Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4284
Peer-review started: August 26, 2014
First decision: September 27, 2014
Revised: November 19, 2014
Accepted: January 8, 2015
Article in press: January 8, 2015
Published online: April 14, 2015
Processing time: 232 Days and 20.8 Hours
AIM: To investigate the timing, safety and efficacy of prophylactic antiviral therapy in patients with hepatitis B virus (HBV) infection undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT).
METHODS: This prospective study recruited a total of 57 patients diagnosed with malignant hematological diseases and HBV infection at the First Affiliated Hospital of Sun Yat-sen University between 2006 and 2013. The patients were classified as hepatitis B surface antigen (HBsAg)-positive or HBsAg-negative/ antiHBc-positive. Patients were treated with chemotherapy followed by antiviral therapy with nucleoside analogues. Patients underwent allo-HSCT when serum HBV DNA was < 103 IU/mL. Following allo-HSCT, antiviral therapy was continued for 1 year after the discontinuation of immunosuppressive therapy. A total of 105 patients who underwent allo-HSCT and had no HBV infection were recruited as controls. The three groups were compared for incidence of graft-vs-host disease (GVHD), drug-induced liver injury, hepatic veno-occlusive disease, death and survival time.
RESULTS: A total of 29 of the 41 subjects with chronic GVHD exhibited extensive involvement and 12 exhibited focal involvement. Ten of the 13 subjects with chronic GVHD in the HBsAg(-)/hepatitis B core antibody(+) group exhibited extensive involvement and 3 exhibited focal involvement. Five of the 10 subjects with chronic GVHD in the HBsAg(+) group exhibited extensive involvement and 5 exhibited focal involvement. The non HBV-infected group did not differ significantly from the HBsAg-negative/antiHBc-positive and the HBsAg-positive groups which were treated with nucleoside analogues in the incidence of graft-vs-host disease (acute GVHD; 37.1%, 46.9% and 40%, respectively; P = 0.614; chronic GVHD; 39%, 40.6% and 40%, respectively; P = 0.98), drug-induced liver injury (25.7%, 18.7% and 28%, respectively; P = 0.7), death (37.1%, 40.6% and 52%, respectively; P = 0.4) and survival times (P = 0.516). One patient developed HBV reactivation (HBsAg-positivity) due to early discontinuation of antiviral therapy.
CONCLUSION: Suppression of HBV DNA to < 103 IU/mL before transplantation, continued antiviral therapy and close monitoring of immune markers and HBV DNA after transplantation may assure the safety of allo-HSCT.
Core tip: The threshold of pre-transplantation hepatitis B virus (HBV) DNA for allo-HSCT was defined as 103 IU/mL. Only 1 patient developed HBV reactivation due to early discontinuation of antiviral therapy. The hepatitis B surface antigen (HBsAg)(+), HBsAg(-)/hepatitis B core antibody(+) and non-HBV infected groups showed no statistically significant differences in the incidence of graft-vs-host disease, drug-induced liver injury, hepatic veno-occlusive disease death, survival times and post-transplantation cumulative survival rates. In summary, suppression of HBV DNA to < 103 IU/mL before transplantation, continued antiviral therapy and close monitoring of immune markers of hepatitis B and HBV DNA after transplantation may assure the safety of allogeneic hematopoietic stem cell transplantation.
- Citation: Liao YP, Jiang JL, Zou WY, Xu DR, Li J. Prophylactic antiviral therapy in allogeneic hematopoietic stem cell transplantation in hepatitis B virus patients. World J Gastroenterol 2015; 21(14): 4284-4292
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4284.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4284
Hepatitis B virus (HBV) infection is a major public health challenge worldwide, with almost 2 billion people showing serological evidence of infection[1]. China is a region of high endemicity and the percentage of hepatitis B surface antigen (HBsAg) carriers in the population aged 1-59 years old is estimated at 7.18%[2,3]. The major goals of antiviral therapy include: (1) loss of HBsAg; (2) seroconversion to anti-HBs; (3) seroconversion to anti-HBe in HBeAg-positive patients; and (4) loss of viral DNA[4].
Immune dysfunction arising from hematological diseases, intensive chemotherapy, immunosuppressive therapy, monoclonal antibody therapy or hematopoietic stem cell transplantation (HSCT) can result in exacerbation or reactivation of HBV infection after viral resolution[5-7]. Reactivation occurs when intracellular covalently closed circular HBV DNA is not completely eliminated after spontaneous resolution of acute or chronic HBV infection or after antiviral therapy[8].
HSCT is the current standard of care for patients with severe hematological disorders, where either a sibling donor or an unrelated matched donor can be used as a source of stem cells (allogeneic HSCT). In areas of high HBV endemicity where almost 15% of the patients receiving HSCT are HBsAg-positive prior to transplantation, HBV reactivation can significantly impact the post-transplant prognosis[9]. More than half of the HBV carriers who receive allo-HSCT develop HBV reactivation[10] and the incidence of impaired liver function, severity of liver injury and hepatitis-related mortality are significantly higher in HBV infected patients receiving allo-HSCT compared with non HBV-infected patients. In addition, HBV reactivation may cause the discontinuation of effective therapy of hematological diseases, which could then indirectly influence the prognosis of these patients[11]. Combined HBV infection was therefore once regarded a contraindication to allo-HSCT.
Nucleoside analogs such as lamivudine and entecavir have been used for the treatment of acute and chronic HBV infection[12,13], as well as for chemotherapy-induced HBV reactivation[14,15]. However, the timing of initiation and optimal duration of antiviral therapy with nucleoside analogues is still controversial. A recent report described late-onset HBV reactivation after long-term prophylactic treatment with lamivudine in an NHL patient who underwent HSCT[16]. It is also unclear if prophylactic use of nucleoside analogues for antiviral therapy is necessary for patients who are HBsAg-negative/antiHBc-positive. Additionally, there is not much information available about the HBV DNA levels in allo-HSCT donors and recipients at the time of transplantation which would predict viral reactivation.
In this study, we aimed to investigate the timing, safety and influence of antiviral therapy in HBsAg-positive and HBsAg-negative/antiHBc-positive HBV patients after allo-HSCT. The primary endpoint of the study was cumulative survival. Secondary endpoints included occurrence of complications, presence of acute or chronic graft-vs-host disease (GVHD), liver damage and veno-occlusive disease (VOD).
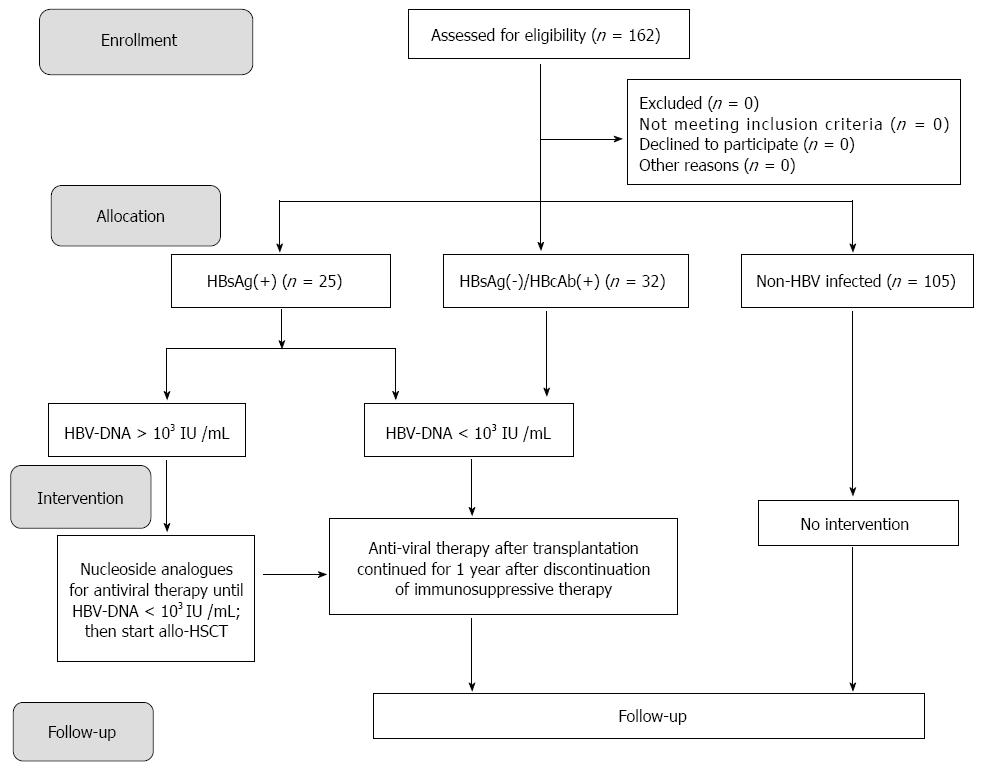
This prospective cohort study recruited a total of 162 patients who received allo-HSCT at the First Affiliated Hospital of Sun Yat-sen University between September 2006 and December 2013. The study was reviewed and approved by the Institutional Review Board of the First Affiliated Hospital, Sun Yat-sen University. All study participants, or their legal guardian, provided informed written consent prior to study enrollment. There were 77 patients with acute myeloid leukemia (AML), 42 patients with acute lymphoblastic leukemia (ALL), 21 patients with aplastic anemia, 3 patients with non-Hodgkin’s lymphoma (NHL), 6 patients with myelodysplastic syndrome (MDS), 11 patients with chronic myeloid leukemia (CML), 1 patient with paroxysmal nocturnal hemoglobinuria (PNH) and 1 patient with myelofibrosis (MF). Based on the presence of immune markers for hepatitis B, patients were classified into: (1) non-HBV infection group (n = 105); (2) HBsAg-positive group (n = 25); and (3) Hepatitis B core antibody (HBcAb)-positive group (n = 32).
(1) All patients were diagnosed based on the criteria for the diagnosis and the evaluation of therapeutic efficacy of hematological diseases[17]; (2) all transplantation procedures were performed according to the guidelines published in the Hematopoietic Stem Cell Transplantation Standard Practice Manual, Fred Hutchinson Cancer Research Center (Chinese Edition); Editors: BEN SHE and YI MING (ISBN:7117090782/9787117090780); (3) study participation was voluntary and all patients agreed to receive allo-HSCT; and (4) all patients were aged < 60 years.
(1) Presence of heart disease and/or kidney diseases or risk factors for these diseases; (2) presence of mental diseases; (3) patients who were pregnant or breast feeding; (4) presence of decompensated liver function prior to transplantation; (5) liver function indicating Child-Pugh grade B-C; (6) glutamate aminotransferase (ALT) levels which were higher than twice the upper limit of normal (> 2ULN); (7) increase in bilirubin levels prior to transplantation; (8) presence of concomitant hepatitis A, C, D or E; and (9) patients who were not compliant with the study protocol. Written informed consent was obtained from all patients and the study was approved by the Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University.
Patients infected with HBV were treated according to the EASL guidelines[4].
Pretreatment protocols: (1) Patients with acute leukemia, CML or MDS received the BuCy protocol prior to allo-HCST (busulfan total dose 16 mg/kg iv drip -8 d to -5 d;cyclophosphamide total dose 120 mg/kg iv drip -4 d to -3 d); (2) patients with aplastic anemia or PNH received the CTX + ATG protocol prior to allo-HCST (cyclophosphamide 50 mg/kg per day iv drip -5 d to -2 d; anti-thymocyte globulin total dose 12.5 mg/kg iv drip -5 d to -2 d); (3) patients with NHL received the BEAM protocol prior to HCST (BCNU total dose 300 mg/m2 -6 d; etoposide total dose 800 mg/m2 -5 d to -2 d; Ara-C total dose 800 mg/m2 -5 d to -2 d; melphalan total dose 140 mg/m2 -1 d)or CBV (BCNU total dose 300 mg/m2 -6 d; etoposide total dose 800 mg/m2 -5 d to -2 d; cyclophosphamide total dose 5 g/m2 iv drip -5 d to -2 d); (4) prevention of GVHD was done with cyclosporine A (60 mg/d); mycophenolate mofetil (1 g/d); methotrexate 15 mg/m2 (+ 1 d) or 10 mg/m2 (+3, 6 and 11 d); and (5) patients receiving non-sibling allogeneic hematopoietic stem cell transplantation were treated with ATG (2.5 mg/kg per day × 3 to 4 d).
Patients with AML were initially treated with anthracyclines and Ara-C at a standard dose for induced chemotherapy. When complete remission was achieved, chemotherapy was consolidated with the original protocol or two courses of Ara-C at a large dose (3 g/m2 q12h d1.3.5). Allo-HSCT was performed after this. Patients with ALL were initially treated with VDLP protocol for induced chemotherapy. When complete remission was achieved, chemotherapy was consolidated with the original protocol or anthracyclines in combination with Ara-C at a standard dose or two courses of the Hyper-CVAD protocol. Allo-HSCT was performed after this. Patients with aplastic anemia received sibling allo-HCST soon after diagnosis.
Serum immune markers for hepatitis B were detected and quantitated using enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Rongsheng Biotech Co., Ltd) according to the manufacturer’s instructions. HBV DNA was quantitated using a PCR kit with a 100 IU/mL detection limit (Shenzhen Daji Biotech Co., Ltd).
Based on the presence of serum markers, patients in the HBsAg-positive group and those in the HBcAb-positive group were treated with nucleoside analogues (entecavir 0.5 mg qd). HSCT was performed when the HBV DNA was lower than 103 IU/mL. Antiviral therapy was continued after transplantation for a period of 1 year after discontinuation of immunosuppressive therapy. After transplantation, evaluation of liver function, quantitation of serum markers for hepatitis B and quantitation of HBV DNA was done monthly for 6 years, once every 3 mo after that for 2 years when abnormalities were absent and thereafter every 6 mo when there were no abnormalities.
HBV reactivation, presence of hepatic VOD, GVHD and liver failure were diagnosed as previously described[17-20].
Continuous variables were presented as mean and standard deviations (SDs). One-way ANOVA analysis was used for group comparisons in variables with normal distribution. Kruskal-Wallis tests were used for group comparisons in variables without normal distribution. The normal distributions were detected by Kolmogorov-Smirnov tests. Categorical variables were presented as counts and percentages. χ2 tests or Fisher’s exact tests were used for group comparison among categorical variables. Kaplan-Meier curves with log-rank test were used to evaluate the cumulative survival rates and the differences between the HBsAg(+) group, the HBsAg(-)/HBcAb(+) group and the non-HBV infected group. Statistical analyses were performed using SPSS software version 17 (SPSS Inc, Chicago, IL, United States). A two-tailed P of < 0.05 was considered significant. The statistical methods of this study were reviewed by a biostatistician from MedCom BioStat Inc (Taipei, Taiwan).
Of the 162 subjects included in this study, 105 were not infected with HBV, 32 subjects were HBsAg(-)/HBcAb(+) and 25 subjects were HBsAg(+) (Figure 1). The baseline characteristics of subjects are presented in Table 1. The included patients were aged 12-56 years (median: 32 years). There were no significant differences in gender, age, type of disease and methods of transplantation between the HBsAg(+), HBsAg(-)/HBcAb(+) and non-HBV infected groups.
| Non-HBV infected (n = 105) | HBsAg(-)/HBcAb(+) (n = 32) | HBsAg(+) (n = 25) | P value | |
| Gender | 0.582 | |||
| Male | 69 (65.71) | 22 (68.75) | 14 (56) | |
| Female | 36 (34.29) | 10 (31.25) | 11 (44) | |
| Age (yr) | 31.35 ± 11.88 | 32 ± 10.31 | 32.48 ± 9.66 | 0.887 |
| Type of disease1 | 0.576 | |||
| Acute myeloid leukemia | 44 (41.90) | 18 (56.25) | 15 (60) | |
| Acute lymphoblastic leukemia | 30 (28.57) | 6 (18.75) | 6 (24) | |
| Aplastic anemia | 16 (15.24) | 4 (12.50) | 1 (4) | |
| Others | 15 (14.29) | 4 (12.50) | 3 (12) | |
| Methods of transplantation | 0.548 | |||
| Sibling | 80 (76.19) | 21(65.63) | 20 (80) | |
| Unrelated | 21 (20.00) | 9 (28.13) | 3 (12) | |
| Haploidy | 4 (3.81) | 2 (6.25) | 2 (8) |
The complications observed in subjects from different groups are presented in Table 2. Of the 41 subjects with chronic GVHD in the non-HBV infected group, 29 subjects exhibited extensive involvement and 12 exhibited focal involvement. Of the 13 subjects with chronic GVHD in the HBsAg(-)/HBcAb(+) group, 10 subjects exhibited extensive involvement and 3 exhibited focal involvement. Of the 10 subjects with chronic GVHD in the HBsAg(+) group, 5 subjects exhibited extensive involvement and 5 exhibited focal involvement.
| Non-HBV infected (n = 105) | HBsAg(-)/HBcAb(+) (n = 32) | HBsAg(+) (n = 25) | P value | |
| GVHD | ||||
| Acute | 39 (37.14) | 15 (46.88) | 10 (40) | 0.614 |
| Chronic | 41(39.05) | 13 (40.63) | 10 (40) | 0.986 |
| Involvement1 | 0.366 | |||
| Extensive | 29 (70.73) | 10 (76.92) | 5 (50) | |
| Limited | 12 (29.27) | 3 (23.08) | 5 (50) | |
| Grade1 | 0.089 | |||
| I | 2 (5.13) | 3 (20) | 2 (20) | |
| II | 19 (48.72) | 2 (13.33) | 2 (20) | |
| III | 16 (41.03) | 7 (46.67) | 4 (40) | |
| IV | 2 (5.13) | 3 (20) | 2 (20) | |
| Drug-induced liver injury | 27 (25.71) | 6 (18.75) | 7 (28) | 0.717 |
| Organ injury | 34 (82.93) | 11 (68.75) | 9 (90) | 0.372 |
| Death | 39 (37.14) | 13 (40.63) | 13 (52) | 0.401 |
| Time of granular cells engraftment (d), median (range) | 12 (7-35) | 12 (9-20) | 10 (8-19) | 0.091 |
| Time of megakaryocytic cells engraftment (d), median (range) | 15 (9-50) | 15 (9-49) | 15 (9-48) | 0.880 |
A total of 39 subjects in the non-HBV infected group (37.1%), 15 subjects in the HBsAg(-)/HBcAb(+) group (46.9%) and 10 subjects in the HBsAg(+) group (40%) had acute GVHD. Two subjects (5.1%) in the non-HBV infected group, 3 subjects (20%) in the HBsAg(-)/HBcAb(+) group and 2 subjects (20%) in the HBsAg(+) group had level I GVHD. Nineteen subjects (48.7%) in the non-HBV infected group, 2 subjects (13.3%) in the HBsAg(-)/HBcAb(+) group and 2 subjects (20%) in the HBsAg(+) group had level II GVHD. Sixteen subjects (41%) in the non-HBV infected group, 7 subjects (46.7%) in HBsAg(-)/HBcAb(+) group and 4 subjects (40%) in HBsAg(+) group had level III GVHD. Two subjects (5.1%) in the non-HBV infected group, 3 subjects (20%) in the HBsAg(-)/HBcAb(+) group and 2 subjects (20%) in the HBsAg(+) group had level IV GVHD. Twenty-seven subjects (25.7%) in the non-HBV infected group, 6 subjects (18.7%) in the HBsAg(-)/HBcAb(+) group and 7 (28%) subjects in the HBsAg(+) group had drug-induced liver injury. Thirty four subjects (82.9%) in the non-HBV infected group, 11 subjects (68.7%) in the HBsAg(-)/HBcAb(+) group and 9 (90%) subjects in the HBsAg(+) group had organ injury. There were 39 deaths (37.1%) in the non-HBV infected group, 13 deaths (40.6%) in the HBsAg(-)/HBcAb(+) group and 13 deaths (52%) in the HBsAg(+) group. Only 1 patient in the HBcAb-positive group died of GVHD (liver involvement). The cause of death in the remaining patients was unrelated to liver failure.
In the HBV-infected group, 1 patient who was HBsAg-positive received antiviral therapy with entecavir after the initial diagnosis and antiviral therapy was discontinued 2 mo after discontinuation of immunosuppressive therapy. Five months after discontinuation of immunosuppressive therapy, there was an increase in transaminase and bilirubin levels and HBV-DNA increased to 106 IU/mL. Antiviral therapy was resumed and there was a recovery of liver function. There was no HBV reactivation in any of the remaining patients. There were no significant differences in GVHD, drug-induced liver injury, organ injury, death, time of engraftment of granular cells and time of engraftment of megakaryocytic cells between the three groups (Table 2).
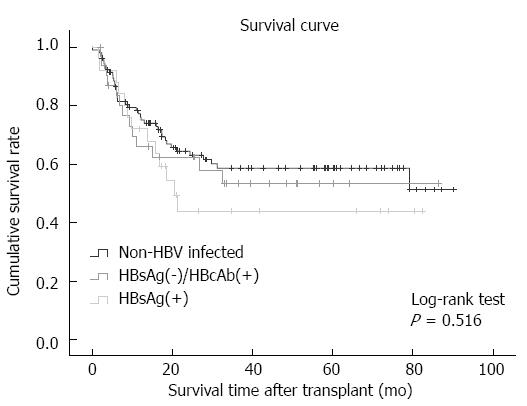
The cumulative survival rates in the different groups are presented in Figure 2. There were no statistically significant differences in the post-transplantation cumulative survival rates between the HBsAg(+), HBsAg(-)/HBcAb(+) and non-HBV infected groups (P = 0.516) (Figure 2).
In this study, we prospectively studied the timing, safety and efficacy of prolonged prophylactic antiviral therapy for HBsAg-positive and HBsAg-negative/antiHBc-positive patients with hematological malignancies who underwent allo-HSCT. Non HBV-infected patients with hematological malignancies were used as controls. Our data showed that HBV patients who received prophylactic antiviral therapy with nucleoside analogues showed no significant differences in the incidence of graft-vs-host disease (GVHD), drug-induced liver injury, organ injury, death or survival time compared to the non-HBV control group. We also showed that using prophylactic antiviral therapy to lower the HBV DNA load to < 103 IU/mL before transplantation improved the safety and efficacy of allo-HSCT by suppressing HBV reactivation.
In the present study, 16.4% of our study patients were HBsAg-positive. These data were consistent with other recent reports[21,22]. Serial evaluations of anti-HBs levels and HBV DNA are necessary to accurately determine the occurrence of reverse seroconversion and HBV reactivation[23]. The incidence of HBV reactivation in patients receiving HSCT has been shown to range from 21%-53%[17,24] and HBV reactivation-related mortality ranged from 5%-40%[25,26]. It was previously reported that HBV-exposed recipients who were HBsAg-negative pre-transplant did not show HBV reactivation after stopping prophylactic lamivudine treatment, while one HBV-carrier recipient had HBV reactivation and fulminant liver failure after stopping lamivudine prophylaxis as late as 31 mo post-transplant[27]. Prophylactic antiviral therapy remains controversial for this population of patients with prior HBV exposure who are considered cured[25].
The major challenges to treating patients who undergo HBV reactivation after HSCT are: (1) high viral loads; (2) immunosuppressed status of the recipient; (3) comorbidities; and (4) risk of generating drug-resistant variants. Although the Standard Practice Manual for HSCT (Seattle) recommends prophylactic antiviral therapy with lamivudine in HBsAg-positive patients receiving allo-HSCT[28], the duration of antiviral therapy is not defined. The Queen Mary Hospital in Hong Kong recommends continuation of prophylactic antiviral therapy after transplantation for at least 12 mo after discontinuation of immunosuppressive therapy in HBsAg-positive patients and the duration of antiviral therapy for patients with a high load of HBV DNA may be longer[29]. Prolonged prophylactic antiviral therapy is especially important in the light of recent reports of delayed immune recovery in HSCT recipients[30] and a case report of HBV reactivation 9 mo after discontinuation of lamivudine treatment in an NHL patient who underwent allo-HSCT[16]. A recent study reported that prolonged prophylactic treatment with entecavir in HBsAg-positive patients or prolonged preemptive treatment with entecavir in HBV-resolved patients prevented HBV reactivation associated with immunosuppression in patients with hematological malignancies[24]. Entecavir treatment was administered for 6 mo after the disappearance of HBsAg and HBV DNA from the serum.
Based on these data, we used antiviral therapy with nucleoside analogues for patients who were HBsAg-positive and those who were HBsAg-negative/HBcAb-positive in this study. HBV can remain in a stable form in the nucleus of affected hepatocytes as covalently closed circular DNA (cccDNA) and the first stage of HBV reactivation which occurs during therapy with potent cytotoxic drugs or with immunosuppressants is characterized by increased viral replication and elevated levels of serum HBeAg and HBV DNA. In HBsAg negative/HBcAb positive patients with normal immune function, HBV-specific CD8+T cells in the peripheral blood may attack infected hepatocytes via the Fas or perforin pathways and inhibit viral replication via release of IFN-γ and TNF-α[26]. However, after allo-HSCT, the incidence of recurrence of HBV infection and related mortality are very high since low levels of autogenous IgG levels may cause an increase in HBsAg levels and recurrence of HBV infection[7,31]. It is important to note that although the incidence of recurrence of HBV infection in these patients is lower than that seen in HBsAg-positive patients, recurrence can lead to fulminant hepatitis which has a high mortality. Rituximab therapy and HSCT are important risk factors of this condition. Previous studies have shown that the incidence of recurrence of HBV infection after HSCT was 10%-20%[22,32-36]. A retrospective long-term study reported that the incidence of recurrence of HBV infection after immunosuppression was 9.0%, 21.7% and 42.9% at 1, 2 and 4 years, respectively[23]. The Consensus on the Management of Lymphoma Patients with HBV Infection in China (2013)[37] recommends monitoring of hepatitis B immune markers and HBV DNA in patients with good compliance and initiation of antiviral therapy when HBV DNA is detectable. Prophylactic antiviral therapy before chemotherapy is recommended for patients with poor compliance. Prophylactic antiviral therapy is also recommended for patients with a high risk for recurrence of HBV infection (including those receiving rituximab therapy or HSCT and those with concomitant hepatic cirrhosis).
In the present study, only 1 patient developed HBV reactivation (HBsAg-positivity) due to early discontinuation of antiviral therapy and HBV reactivation (HBV-DNA levels of 106 IU/mL at 5 mo after discontinuation of immunosuppressive therapy) occurred at the stage of immune reconstitution. However, HBV-DNA was < 500 IU/mL in the rest of the patients. Our data were consistent with a previous report showing that prophylactic lamivudine treatment administered for varying periods of time, ranging from 24 wk post-transplant until 18 mo after transplant, inhibited HBV reactivation[27]. These data suggested that antiviral therapy should be continued for 1 year after discontinuation of immunosuppressive therapy. However, it is important to note that prolonged lamivudine treatment for HBV reactivation has been shown to be associated with the generation of treatment-resistant variants, while tenofovir and adefovir are thought to negatively impact the renal function of patients[30,38-40]. Our choice of entecavir was based on data showing that entecavir was associated with significantly lower levels of resistance compared to lamivudine[40]. In this study, entecavir treatment was started at the time of chemotherapy initiation and was stopped at 1 year after discontinuation of immunosuppressive therapy. There was no significant difference in hematopoietic reconstitution, hematopoietic stem cell engraftment status or incidence of GVHD between the non-hepatitis B group (which received no entecavir therapy) and the hepatitis B group, suggesting that entecavir was safe for the transplantation of hematopoietic stem cells in HBV patients. There was also no significant difference in the incidence of drug-induced liver injury, hepatic vein obstruction syndrome and liver involvement of GVHD between the two groups, suggesting the efficacy of entecavir therapy for this group of patients. Our data agreed with an earlier report demonstrating the safety and tolerability of entecavir in HBV patients undergoing HSCT[41].
A high load of HBV DNA (> 105 copies/mL) prior to chemotherapy[6] or HSCT[5] is an important risk factor for HBV reactivation. In patients receiving chemotherapy, the incidence of recurrence of HBV infection was 37.8% in patients with detectable HBV DNA and 10.9% in patients with undetectable HBV DNA[7]. However, the exact threshold remains controversial. Three different studies proposed that the threshold of viral load dictating recurrence was 0.5 × 103 IU/mL, 1 × 103 IU/mL and 2 × 104 IU/mL, respectively[7,42,43]. These data suggested that it would be advantageous to use antiviral therapy with nucleos(t)ide analogues to reduce the viral load before transplantation. Quantification of viral DNA is a useful tool to identify patients who should receive antiviral therapy with nucleoside analogues prior to transplantation. In the present study, we defined the threshold of pre-transplantation HBV DNA as 103 IU/mL and HBV loads and medication compliance of patients with values above this threshold were closely monitored. Our data showed no hepatitis-related liver failure in patients with HBV DNA loads below the threshold value.
This study had several limitations. The factors initiating HBV reactivation were not identified or characterized and this is an important future goal. It is also essential to investigate the optimal course of antiviral therapy. Based on our study, we also could not conclude whether HBV reactivation in elderly patients (> 60 years old) can be also safely prevented with the threshold value of 103 IU/mL). Another limitation of this study was that we did not determine HBV genotypes. In our future studies, we would like to stratify HBV-infected patients based on the type of disease, HBV genotype, severity of GVHD and degree of HBV activity, and to explore individualized antiviral therapeutic strategies.
In summary, our data demonstrated that prolonged prophylactic antiviral therapy to lower the HBV DNA load to 103 IU/mL before transplantation and close monitoring of HBV DNA and immune markers of HBV after transplantation was beneficial in HBsAg-negative antiHBc-positive patients undergoing allo-HSCT. Antiviral therapy administered prior to transplantation was safe and had no influence on hematopoietic reconstitution. It is important to investigate whether the prolonged duration of antiviral therapy could cause resistant variants of HBV.
In areas of high hepatitis B virus (HBV) endemicity where almost 15% of the patients receiving hematopoietic stem cell transplantation are HBsAg-positive prior to transplantation, HBV reactivation can significantly impact the post-transplant prognosis. Nucleoside analogs such as lamivudine and entecavir have been used for the treatment of acute and chronic HBV infection, as well as for chemotherapy-induced HBV reactivation. However, the timing, safety and influence of prophylactic antiviral therapy in HBsAg-positive and in HBsAg-negative/antiHBc-positive HBV patients after allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains unclear.
Recent guidelines recommend continuation of prophylactic antiviral therapy after transplantation for at least 12 mo after discontinuation of immunosuppressive therapy in HBsAg-positive patients and the duration of antiviral therapy for patients with a high load of HBV DNA may be longer. Prolonged prophylactic treatment with entecavir in HBsAg-positive patients or prolonged preemptive treatment with entecavir in HBV-resolved patients prevented HBV reactivation associated with immunosuppression in patients with hematological malignancies. A high load of HBV DNA (> 105 copies/mL) prior to chemotherapy or HSCT is an important risk factor for HBV reactivation.
We used entecavir therapy for patients who were HBsAg-positive as well as those who were HBsAg-negative/HBcAb-positive. Entecavir treatment was started at the time of chemotherapy initiation and was stopped at 1 year after discontinuation of immunosuppressive therapy. There was no significant difference in hematopoietic reconstitution, hematopoietic stem cell engraftment status or incidence of graft-vs-host disease (GVHD) between the non-hepatitis B group (which received no entecavir therapy) and the hepatitis B group, suggesting that entecavir was safe for the transplantation of hematopoietic stem cells in HBV patients. There was also no significant difference in the incidence of drug-induced liver injury, hepatic vein obstruction syndrome and liver involvement of GVHD between the two groups, suggesting the efficacy of entecavir therapy. We defined the threshold of pre-transplantation HBV DNA as 103 IU/mL and HBV loads and medication compliance of patients with values above this threshold were closely monitored. Our data showed no hepatitis-related liver failure in patients with HBV DNA loads below the threshold value.
Prolonged prophylactic antiviral therapy to lower the HBV DNA load to 103 IU/mL before transplantation and close monitoring of HBV DNA and immune markers of HBV after transplantation were beneficial in HBsAg-negative antiHBc-positive patients undergoing allo-HSCT.
Allogeneic hematopoietic stem cell transplantation is a procedure where allogeneic stem cells are transfused in order to restore hematopoietic function in patients who are immunosuppressed, commonly during chemotherapy. HBV reactivation occurs when intracellular covalently closed circular HBV DNA is not completely eliminated after spontaneous resolution of acute or chronic HBV infection or after antiviral therapy.
The authors investigated the safety and efficacy of prophylactic antiviral treatment in HBV-infected patients who received allogeneic hematopoietic stem cell transplantation. Prevention of HBV reactivation is important in the management of HSCT and antiviral therapy with nucleoside analogues plays a key role in the clinical practice. In the present study, they defined the threshold of pre-transplantation HBV-DNA as 103 IU/mL and prospectively followed-up the clinical courses of the patients.
P- Reviewer: Doki N, Enomoto H, Makvandi M S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | Hepatitis B vaccines. Wkly Epidemiol Rec. 2009;84:405-419. [PubMed] |
| 2. | He F. Ministry of Health Official Reports: the results of the national serological survey of heptitis B. Zhongguo Yiliaoqixie Xinxi. 2008;23:1. |
| 3. | Shitani M, Sasaki S, Akutsu N, Takagi H, Suzuki H, Nojima M, Yamamoto H, Tokino T, Hirata K, Imai K. Genome-wide analysis of DNA methylation identifies novel cancer-related genes in hepatocellular carcinoma. Tumour Biol. 2012;33:1307-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2401] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 5. | Lau GK, Leung YH, Fong DY, Au WY, Kwong YL, Lie A, Hou JL, Wen YM, Nanj A, Liang R. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood. 2002;99:2324-2330. [PubMed] |
| 6. | Ling R, Harrison TJ. Production of hepatitis B virus covalently closed circular DNA in transfected cells is independent of surface antigen synthesis. J Gen Virol. 1997;78:1463-1467. [PubMed] |
| 7. | Yeo W, Zee B, Zhong S, Chan PK, Wong WL, Ho WM, Lam KC, Johnson PJ. Comprehensive analysis of risk factors associating with Hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004;90:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 246] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Onozawa M, Hashino S, Izumiyama K, Kahata K, Chuma M, Mori A, Kondo T, Toyoshima N, Ota S, Kobayashi S. Progressive disappearance of anti-hepatitis B surface antigen antibody and reverse seroconversion after allogeneic hematopoietic stem cell transplantation in patients with previous hepatitis B virus infection. Transplantation. 2005;79:616-619. [PubMed] |
| 9. | Hsiao LT, Chiou TJ, Gau JP, Liu JH, Tzeng CH, Chen PM. Hepatitis B infection in haematopoietic stem cell transplantation: still unresolved. Hong Kong Med J. 2009;15:42-44. [PubMed] |
| 10. | Liang TJ, Baruch Y, Ben-Porath E, Enat R, Bassan L, Brown NV, Rimon N, Blum HE, Wands JR. Hepatitis B virus infection in patients with idiopathic liver disease. Hepatology. 1991;13:1044-1051. [PubMed] |
| 11. | Moses SE, Lim Z, Zuckerman MA. Hepatitis B virus infection: pathogenesis, reactivation and management in hematopoietic stem cell transplant recipients. Expert Rev Anti Infect Ther. 2011;9:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1347] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 13. | Andreone P, Caraceni P, Grazi GL, Belli L, Milandri GL, Ercolani G, Jovine E, D’Errico A, Dal Monte PR, Ideo G. Lamivudine treatment for acute hepatitis B after liver transplantation. J Hepatol. 1998;29:985-989. [PubMed] |
| 14. | Yeo W, Chan PK, Ho WM, Zee B, Lam KC, Lei KI, Chan AT, Mok TS, Lee JJ, Leung TW. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004;22:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, Liu CY, Yang MH, Tzeng CH, Lee PC. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31:2765-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 16. | Milazzo L, Corbellino M, Foschi A, Micheli V, Dodero A, Mazzocchi A, Montefusco V, Zehender G, Antinori S. Late onset of hepatitis B virus reactivation following hematopoietic stem cell transplantation: successful treatment with combined entecavir plus tenofovir therapy. Transpl Infect Dis. 2012;14:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Smith LH, Wyngaarden JB, Cooper JAD, Pappas PG. Cecil Review of General Internal Medicine. 6th ed. St. Louis: Saunders (W.B.) Co Ltd 1988; . |
| 18. | Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, Hui P, Leung NW, Zee B, Johnson PJ. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62:299-307. [PubMed] |
| 19. | Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, Sensenbrenner LL, Santos GW, Saral R. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778-783. [PubMed] |
| 20. | Thomas E, Storb R, Clift RA, Fefer A, Johnson FL, Neiman PE, Lerner KG, Glucksberg H, Buckner CD. Bone-marrow transplantation (first of two parts). N Engl J Med. 1975;292:832-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1277] [Cited by in RCA: 1122] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Li J, Liu J, Huang B, Zheng D, Chen M, Zhou Z, Xu D, Zou W. Hepatitis B virus infection status is an independent risk factor for multiple myeloma patients after autologous hematopoietic stem cell transplantation. Tumour Biol. 2013;34:1723-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Mikulska M, Nicolini L, Signori A, Rivoli G, Del Bono V, Raiola AM, Di Grazia C, Dominietto A, Varaldo R, Ghiso A. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive allogeneic haematopoietic stem cell transplant recipients: risk factors and outcome. Clin Microbiol Infect. 2014;20:O694-O701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Papamichalis P, Alexiou A, Boulbou M, Dalekos GN, Rigopoulou EI. Reactivation of resolved hepatitis B virus infection after immunosuppression: is it time to adopt pre-emptive therapy? Clin Res Hepatol Gastroenterol. 2012;36:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Tamori A, Hino M, Kawamura E, Fujii H, Uchida-Kobayashi S, Morikawa H, Nakamae H, Enomoto M, Murakami Y, Kawada N. Prospective long-term study of hepatitis B virus reactivation in patients with hematologic malignancy. J Gastroenterol Hepatol. 2014;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Martyak LA, Taqavi E, Saab S. Lamivudine prophylaxis is effective in reducing hepatitis B reactivation and reactivation-related mortality in chemotherapy patients: a meta-analysis. Liver Int. 2008;28:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, Csako G. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519-528. [PubMed] |
| 27. | Moses SE, Lim ZY, Sudhanva M, Devereux S, Ho AY, Pagliuca A, Zuckerman M, Mufti GJ. Lamivudine prophylaxis and treatment of hepatitis B Virus-exposed recipients receiving reduced intensity conditioning hematopoietic stem cell transplants with alemtuzumab. J Med Virol. 2006;78:1560-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Flowers ME, McDonald G, Carpenter P, Boeckh M, Sanders J, Stern J, Holmberg L, Schubert M, Martin PJ. Long-term follow-up after hematopoietic stem cell transplant general guidelines for referring physicians. Seattle: Fred Hutchinson Cancer Research Center/ Seattle Cancer Care Alliance 2014; 76-79. [ Last accessed in April 2015] Available from: http://www.fhcrc.org/content/dam/public/Treatment-Suport/Long-Term-Follow-Up/physician.pdf. |
| 29. | Liang R. How I treat and monitor viral hepatitis B infection in patients receiving intensive immunosuppressive therapies or undergoing hematopoietic stem cell transplantation. Blood. 2009;113:3147-3153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Allen MI, Deslauriers M, Andrews CW, Tipples GA, Walters KA, Tyrrell DL, Brown N, Condreay LD. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Lamivudine Clinical Investigation Group. Hepatology. 1998;27:1670-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 630] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 31. | Dai MS, Chao TY, Kao WY, Shyu RY, Liu TM. Delayed hepatitis B virus reactivation after cessation of preemptive lamivudine in lymphoma patients treated with rituximab plus CHOP. Ann Hematol. 2004;83:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Dhédin N, Douvin C, Kuentz M, Saint Marc MF, Reman O, Rieux C, Bernaudin F, Norol F, Cordonnier C, Bobin D. Reverse seroconversion of hepatitis B after allogeneic bone marrow transplantation: a retrospective study of 37 patients with pretransplant anti-HBs and anti-HBc. Transplantation. 1998;66:616-619. [PubMed] |
| 33. | Knöll A, Boehm S, Hahn J, Holler E, Jilg W. Reactivation of resolved hepatitis B virus infection after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;33:925-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Hammond SP, Borchelt AM, Ukomadu C, Ho VT, Baden LR, Marty FM. Hepatitis B virus reactivation following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:1049-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Viganò M, Vener C, Lampertico P, Annaloro C, Pichoud C, Zoulim F, Facchetti F, Poli F, Scalamogna M, Deliliers GL. Risk of hepatitis B surface antigen seroreversion after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Seth P, Alrajhi AA, Kagevi I, Chaudhary MA, Colcol E, Sahovic E, Aljurf M, Gyger M. Hepatitis B virus reactivation with clinical flare in allogeneic stem cell transplants with chronic graft-versus-host disease. Bone Marrow Transplant. 2002;30:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Experimental Diagnosis Group, Hematology Society Chinese Medical Association. Consensus of Chinese experts on morphologic analysis of blood cells. Zhongguo Xueyexue Zazhi. 2013;34:558-560. |
| 38. | Chen FW, Coyle L, Jones BE, Pattullo V. Entecavir versus lamivudine for hepatitis B prophylaxis in patients with haematological disease. Liver Int. 2013;33:1203-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Marinone C, Mestriner M. HBV disease: HBsAg carrier and occult B infection reactivation in haematological setting. Dig Liver Dis. 2011;43 Suppl 1:S49-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Ghany MG, Doo EC. Antiviral resistance and hepatitis B therapy. Hepatology. 2009;49:S174-S184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 41. | Aoki J, Kimura K, Kakihana K, Ohashi K, Sakamaki H. Efficacy and tolerability of Entecavir for hepatitis B virus infection after hematopoietic stem cell transplantation. Springerplus. 2014;3:450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Lau GK, Suri D, Liang R, Rigopoulou EI, Thomas MG, Mullerova I, Nanji A, Yuen ST, Williams R, Naoumov NV. Resolution of chronic hepatitis B and anti-HBs seroconversion in humans by adoptive transfer of immunity to hepatitis B core antigen. Gastroenterology. 2002;122:614-624. [PubMed] |
| 43. | Hui CK, Cheung WW, Au WY, Lie AK, Zhang HY, Yueng YH, Wong BC, Leung N, Kwong YL, Liang R. Hepatitis B reactivation after withdrawal of pre-emptive lamivudine in patients with haematological malignancy on completion of cytotoxic chemotherapy. Gut. 2005;54:1597-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |