0.55 d vs 3.83 ± 0.59 d, P < 0.001) and exhaustion (4.17 ± 0.75 d vs 5.37 ± 0.81 d, P < 0.001). The mean visual analogue score on postoperative day 4 was less in the TLPD group (3.5 ± 9.7 vs 4.47 ± 1.11, P < 0.05). No differences in surgery-related morbidities and mortality were observed between the two groups. Patients in the TLPD group recovered more quickly and required a shorter hospital stay after surgery (9.97 ± 3.74 d vs 11.87 ± 4.72 d, P < 0.05). A significant difference in the total cost was found between the two groups (TLPD 81317.43 ± 2027.60 RMB vs laparotomy 78433.23 ± 5788.12 RMB, P < 0.05). TLPD had a statistically higher cost for both surgery (24732.13 ± 929.28 RMB vs 19317.53 ± 795.94 RMB, P < 0.001) and anesthesia (6192.37 ± 272.77 RMB vs 5184.10 ± 146.93 RMB, P < 0.001), but a reduced cost for admission examination (50392.93 ± 1761.22 RMB vs 53931.60 ± 5556.94 RMB, P < 0.05).
CONCLUSION: TLPD is safe when performed by experienced pancreatobiliary surgeons during the initial learning curve, but has a higher cost than open pancreaticoduodenectomy.
Core tip: Open pancreaticoduodenectomy is the classic procedure for pancreatic and periampullary malignancies and some benign diseases. However, laparoscopic pancreaticoduodenectomy has only been in application for ten years, and has been popular for only a few years. The technique used in the laparoscopic procedure is quite different from laparotomy. Thus, experienced laparotomy surgeons are required to adapt to these changes. The safety of laparoscopic pancreaticoduodenectomy by experienced surgeons in laparotomy during the initial learning curve was demonstrated in our study and resulted in faster postoperative recovery.
-
Citation: Tan CL, Zhang H, Peng B, Li KZ. Outcome and costs of laparoscopic pancreaticoduodenectomy during the initial learning curve
vs laparotomy. World J Gastroenterol 2015; 21(17): 5311-5319 - URL: https://www.wjgnet.com/1007-9327/full/v21/i17/5311.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i17.5311
Total laparoscopic pancreaticoduodenectomy (TLPD) is the most advanced laparoscopic procedure for pancreatectomy. TLPD seems to be as safe as open pancreaticoduodenectomy (OPD) by skilled surgeons[1-4], but the technical difficulties still prevent many surgeons from attempting this technique. Since Gagner and Pomp[5] first reported TLPD in 1994, only 285 reported cases have been reported as of 2011[6]. In this research, we retrospectively analyzed surgical outcomes following TLPD and the safety and cost during the initial learning curve vs OPD.
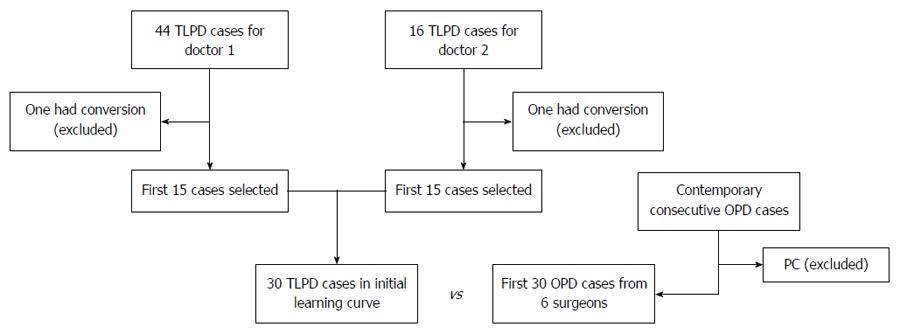
This is a retrospective review of consecutive patients who underwent TLPD performed by experienced surgeons between December 2009 and April 2014. Following Institutional Review Board approval, a retrospective review of our database was performed. For enrollment, TLPD patients were required to have no sign of malignant pancreatic disease during the initial learning curve. The experiences of the initial 15 TLPD cases for each surgeon were considered the initial learning curve in this review. The same number of consecutive OPD cases with the same spectrum of diseases was selected and these patients served as the control group (Figure 1).
