Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4379
Peer-review started: August 8, 2014
First decision: August 27, 2014
Revised: September 30, 2014
Accepted: November 19, 2014
Article in press: November 19, 2014
Published online: April 14, 2015
Processing time: 251 Days and 12.6 Hours
Mesenchymal stem cells (MSC) are cells of stromal origin which exhibit unlimited self-renewal capacity and pluripotency in vitro. It has recently been observed that MSC may also exert a profound immunosuppressive and anti-inflammatory effect both in vitro and in vivo with consequent potential use in autoimmune disorders. We present the case of a patient suffering from childhood-onset, multidrug resistant and steroid-dependent Crohn’s disease who underwent systemic infusions of MSC, which led to a temporary reduction in CCR4, CCR7 and CXCR4 expression by T-cells, and a temporary decrease in switched memory B-cells, In addition, following MSC infusion, lower doses of steroids were needed to inhibit proliferation of the patient’s peripheral blood mononuclear cells. Despite these changes, no significant clinical benefit was observed, and the patient required rescue therapy with infliximab and subsequent autologous hematopoietic stem cell transplantation. The results of biological and in vitro observations after MSC use and the clinical effects of infusion are discussed, and a brief description is provided of previous data on MSC-based therapy in autoimmune disorders.
Core tip: This is the first report of a lack of clinical response using interferon-γ pre-treated mesenchymal stem cell infusion in a patient with intractable Crohn’s disease. Data on the clinical effects of mesenchymal stem cell treatment in autoimmune disorders are sparse and usually report a good clinical response; however, since very few reports have been published, we think negative results are also worthy of attention. The use of mesenchymal stem cells pre-treated with interferon-γ is also of interest, and the lack of clinical benefit of bone marrow-derived interferon-γ pre-treated mesenchymal stem cell infusion has also been described, despite the lymphocyte immune regulation effect.
- Citation: Taddio A, Tommasini A, Valencic E, Biagi E, Decorti G, De Iudicibus S, Cuzzoni E, Gaipa G, Badolato R, Prandini A, Biondi A, Ventura A. Failure of interferon-γ pre-treated mesenchymal stem cell treatment in a patient with Crohn’s disease. World J Gastroenterol 2015; 21(14): 4379-4384
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4379.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4379
Mesenchymal stem cells (MSC) are cells of stromal origin which exhibit unlimited self-renewal capacity and pluripotency in vitro; more specifically, several reports have described the capacity of MSC to differentiate in vitro into multiple cellular lineages, and their use in regenerative medicine has been extensively investigated in various clinical settings[1].
Much interest has been generated recently by the observation that MSC may also exert a pronounced immunosuppressive and anti-inflammatory effect, both in vitro and in vivo. The mechanisms behind this immunosuppressive effect are still unclear, but they seem to involve events mediated by both soluble factors and cell contact[2,3].
There are few studies describing treatment with systemic MSC in humans, and most of these are in patients treated for acute graft-vs-host disease (GvHD), indicating that infusions of MSC expanded in vitro, irrespective of the donor, might be an effective treatment for patients with steroid-resistant, acute GvHD[4]. MSC treatment has also been tried out in other autoimmune diseases such as systemic lupus erythematosus and Sjögren’s disease[5,6], with encouraging results. Moreover, it has been demonstrated that systemic infusions of MSC lessen the clinical and histopathological severity of experimental colitis, reducing weight loss, diarrhea, and inflammation[7]. This potential role of MSC in modulating the immune response and tissue regeneration led to the idea of using MSC for a new cellular approach in the treatment of Crohn’s disease (CD) in humans, with conflicting results[8]. Preclinical data obtained from cellular and animal models have suggested that the profile of immune activation in the host prior to administration of MSC can be a critical factor in the outcome of immune suppression. Indeed, treatment with interferon-γ (IFN-γ) can enhance the immunosuppressive action of MSC, especially when the MSC are used in a setting of ongoing immune activation[9], and several reports have suggested that exposure to activated lymphocytes, or to IFN-γ, is necessary to activate the immunosuppressive properties of MSC[10-12]. Based on these results, we hypothesized that MSC could be briefly incubated with IFN-γ in a clinical-grade setting in order to improve their capability for treating severe immune disorders. We present the case of a patient with intractable, childhood-onset, steroid-dependent CD, who underwent MSC infusion to no effect.
The patient was a 31-year-old woman suffering from CD. This diagnosis was made when she was 11 years old, and was characterized by diarrhea, weight loss, asthenia, and high levels of inflammatory markers. On that occasion, colonoscopy and esophagogastroduodenoscopy were performed and biopsy specimens confirmed the preliminary diagnosis of ileal and perianal CD.
The patient was initially treated with steroids and enteral nutrition, but frequent relapses occurred when steroids were tapered leading rapidly to ileo-colic resection due to stenosis. As a consequence, thalidomide was started and there was some clinical improvement, but treatment was suspended when the patient showed evidence of thalidomide-related peripheral toxicity. Over the following years, several attempts were made to treat her with azathioprine, cyclosporine, and methotrexate, but the clinical response was poor. When she was 24 years old, she received treatment with infliximab, with some clinical improvement, but she then developed a new stenosis requiring surgical procedures and the drug was withdrawn. She was then started on adalimumab, but no clinical benefits were observed and she developed herpetic esophagitis. A second trial with thalidomide was suspended because of the re-appearance of neurological complications and dapsone was eventually started, on the basis of some anecdotal clinical reports, again with a poor clinical response.
Given the long clinical history, characterized by numerous complications and multiple surgical interventions, a disease that was steroid-dependent and still active, despite ample use of immunosuppressants, and the absence of opportunistic infections and small intestine bacterial overgrowth, we decided to try MSC infusions.
At the time of the first infusion, the physical examination was unremarkable, but the patient had oral ulcers with a feeding inability requiring nocturnal enteral nutrition, abdominal pain, watery diarrhea (35 episodes per week), and perianal ulcers. Colonoscopy showed inflammation of the mucosa. Laboratory examinations were normal, including acute phase reactants (erythrocyte sedimentation rate: 18 mm/h; normal value 0-20; C-reactive protein: 0.34 mg/dL; normal value < 0.5) except for the presence of chronic normocytic anemia (hemoglobin: 9.2 g/dL; mean cell volume: 78 fl), hypergammaglobulinemia (IgG: 1780 mg/dL) and increased levels of fecal calprotectin (2200 mg/kg; normal value < 50). Magnetic resonance imaging of the gastrointestinal tract showed free fluid, peritoneal fat stranding with enhancement, diffuse thickening of the bowel wall, and stratified enhancement of sigmoid and ascending colon; ileal and ileo-colic strictures were also present. The CD activity index (AI) was 270. The patient was treated with dapsone (50 mg bid) and prednisone (25 mg/d).
The patient underwent the two scheduled MSC infusions (2 × 106 cells/kg per infusion) which were well tolerated. Ten days after the second infusion, despite CDAI improvement (215), there was exacerbation of abdominal pain and worsening of diarrhea, and the clinical picture deteriorated rapidly. Laboratory examinations remained normal, but calprotectin levels were still high, and the patient underwent rescue therapy with infliximab (10 mg/kg per infusion) with transient slight improvement; she was then directed to an autologous hematopoietic stem cell transplantation (HSTC) program.
The MSC were produced in the officially approved cell factory Laboratorio di Terapia Cellulare e Genica “Stefano Verri” in Monza (Italy), compliant with European good manufacturing practices, as determined by AIFA (Agenzia Italiana del Farmaco, Rome). The procedures were approved by the Istituto Superiore di Sanità (approval by ISS, Rome, 5.09.2007, n 45253(06)-PRE.21-882).
The medical ethical committee of the IRCCS “Burlo Garofolo” (Trieste, Italy) approved the use of IFN-γ pretreated MSC for compassionate use. The patient gave her written informed consent.
We present a case of refractory, drug-resistant, steroid-dependent childhood-onset CD which was then treated with two infusions of MSC, with rapid deterioration of the clinical picture and subsequent need for medical rescue therapy before autologous HSTC.
To our knowledge, this is the 15th patient affected by CD to be treated with systemic MSC infusion. The first report is by Duijvestein et al[8], in which 10 patients with chronic severe steroid-refractory CD were recruited for MSC treatment in a two-infusion protocol schedule. Two patients were withdrawn from the study and did not complete the protocol. Among the 8 patients who received both infusions, 5 showed improvement in the CDAI, but only 3 showed a clinical response (defined as a drop in CDAI by more than 70), while 3 patients required surgery over a 14-wk period following infusions. It should be pointed out that remission was not achieved in any of the patients. Liang et al[9] treated 7 patients, of whom 4 had CD and 3 ulcerative colitis. According to the authors, 5 patients had achieved remission at the 3-mo follow-up, and this remission lasted for over 24 mo in 2 patients. However, only one patient stopped steroid treatment completely, while 3 patients tapered partially and the other 3 could not reduce their total steroid dosage, an indirect indication of incompletely controlled disease.
In the current case, there was no observable clinical improvement after infusion, and indeed, soon after the second infusion, the patient required rescue therapy with infliximab before undergoing HSCT. We would also like to stress that in order to enhance the clinical response in our patient, the MSCs were pre-treated with IFN-γ, because it has been shown that IFN-γ activation of MSCs increases their immunosuppressive capabilities in vitro and (we therefore hoped), their therapeutic efficacy in vivo[13]. Unfortunately, we found no evidence of any positive clinical response.
It might be argued that the lack of clinical response to MSC infusion in our case could be due to weak biological properties of the cells. It should be pointed out that when the cells were thawed after cryopreservation before their infusion, a small sample was analyzed to check MSC vitality and immunosuppressive capacity: no difference was found before and after cryopreservation. In addition, a much higher percentage of natural killer (NK) cells was found in the patient’s serum after the second infusion (4.52% before infusion, compared to 14.5% after, which could be interpreted as a “physiological” response to MSC infusion. In fact, it is known that MSC are susceptible to lysis by NK cells, and that there is a natural tendency for NK cells to proliferate in order to counteract the biological effect of MSC[14].
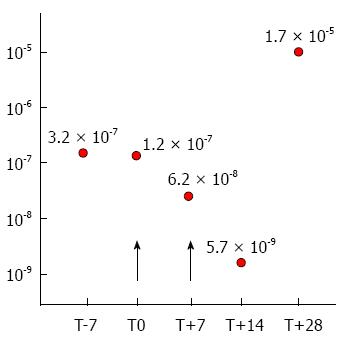
Another interesting finding which confirmed the biological effect of MSC infusion was that methylprednisolone IC50 levels (i.e., the concentration required to reduce in vitro mononuclear proliferation to 50%) were similar before MSC treatment (T-7 and T0), while lower concentrations of the steroid were needed to inhibit proliferation of the patient’s peripheral blood mononuclear cells after infusion (T+7 and T+14). After the last infusion, however (T+28), an increase in the IC50 was observed which coincided with the patient’s worsening symptoms (Figure 1)[15].
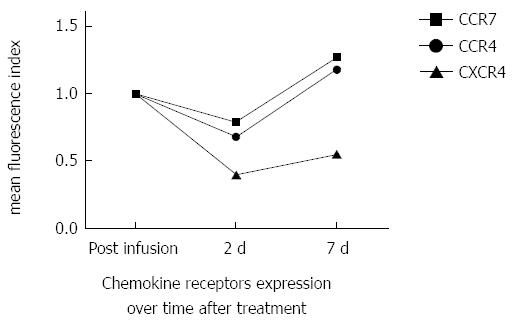
Analysis of T-lymphocytes before the infusion and at days +2 and +7 respectively showed a transient reduction in CCR4, CCR7 and CXCR4 expression at day +2 compared with basal levels, while expression of CXCR4 was still reduced after 7 d (Figure 2). In addition, analysis of B-cells showed a transient decrease in switched B-cells, while the maturation of monocyte-derived dendritic cells, as measured by analysis of CD80, CD86, and CD83 expression, was not affected (data not shown). These results suggest that MSC infusion may have led to a mobilization of select T and B cell subsets to tissues within 2 d; these observations support previous reports suggesting a mechanism of B-cell suppression, with immunoglobulin impairment production induced by release of soluble factors[16].
In our case, despite the evidence of some in vitro properties and in vivo biological effects, no positive clinical response to MSC therapy was found. Of course, this is just a single case report and large multicenter trials, presently ongoing, need to be encouraged[17]. We should also stress the fact that MSCs were used in this case to treat a very severe form of CD, resistant to multiple attempts with standard treatments, and in a late phase of clinical progression. Studies of GvHD patients after allogeneic HSCT seem to support the hypothesis that MSC therapy should be promoted in the very early stage of disease immediately after steroid failure, while patients with more advanced or chronic GvHD failed to show any clinical benefit[18]. In line with this observation, we recently demonstrated that mediators derived from activated lymphocytes can suppress MSC which have not been previously activated[19]. In fact, the presence of soluble mediators in the environment can be a critical factor in the survival and action of MSC[20,21]. IFN-γ treatment has been proposed to enhance the action of MSC against already-activated lymphocytes, but this treatment may not to be sufficient to protect MSC from the effect of high concentrations of inflammatory mediators. Therapy with MSC has created great hope for advances in the treatment of autoimmune disorders, but at this moment in time, there is still only weak evidence of their clinical effectiveness. In fact, there is a great paucity of data available regarding clinical trials with MSC therapy in the field of autoimmune disorders. To confirm this, a PubMed search was carried out for the following terms “autoimmune disorders” and “mesenchymal stem cells”. We found 485 articles, consisting mostly of reviews (177/485; 36.5%) and animal models and in vitro studies (285/485; 58.8%), while there were only 23 articles consisting of clinical trials and case reports (4.7%).
It is also important to bear in mind that negative findings tend not to be reported in the medical literature, which is why we think that describing this experience should be of interest to other physicians and researchers. Clearly, the data in our possession is still insufficient to be able to consider systemic infusion of MSC in the treatment of CD, at least in its very advanced, progressive and multiple drug-resistant forms. Our experience suggests that patients with intractable drug-resistant CD should still undergo HSCT, while MSC therapy may perhaps be considered in the earlier stages of the disease. The use of MSC therapy in CD fistulas, on the other hand, may be of some benefit[22].
After a long series of attempts at treatment with immunosuppressants and surgery, a 31-year-old woman with intractable Crohn’s disease presented with oral ulcers, a feeding inability requiring nocturnal enteral nutrition, abdominal pain, watery diarrhea and perianal ulcers.
The diagnosis was Crohn’s disease.
Given the clinical history of the patient, characterized by numerous complications, a series of surgical interventions, a dependency on steroids, and long-term use of immunosuppressants, we ruled out the possible presence of opportunistic infections, malignancies and small intestine bacterial proliferation.
The patient had chronic normocytic anemia (Hb: 9.2 g/dL; MCV: 78 fl), hypergammaglobulinemia (IgG: 1780 mg/dL) and increased levels of fecal calprotectin (2200 mg/kg; n.v. < 50).
The patient underwent magnetic resonance imaging of the gastrointestinal tract, which showed free fluid, peritoneal fat stranding with enhancement, diffuse thickening of the bowel wall, and stratified enhancement of the sigmoid and ascending colon; ileal and ileo-colic strictures were also found.
Biopsy specimens from both the colon and ileum were consistent with a diagnosis of ileal and perianal Crohn’s disease.
Interferon-γ pre-treated mesenchymal stem cells were infused at a dosage of 2 × 106 cells/kg. Two infusions were scheduled.
Mesenchymal stem cells are cells of a stromal origin which exhibit unlimited self-renewal capacity and pluripotency in vitro and have a pronounced anti-inflammatory and immunosuppressive effect.
We describe a case of a patient with intractable Crohn’s disease in which no clinical benefit was obtained from the use of interferon-γ pre-treated mesenchymal stem cells despite lymphocyte dysregulation. The usefulness of mesenchymal stem cell treatment in autoimmune disorders is still to be confirmed; the literature is scarce and results are uncertain.
The authors describe a case of intractable Crohn’s disease which failed to respond to parenteral mesenchymal stem cell therapy. The article highlights the failure of interferon-γ pre-treated mesenchymal stem cell infusion, points to the effect on lymphocyte regulation and discusses the results obtained using mesenchymal stem cells to treat autoimmune diseases.
P- Reviewer: Dimcevski G S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Liu XM
| 1. | Bernardo ME, Pagliara D, Locatelli F. Mesenchymal stromal cell therapy: a revolution in Regenerative Medicine? Bone Marrow Transplant. 2012;47:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [PubMed] |
| 3. | Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619-4621. [PubMed] |
| 4. | Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2047] [Cited by in RCA: 2028] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 5. | Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, Hou Y, Zeng X, Gilkeson GS, Sun L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 326] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 6. | Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, Ding G, Gao R, Zhang C, Ding Y. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012;120:3142-3151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 497] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 8. | Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 470] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 9. | Liang J, Zhang H, Wang D, Feng X, Wang H, Hua B, Liu B, Sun L. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut. 2012;61:468-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Renner P, Eggenhofer E, Rosenauer A, Popp FC, Steinmann JF, Slowik P, Geissler EK, Piso P, Schlitt HJ, Dahlke MH. Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function. Transplant Proc. 2009;41:2607-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466-2477. [PubMed] |
| 12. | Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353-363. [PubMed] |
| 13. | Valencic E, Piscianz E, Andolina M, Ventura A, Tommasini A. The immunosuppressive effect of Wharton’s jelly stromal cells depends on the timing of their licensing and on lymphocyte activation. Cytotherapy. 2010;12:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484-1490. [PubMed] |
| 15. | Cuzzoni E, De Iudicibus S, Bartoli F, Ventura A, Decorti G. Association between BclI polymorphism in the NR3C1 gene and in vitro individual variations in lymphocyte responses to methylprednisolone. Br J Clin Pharmacol. 2012;73:651-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367-372. [PubMed] |
| 17. | Corcione A; ClinicalTrials. gov. Mesenchymal stem cells and crohn’s disease. Available from: http://www.clinicaltrials.gov/ct2/results?term=mesenchymal stem cells and crohn’s disease&Search=Search. |
| 18. | Introna M, Lucchini G, Dander E, Rovelli A, Balduzzi A, Longoni D, Pavan F, Masciochi F, Algarotti A, CMicò C. Safe and Effective Treatment of Graft Vs Host Disease with Platelet Lysate-Expanded Human Mesenchymal Stromal Cells: A Phase 1 Study On 47 Adult and Pediatric Patients. Blood. 2012;120:743. |
| 19. | Valencic E, Loganes C, Cesana S, Piscianz E, Gaipa G, Biagi E, Tommasini A. Inhibition of mesenchymal stromal cells by pre-activated lymphocytes and their culture media. Stem Cell Res Ther. 2014;5:3. [PubMed] |
| 20. | Freytes DO, Kang JW, Marcos-Campos I, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 21. | Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17:1594-1601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 554] [Cited by in RCA: 558] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 22. | Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 413] [Article Influence: 29.5] [Reference Citation Analysis (0)] |