Preoperative investigations included routine blood tests, neoplastic markers (carcinoembryonic antigen, α-fetoprotein, cancer antigen 19-9), chest X-ray, abdominal ultrasound, computed tomography, or magnetic resonance imaging. No patient had preoperative biliary drainage. The nasogastric tube was removed on postoperative day 1 if the patient had been extubated and had no sign of delayed gastric emptying. All patients received prophylactic antibiotics intraoperatively and for three days postoperatively, except those with infective complications. Self-controlled analgesia comprised of sufentanil 200 μg, tramadol 1500 mg, and granisetron 12 mg was given to adult patients for three days postoperatively. The visual analogue score (VAS) was evaluated on postoperative day 4. Peripancreatic drainage fluid was collected to measure amylase levels on postoperative day 3 and every three days thereafter as needed. Prophylactic Stilamin (EMD Serono of Merck KGaA, Darmstadt, Germany) was administered to all patients for at least six days to prevent pancreatic fistula (PF).
Major postoperative morbidities were defined and graded using criteria recommended by the International Study Group of Pancreatic Surgery, including postoperative pancreatic fistula, delayed gastric emptying, and postpancreatectomy hemorrhage[7-9]. Biliary leak was defined as drainage of any volume of fluid from percutaneous drains or the wound consistent with bile, which was defined by a bilirubin concentration greater than the serum concentration. Enteric leaks were identified using radiographic contrast imaging. Fluid collection in intra-abdominal or pleural regions was performed via radiographic imaging, which was differentiated from abscess by positive microbial cultures. Pneumonia was diagnosed based on chest X-ray changes following antibiotic therapy.
The Pearson χ2 test was used for categorical variables. Fisher’s exact test was used in cases with a variable count < 5. For continuous variables, the Student’s t test was used for normally distributed variables and the Wilcoxon rank-sum test was used for non-normally distributed variables. P < 0.05 was considered statistically significant. All analyses were performed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, United States).
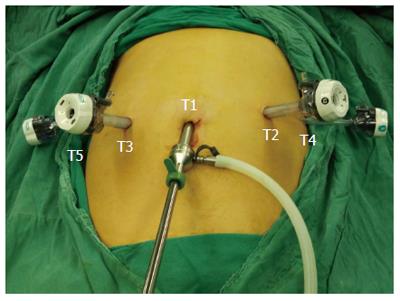
Two surgeons performed TLPD using a similar procedure during the initial learning curve. The patients were placed in the modified lithotomy reverse Trendelenburg position with thighs parallel to the ground. The surgeon stood between the legs of the patient. The surgeon operating the camera stood on the right side of the patient and the assistant surgeon stood on the left side of the patient. The port positions are shown in Figure 2. Five trocars were used: a 12-mm telescope trocar in the navel; two 12-mm trocars along the left and right midclavear lines, lateral to the rectus muscles, 2 cm above the naval trocar; and two 5-mm trocars along the left and right anterior axillary line.
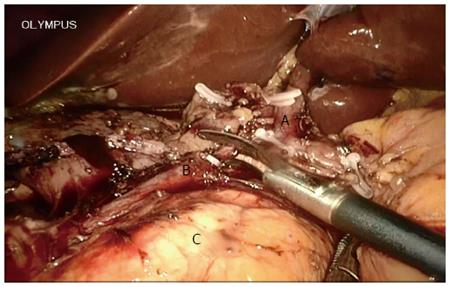
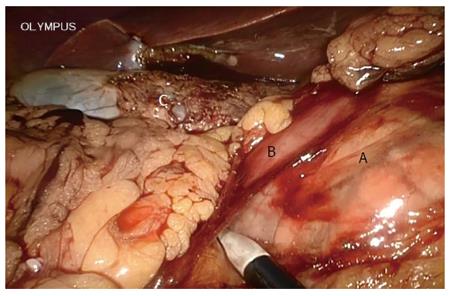
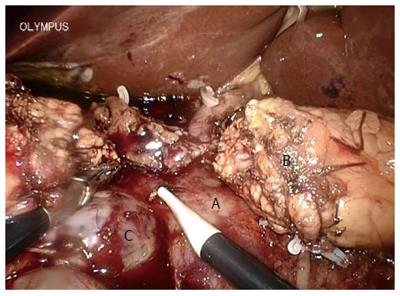
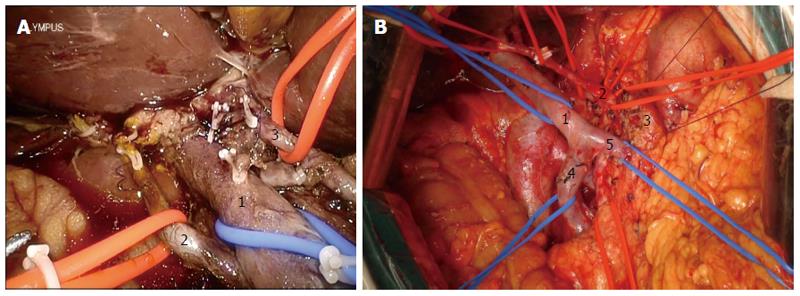
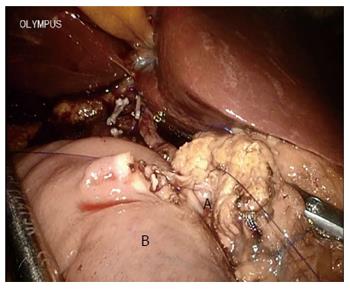
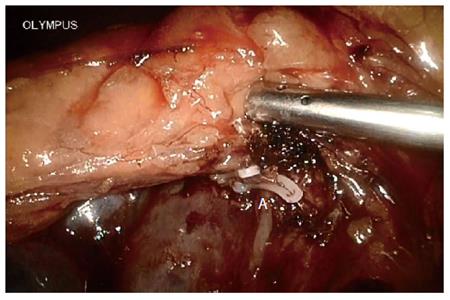
A systematic examination of the peritoneal cavity was performed, and any suspicious serosal lesions were biopsied. The extent and mobility of the primary tumor were assessed. Malignant carcinoma in the pancreas required conversion during the initial learning curve. The gastrocolic omentum was mobilized to gain entry to the lesser sac. The gastrohepatic omentum was opened to visualize the caudate lobe. The right gastric artery was identified and divided. The gastrocolic trunk was clipped and then cut. The stomach was cut on the left side of the pylorus using an endo linear cutter (Ethicon of Johnson and Johnson, New Brunswick, NJ, United States). Calot’s triangle was dissected, the cystic artery and duct were ligated, and cholecystectomy was performed at this stage. All the fibro-fatty tissue along the common bile duct was moved down caudally. The common hepatic duct was incised and blocked. The gastroduodenal artery, identified at the groove between the neck and head of the pancreas, was ligated and divided at its origin (Figure 3). The duodenum was then mobilized using the Kocher maneuver (Figure 4), and the inferior vena cava and aorta were exposed. The hepatic flexure and transverse colon were mobilized down, exposing the entire second and third part of the duodenum up to the neck of the pancreas. Blunt dissection in the tissue plane between the anterior surface of the superior mesenteric vein (SMV) and the posterior surface of the neck of the pancreas created a tunnel. An umbilical tape was passed through this tunnel and the pancreatic neck was lifted off the SMV/portal vein. The tunnel was then extended towards the body of the pancreas for 2 cm. The duodenojejunal flexure was mobilized and the distal jejunum divided using an endo linear cutter. The free end of the jejunum was passed under the root of the mesentery (retrocolic) to the supracolic compartment. The neck of the pancreas was carefully divided using ultrasonic shears (Ethicon). Both the superior and inferior pancreaticoduodenal veins were ligated. The uncinate process was separated along the right aspect portal vein, and the specimen was retracted to the right (Figure 5). The common hepatic artery was skeletonized by dissecting out the fibro-fatty tissue along with lymph nodes from the origin of the gastroduodenal artery. The periportal node was then cleared (Figure 6A).
Child’s reconstruction was performed in all patients. The order of anastomosis on the jejunal limb was pancreaticojejunostomy (PJ), hepaticojejunostomy, and gastrojejunostomy. The cut-end of the jejunum was brought to the cut-end of the pancreas in a retrocolic fashion. In most patients, an end-to-side modified mucosa-to-mucosa PJ was performed during the initial learning curve[10] (Figure 7). No stents were placed in the pancreatic duct. The end of the common bile duct was trimmed, and an end-to-side choledochojejunostomy was performed. Finally, an end-to-side gastrojejunostomy anastomosis was performed in the infracolic region by placing the stomach in an antecolic fashion.
After the initial learning curve, surgeons preferred the pylorus preserving pancreatoduodenectomy. The duct-to-mucosa PJ was chosen for dilated pancreatic ducts.
In total, 60 cases were scheduled for TLPD between December 2009 and April 2014, which were performed by two experienced pancreatobiliary surgeons. Two patients were excluded due to conversion during the selection process. Both conversions occurred during the initial learning curve. In the remaining 58 cases, the mortality rate was 1.7% (1/58), and morbidity was 55.2% (32/58). The first 15 consecutive successful TLPD cases for each surgeon were considered the initial learning curve in this review. In total, 30 TLPD cases considered the initial learning curve without pancreatic malignancy were included. The remaining 28 cases were considered to be outwith the learning curve, and 3 cases of pancreatic malignancy were included. Thirty TLPD cases were selected as the initial learning curve in this study, and 30 consecutive cases of OPD with the same spectrum of diseases served as controls. These 30 cases underwent surgery performed by six experienced surgeons.
The baseline patient characteristics are presented in Table 1. Pathologic diagnoses included malignant lesions in 90% (27/30) of TLPD cases, including periampullary adenocarcinoma (n = 25), distal common bile duct adenocarcinoma (n = 1), and duodenum adenocarcinoma (n = 1), and in 87% (26/30) of OPD cases, including periampullary adenocarcinoma (n = 23), distal common bile duct adenocarcinoma (n = 2), and malignant branch-duct intraductal papillary mucinous neoplasm (n = 1). Comorbidities were similar in both groups. The American Society of Anesthesiologists scores were also similar in both groups.
| Characteristic | TLPD | OPD | P value |
| Age, yr (range) | 59.3 ± 9.3 (44-79) | 59.9 ± 10.4 (36-78) | 0.804 |
| Male/female | 18/12 | 23/7 | 0.165 |
| Malignant/benign | 27/3 | 26/4 | 0.688 |
| Comorbidities | |||
| Hypertension | 3 | 4 | 1.000 |
| Chronic pancreatitis | 0 | 1 | 1.000 |
| Hepatocirrhosis | 0 | 2 | 0.472 |
| Diabetes | 3 | 2 | 1.000 |
| Pulmonary disease | 1 | 2 | 1.000 |
| Cardiac disease | 0 | 1 | 1.000 |
| ASA score | 0.943 | ||
| ASA 1 | 6 | 6 | |
| ASA 2 | 19 | 18 | |
| ASA 3 | 5 | 6 |
The mean operative time was longer in the TLPD group compared with the OPD group (P < 0.001) (Table 2). No statistical differences in the need for perioperative transfusion or mean number of lymph nodes harvested were observed between the two groups. The mean number of days to the return of bowel sounds was less in the TLPD group (P < 0.001). The mean number of days in which the patients were exhausted was also less in the TLPD group (P < 0.001). The mean VAS score on postoperative day 4 was less in the TLPD group (P = 0.010). There was no statistically significant difference in PF rates between the groups, although grade A PF tended to be higher in the TLPD group. The rates of other surgery-related morbidities were not significantly different between the two groups. No difference in mortality rates was observed between the groups. Patients in the TLPD group had a significantly shorter hospital stay after surgery compared to in the OPD group (P = 0.002).
| Characteristic | TLPD | OPD | P value |
| Operative time (min) | 513.17 ± 56.13 | 371.67 ± 85.53 | < 0.001 |
| Perioperative transfusion needed, n | 0 | 1 | 1 |
| No. of lymph nodes harvested | 8.67 ± 1.71 | 9.58 ± 2.21 | 0.102 |
| Return of bowel sounds (d) | 2.03 ± 0.55 | 3.83 ± 0.59 | < 0.001 |
| Exhaustion (d) | 4.17 ± 0.75 | 5.37 ± 0.81 | < 0.001 |
| VAS score | 3.50 ± 9.70 | 4.47 ± 1.11 | 0.010 |
| LOS (d) | 9.97 ± 3.74 | 11.87 ± 4.72 | 0.002 |
| Surgery-related morbidity, n | |||
| Pneumonia | 3 | 5 | 0.704 |
| Intra-abdominal abscess | 0 | 1 | 1.000 |
| Postpancreatectomy hemorrhage | 1 | 1 | 1.000 |
| Biliary leak | 1 | 0 | 1.000 |
| Pancreatic fistula | 10 | 6 | 0.099 |
| A | 9 | 3 | 0.053 |
| B | 0 | 2 | |
| C | 1 | 1 | |
| Delayed gastric emptying, n | 2 | 3 | 1.000 |
| MODS, n | 0 | 1 | 1.000 |
| Mortality, n | 0 | 1 | 1.000 |
A significant difference was observed in the total cost of pancreaticoduodenectomy in the TLPD group compared with the OPD group (81317.43 ± 2027.60 RMB vs 78433.23 ± 5788.12 RMB, P = 0.014) (Figure 8). When the total cost was broken down into cost of surgery, anesthesia, and admission evaluation, the TLPD group had a statistically higher cost for both surgery (24732.13 ± 929.28 RMB vs 19317.53 ± 795.94 RMB, P < 0.001) and anesthesia (6192.37 ± 272.77 RMB vs 5184.10 ± 146.93 RMB, P < 0.001), but decreased cost for admission evaluation (50392.93 ± 1761.22 RMB vs 53931.60 ± 5556.94 RMB, P = 0.034), compared with the OPD group.
During the past 25 years, significant advances have been achieved in OPD surgery. A recent analysis of 424 patients who underwent pancreatic resection showed a 90-d mortality rate of 1.7%, and one- and five-year survival rates of 76% and 23%, respectively[11]. Although minimally invasive surgery has been used in many pancreatic operations, the most controversial topic in pancreatic surgery is still the utility of minimally invasive PD[12-14]. The literature shows that TLPD can be performed safely, with good clinical and oncologic outcomes[4,14-16]. However, the procedure is technically demanding and can only be performed safely by skilled surgeons[13,17]. Gumbs et al[6] conducted a review of the literature on laparoscopic Whipple procedures from articles published between 1994 and 2010, and found a total of only 285 cases. It is unknown whether there are any differences in the surgical outcomes and cost between TLPD during the initial learning curve and OPD by experienced OPD surgeons, only that it is safe after the learning curve. This study analyzed and compared the surgical outcomes and cost during the initial learning curve of TLPD with OPD by experienced pancreatobiliary surgeons.
Surgeons may have had some experience of other laparoscopic surgeries before TLPD, such as laparoscopic cholecystectomy, laparoscopic splenectomy, and laparoscopic distal pancreatectomy. However, there is little skill required regarding the sutures used in these laparoscopic surgeries. PD requires meticulous manipulation and complicated reconstruction, and a number of sutures are needed during the procedure. This is a big challenge for surgeons beginning TLPD. From the results of the present study, an obvious difference in surgical outcomes was observed between the TLPD and OPD groups, and surgeons require more time to complete TLPD compared with OPD.
Consideration for TLPD required no sign of malignant pancreatic disease during initial learning curve in this review. OPD was chosen when pancreatic head malignant disease was suspected. As it is very important to clean the retroperitoneal margin during skeletonization of the lateral, posterior, and anterior borders of the superior mesenteric artery as recommended by the National Comprehensive Cancer Network guidelines, this may have been related to the survival rate[18-20]. In fact, it was hard to achieve optimal dissection and skeletonization of the hepatic artery in the TLPD group during the initial learning curve. Although we found that the number of lymph nodes harvested was similar in the TLPD and OPD groups, the soft tissue around the vessels in the TLPD group could not be cleaned as well as that in the OPD group (Figure 6B), including the superior mesenteric artery[21]. This may be not an obstacle after the learning curve. In large centers performing TLPD, even major veins can be resected and reconstructed during TLPD[3,22]. More lymph nodes were retrieved in TLPD patients compared with OPD patients for malignant disease in the study by Asbun et al[23]. Some reports also show a higher R0 rate and increased lymph node retrieval in minimally invasive PD[24,25]. In one report from Palanivelu et al[26], the five-year actuarial survival rate for pancreatic head adenocarcinoma following TLPD was 19.1%[26]. This was close to the five-year survival rate of OPD, but their results only included patients with early stage pancreatic head adenocarcinoma (T1N0M0 and T2N0M0). In the present study, most malignant lesions in the TLPD group were ampullary adenocarcinomas. Unfortunately, the five-year survival rates for malignancy were lacking in this study due to a short follow-up period in the TLPD group.
Two patients were converted to open surgery during the initial learning curve. One patient was converted because pancreatic head adenocarcinoma was highly suspected during the procedure. The other patient was converted due to uncontrolled bleeding when creating a tunnel between the anterior surface of the SMV and the posterior surface of the neck of the pancreas. When the second patient was converted, it was found that the bleeding was caused by a small artery below the pancreatic body (Figure 9) close to the SMV. Uncontrolled bleeding was caused by damage to the SMV during hurry-scurry hemostasis. So the small artery was firstly clamped and cut before creating the tunnel after this case. The conversion rate was low in this study. We suppose that this was directly related to the experience of the surgeons gained from OPD. Damage to the main vessels was avoided during the procedure, as the anatomic structure was very familiar to the surgeons. Our results showed that the two groups were reasonably well matched for age, sex, tumor characteristics, comorbidities, and American Society of Anesthesiologists grade. The rate in females was a little higher in the TLPD group, however, this was not statistically different. These results are consistent with those in practice, as most patients seen and evaluated for possible PD are candidates for both TLPD and OPD, except those with pancreatic head malignant disease.
Transfusion requirements were equal in the two groups. Although it was more inconvenient to staunch bleeding using sutures in the TLPD group than in the OPD, the Hemlock (Athlone, Johnson and Johnson) was mainly used to prevent or staunch bleeding from small vessels in the TLPD group. The magnified view provided by the laparoscope enhanced the surgeons’ view of small vessels around the specimen and forced the surgeons to perform a more careful dissection. These two factors resulted in few transfusions in the TLPD group. Because the surgeons in this study were very familiar with the PD procedure, blood loss was also low in the OPD group. Therefore, transfusion requirements were low in both the TLPD group and the OPD group.
Bowel function recovered more quickly in the TLPD group compared with the OPD group, although the operative time was longer in the TLPD group. We found that intestinal gurgling was earlier after TLPD compared with OPD. Patients were exhausted more quickly after TLPD, which was expected. Laparoscopic procedures can provide better warmth preservation for the intestine than OPD. This may be one reason for quick bowel function recovery. Laparoscopic procedures can reduce postoperative pain. The mean VAS score on postoperative day 4 was less in the TLPD group. Thus, the postoperative stress reaction may be reduced after TLPD. This may also be another reason for quick recovery of bowel function. Quick bowel function recovery and less postoperative pain resulted in better enteral nutrition and earlier ambulation. These findings match the concept of the enhanced recovery program which has been verified as beneficial for early hospital discharge[27-29]. Thus, as expected, the length of hospital stay was significantly shorter in the TLPD group compared with the OPD group.
Overall surgical-related morbidity and mortality were not significantly different between the TLPD and OPD groups in this study. Reconstruction using TLPD is challenging during the initial learning curve. Exposure and suturing are two main problems during this procedure, especially for small diameter biliary or pancreatic ducts. Grade A PF tended to be higher in the TLPD group in this study. However, grade B and C were similar in both groups. We think that the quality of PJ seriously affected grade A PF in the TLPD group, but we are unsure why the high PF rate was only limited to grade A. From our observations of the PF patients, the volume of PF decreased quickly after exhaustion in a few patients. Is quick bowel function recovery beneficial to PJ resolution? More studies are required to explain this issue. No mortality was observed in the TLPD group. One patient died in the OPD group. This was a 63-year-old male patient who developed grade C PF after surgery, which resulted in postpancreatectomy hemorrhage and infection. This patient died of multiple organ dysfunction syndrome.
The mean total cost was higher in the TLPD group compared with the OPD group. When the total cost was broken down, TLPD was noted to result in significant increases in the cost of both surgery and anesthesia, but a decrease in the cost of admission evaluation. The higher cost of surgery and anesthesia in the TLPD group was due to the required surgical equipment and supplies, and longer surgical time. The lower cost of admission evaluation in the TLPD group due to a shorter hospital stay and reduced requirement for parenteral alimentation. In addition, a group from the United States found that TLPD was associated with a significantly higher surgical cost due to both increased time and supply costs[30]. However, mean hospital admission cost associated with OPD in their study was greater in comparison with TLPD. These results are similar to our findings, although the total cost for TLPD was higher than OPD. We suggest that the higher cost of surgical equipment and supplies in China caused this difference compared with the United States.
In conclusion, it seems safe to perform TLPD during the initial learning curve by experienced pancreatobiliary surgeons. TLPD is beneficial for patient recovery. The cost of TLPD was higher compared with OPD. The cost of TLPD may be lower than OPD if the price of surgical equipment and supplies decreases.
Total laparoscopic pancreaticoduodenectomy (TLPD) has proven to be the most advanced laparoscopic procedure for pancreatectomy. However, the procedure requires not only skill, but also time and physical energy of surgeons. The safety is another problem that prevents many surgeons who are experienced in open procedure from performing this laparoscopic procedure. This procedure has been demonstrated as safe in large medical centers, but the outcome in initial learning curve is not fully known.
Although some specialized surgeons in large medical centers have rich experience in TLPD, especially the extensive TLPD such as major venous resection, many other surgeons have only limited experience with this procedure. This study compared the safety of TLPD during the initial learning curve with open pancreaticoduodenectomy (OPD) in terms of outcome and costs.
Few reports are available on the difference between TLPD in initial learning curve and the open procedure. To understand the exact difference in the surgical outcome and the inpatient cost for this procedure, the authors selected patients who received TLPD in initial learning curve or OPD with the same spectrum of disease performed by surgeons at different experience levels. It seems safe to perform TLPD in the initial learning curve by experienced pancreatobiliary surgeons. The cost of TLPD is higher than OPD, due to an increased cost for both surgery and anesthesia, but is associated with a decreased cost for admission examination. The higher cost of surgery and anesthesia in TLPD incurred from surgical equipment and supplies, and longer operation time.
The study results suggest that it is safe to perform TLPD by experienced pancreatobiliary surgeons. Whether pancreatic cancer could be treated by this procedure in the initial learning curve should be further studied.
Pancreaticoduodenectomy is a complicated abdominal surgical procedure with trauma. The extent of resection comprises part of pancreas, adjacent duodenum, middle and distal common bile duct, part of stomach, and jejunum.
This paper compared the surgical safety and feasibility of TLPD during the initial learning curve with open pancreaticoduodenectomy. TLPD is difficult for most surgeons and references are limited. Therefore, the study is important. Although the patients operated upon during the initial learning curve are included in the statistical analysis, information on mortality and morbidity after initial learning curve should be provided.
P- Reviewer: Ruckert F, Tong WD S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Ma S
| 1. | Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc. 2006;20:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Gumbs AA, Gayet B. The laparoscopic duodenopancreatectomy: the posterior approach. Surg Endosc. 2008;22:539-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, Kendrick ML. Pancreaticoduodenectomy with major vascular resection: a comparison of laparoscopic versus open approaches. J Gastrointest Surg. 2015;19:189-194; discussion 194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Song KB, Kim SC, Hwang DW, Lee JH, Lee DJ, Lee JW, Park KM, Lee YJ. Matched Case-Control Analysis Comparing Laparoscopic and Open Pylorus-Preserving Pancreaticoduodenectomy in Patients With Periampullary Tumors. Ann Surg. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 5. | Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408-410. [PubMed] |
| 6. | Gumbs AA, Rodriguez Rivera AM, Milone L, Hoffman JP. Laparoscopic pancreatoduodenectomy: a review of 285 published cases. Ann Surg Oncol. 2011;18:1335-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3514] [Article Influence: 175.7] [Reference Citation Analysis (34)] |
| 8. | Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 2331] [Article Influence: 129.5] [Reference Citation Analysis (0)] |
| 9. | Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1950] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 10. | Palanivelu C, Rajan PS, Rangarajan M, Vaithiswaran V, Senthilnathan P, Parthasarathi R, Praveen Raj P. Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg. 2009;16:731-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Lewis R, Drebin JA, Callery MP, Fraker D, Kent TS, Gates J, Vollmer CM. A contemporary analysis of survival for resected pancreatic ductal adenocarcinoma. HPB (Oxford). 2013;15:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | de la Fuente SG. Laparoscopic pancreaticoduodenectomies: a word of caution. J Am Coll Surg. 2013;216:1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Qin H, Qiu J, Zhao Y, Pan G, Zeng Y. Does minimally-invasive pancreaticoduodenectomy have advantages over its open method? A meta-analysis of retrospective studies. PLoS One. 2014;9:e104274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, Kendrick ML. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg. 2014;260:633-68; discussion 638-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 15. | Kendrick ML. Laparoscopic and robotic resection for pancreatic cancer. Cancer J. 2012;18:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Fisher SB, Kooby DA. Laparoscopic pancreatectomy for malignancy. J Surg Oncol. 2013;107:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Kang CM, Lee SH, Chung MJ, Hwang HK, Lee WJ. Laparoscopic pancreatic reconstruction technique following laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2015;22:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Tol JA, Brosens LA, van Dieren S, van Gulik TM, Busch OR, Besselink MG, Gouma DJ. Impact of lymph node ratio on survival in patients with pancreatic and periampullary cancer. Br J Surg. 2015;102:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Li J, Zhang B, Cui G, Dai D. [Correlation between characteristics of lymph node metastases and prognosis in pancreatic cancer treated with pancreaticoduodenectomy]. Zhonghua Zhong Liu Zazhi. 2014;36:688-692. [PubMed] |
| 20. | El Nakeeb A, El Shobary M, El Dosoky M, Nabeh A, El Sorogy M, El Eneen AA, Abu Zeid M, Elwahab MA. Prognostic factors affecting survival after pancreaticoduodenectomy for pancreatic adenocarcinoma (single center experience). Hepatogastroenterology. 2014;61:1426-1438. [PubMed] |
| 21. | Young S, Abbitt P, Hughes SJ. Port-site recurrence of pancreatic adenocarcinoma following laparoscopic pancreaticoduodenectomy. J Gastrointest Surg. 2012;16:2294-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Kendrick ML, Sclabas GM. Major venous resection during total laparoscopic pancreaticoduodenectomy. HPB (Oxford). 2011;13:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 24. | Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc. 2012;26:2397-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Zeh HJ, Zureikat AH, Secrest A, Dauoudi M, Bartlett D, Moser AJ. Outcomes after robot-assisted pancreaticoduodenectomy for periampullary lesions. Ann Surg Oncol. 2012;19:864-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Palanivelu C, Jani K, Senthilnathan P, Parthasarathi R, Rajapandian S, Madhankumar MV. Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg. 2007;205:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Coolsen MM, van Dam RM, van der Wilt AA, Slim K, Lassen K, Dejong CH. Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg. 2013;37:1909-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Paton F, Chambers D, Wilson P, Eastwood A, Craig D, Fox D, Jayne D, McGinnes E. Effectiveness and implementation of enhanced recovery after surgery programmes: a rapid evidence synthesis. BMJ Open. 2014;4:e005015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 29. | Abu Hilal M, Di Fabio F, Badran A, Alsaati H, Clarke H, Fecher I, Armstrong TH, Johnson CD, Pearce NW. Implementation of enhanced recovery programme after pancreatoduodenectomy: a single-centre UK pilot study. Pancreatology. 2013;13:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Mesleh MG, Stauffer JA, Bowers SP, Asbun HJ. Cost analysis of open and laparoscopic pancreaticoduodenectomy: a single institution comparison. Surg Endosc. 2013;27:4518-4523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |