Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4268
Peer-review started: September 19, 2014
First decision: October 29, 2014
Revised: January 2, 2015
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: April 14, 2015
Processing time: 209 Days and 14.6 Hours
AIM: To assess the efficacy and safety of weekly docetaxel plus a fixed-dose rate (FDR) of gemcitabine in metastatic esophageal squamous cell carcinoma (SCC).
METHODS: A multi-center, open-label, prospective phase II study was designed. Thirty-three esophageal SCC patients with documented progression after fluoropyrimidine/platinum-based first-line chemotherapy were enrolled and treated with docetaxel 35 mg/m2 and gemcitabine 1000 mg/m2 iv at a FDR (10 mg/m2 per minute) on days 1 and 8. Treatment was repeated every twenty-one days until disease progression, unacceptable toxicity, or consent withdrawal. The primary endpoint was response rate (RR), and secondary endpoints were safety, progression-free survival (PFS) and overall survival (OS).
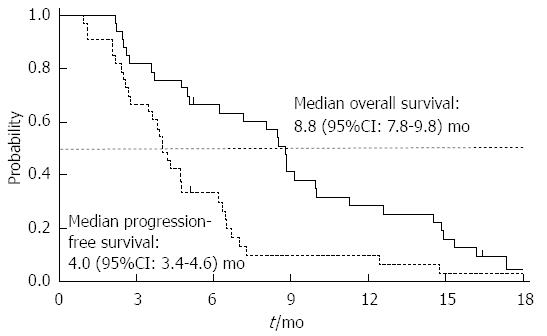
RESULTS: Combination of weekly docetaxel and FDR gemcitabine was well tolerated: the most common treatment-related adverse events were anemia (97%), fatigue (64%) and neutropenia (55%). One patient with multiple lung and lymph node metastases died of respiratory failure after receiving four cycles of chemotherapy, and the possibility of drug-induced pneumonitis could not be completely excluded. Disease control (objective response plus stable disease) in the ITT population was achieved in 88% of patients, and the overall RR was 30% (95%CI: 15%-46%). The median PFS and OS were 4.0 (95%CI: 3.4-4.6) and 8.8 mo (95%CI: 7.8-9.8 mo), respectively.
CONCLUSION: A combination of weekly docetaxel and FDR gemcitabine showed promising antitumor activity and tolerability in previously treated, metastatic esophageal SCC.
Core tip: Esophageal squamous cell carcinoma (SCC) is a lethal disease with a poor prognosis. Currently, there is no standard chemotherapy regimen for metastatic esophageal SCC patients who have failed platinum and fluoropyrimidine combination chemotherapy. In this multi-center, prospective phase II study, we demonstrated that the combination of weekly docetaxel and a fixed-dose rate of gemcitabine is active and well tolerated as a salvage chemotherapy in patients with previously treated metastatic esophageal SCC.
- Citation: Lee MY, Jung KS, Kim HS, Lee JY, Lim SH, Kim M, Jung HA, Kim SM, Sun JM, Ahn MJ, Lee J, Park SH, Yi SY, Hwang IG, Lee SC, Ahn HK, Lim DH, Lee SI, Park KW. Weekly docetaxel and gemcitabine in previously treated metastatic esophageal squamous cell carcinoma. World J Gastroenterol 2015; 21(14): 4268-4274
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4268.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4268
Esophageal cancer is the eighth most common cause of cancer death worldwide. There are two main histological types; adenocarcinoma and squamous cell carcinoma (SCC). Although esophageal adenocarcinoma is more prevalent in Western countries, SCC is the most predominant histologic subtype globally[1]. Esophageal SCC is a highly lethal disease with a five-year survival rate of 15%-19%[2,3]. Treatments for metastatic esophageal SCC are limited; most patients are not eligible for surgery because more than two thirds of patients present with unresectable or metastatic disease at the time of initial diagnosis, and the majority of remaining patients with initially locoregional disease eventually develop distant metastases[4]. Therefore, palliative chemotherapy is the only treatment option for patients with metastatic esophageal SCC to prolong their survival and to improve their quality of life. A combination of cisplatin and infusional 5-fluorouracil (5-FU) is the most commonly used regimen for palliative first-line chemotherapy in metastatic esophageal SCC[4-6]. However, the number of effective cytotoxic agents for the treatment of patients with metastatic esophageal SCC is limited. When patients have failed platinum and fluoropyrimidine combination chemotherapy, it is commonly observed that patients experience a rapid clinical deterioration and decline in their performance status.
Docetaxel is one of the most widely used chemotherapeutic agents in metastatic esophageal SCC patients[7]. Although docetaxel is often combined with cisplatin, particularly in a salvage setting, the cisplatin-based chemotherapy had a clinically important toxicity profiles. To avoid cisplatin-related toxicity, there are several ways including omission or replacement of cisplatin with a cytotoxic agent with similar activity. Gemcitabine, among others, has a notable activity and tolerable toxicity profile in esophageal SCC[8], blocking cancer cells in a different cellular phase than docetaxel[9]. A randomized phase II study in patients with pancreatic cancer suggested that gemcitabine given at a fixed-dose rate (FDR) infusion (i.e., 10 mg/m2 per minute) have did not have a greater activity than a bolus infusion[10]. Combinations of docetaxel and gemcitabine have been studied in treatment of various solid tumors including metastatic breast cancer[11], non-small cell lung cancer (NSCLC)[12], and other malignancies, because both drugs have good antitumor activity with non-overlapping toxicity[13]. For example, a clinical study by Hensley et al[14] demonstrated an impressive 53% response rate in patients with predominantly uterine leiomyosarcoma. In this study, patients received gemcitabine 900 mg/m2 on days 1 and 8 plus docetaxel 100 mg/m2 on day 8 with granulocyte-colony stimulation factor (G-CSF) support every 3 wk. Hematologic toxicity including neutropenia and thrombocytopenia was the most common dose-limiting toxicity and antitumor activity of the regimen was equivalent to cisplatin-based combinations.
Currently, optimal dose schedules for docetaxel plus gemcitabine combination chemotherapy have not been determined. A weekly docetaxel and gemcitabine combination was attractive because of the low incidence of severe myelosuppression in weekly docetaxel compared to the standard 3-weekly docetaxel regimen[15-17], this altered toxicity profile suggested that, as a salvage treatment, there was the potential for better tolerance and increased dose intensity. We conducted a phase II study in order to assess the efficacy and safety of weekly docetaxel plus a FDR of gemcitabine as a salvage chemotherapy in patients with metastatic esophageal SCC. The primary objective was to evaluate the anti-tumor activity in terms of response rate (RR), and secondary objectives were progression-free survival (PFS), overall survival (OS), and the safety profile of the regimen.
This was an open-label, multi-center study of palliative chemotherapy with weekly docetaxel plus a FDR of gemcitabine. Patients with histologically confirmed metastatic or recurrent SCC of the esophagus were enrolled in this prospective phase II study. All patients were required to have experienced progression after at least one cytotoxic chemotherapy regimen involving both fluoropyrimidine and platinum. Patients who experienced recurrence during or within 6 mo after the completion of adjuvant chemotherapy were allowed to enter the study. Only patients older than 18 years of age, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 were eligible for entry into this study. Other eligibility criteria included at least one bi-dimensionally measurable lesion by the RECIST 1.1 criteria[18], a life expectancy of at least 3 mo, and adequate bone marrow, renal, and liver functions. Patients with serious concomitant medical diseases prior to exposure to docetaxel or gemcitabine, who were pregnant or breast feeding, who had a history of significant neurologic or psychiatric disorders, or evidence of serious gastrointestinal bleeding were considered ineligible. A treatment-free interval of at least 4 wk was required to enter the study. Before the study was initiated, the protocol was approved by the Institutional Review Board of all the participating hospitals in accordance with the ethical principles of the Declaration of Helsinki and local guidelines, and all patients provided written informed consent prior to participation in any study-specific procedures.
The pre-treatment evaluation included medical history and a physical examination, complete blood count with differentials, chemistry, chest X-ray, computed tomography (CT) scans of the thorax and any other diagnostic procedures as clinically indicated. Gemcitabine 1000 mg/m2 was administered intravenously at a FDR 10 mg/m2 per minute on days 1 and 8. Docetaxel 35 mg/m2 was also administered intravenously over 60 min on days 1 and 8. Premedications included adequate antiemetic therapy and dexamethasone 15 mg before each docetaxel infusion. All chemotherapy was administered on an outpatient basis unless hospitalization was required for other reasons, and the cycle was repeated every twenty-one days until there was documented progression of the disease, unacceptable toxicity, or the patients refusal. All patients received full supportive care, which included blood product transfusion, antiemetics, and analgesics as appropriate. Dose reductions were based on toxicities that were graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) scale version 4.0. Clinic visits and toxicity evaluation were performed on days 1 and 8 of each cycle. Each chemotherapy cycle was started only if neutrophil counts were ≥ 1500/mm3, platelet counts were ≥ 75000/mm3, and all non-hematologic toxicities had been reduced to grade 0 to 1. In the case of a delay of more than twenty-one days due to toxicity, the protocol treatment was discontinued. If the patient experienced febrile neutropenia, grade 4 neutropenia lasting > seven days, grade 4 thrombocytopenia, or grade 3 or 4 non-hematologic toxicities with the exception of nausea, vomiting and alopecia, subsequent doses of gemcitabine and docetaxel were lowered by 20% from the doses in the preceding cycles. Dose reduction on day 8 of each cycle was permitted in accordance with the protocols. In brief, the 8th day doses of gemcitabine and docetaxel were reduced if neutrophil counts were between 1000/mm3 and 1500/mm3, or if platelet counts were between 50000/mm3 and 75000/mm3. Gemcitabine and docetaxel doses were omitted on day 8 when neutrophil counts were < 1000/mm3 or platelet counts were < 50000/mm3.
Appropriate imaging studies including contrast-enhanced CT scans were conducted every two cycles to evaluate treatment response. As the primary endpoint of this study was objective RR, the clinical tumor response was assessed according to the RECIST criteria version 1.1[18]. Upon progression disease (PD), further salvage treatments were permitted at the investigators’ discretion, and the nature of any treatments was recorded.
The secondary endpoints were PFS, OS and safety. PFS and OS were measured from day 1 on first study treatment cycle until the day of documented PD and death, respectively. OS and PFS were assessed using the Kaplan-Meier method, and the 95%CI for the median time to an event were calculated. Sample sizes were calculated to reject a 10% response rate in favor of a target response rate of 30%, with a significance level of 0.05 and a power of 90%. Considering a 10% drop-out rate, a total of 33 patients was planned. The statistical methods of this study were reviewed by Kyunga Kim (PhD in Statistics) from Biostatistics and Clinical Epidemiology Center at Samsung Medical Center.
The current phase II study was opened in August 2011 among oncology departments in six tertiary Korean centers. The last patients entered the study in Mar 2014. The baseline characteristics of the 33 patients are listed in Table 1. All patients had histologically proven SCC arising from the esophagus. In a majority of patients the primary site was the middle (n = 26) or distal thoracic esophagus (n = 6). Twenty-three (70%) patients had undergone esophagectomy, and three patients (9%) had received curative-aim chemoradiotherapy. The median age was 59 years with a range of 44 to 76 years, and all patients had symptoms at baseline (ECOG performance status 1 in 32/33 patients and 2 in one patient). The most common first-line chemotherapy regimen was 5-FU plus cisplatin (76%) followed by capecitabine plus cisplatin or paclitaxel (24%). In eight patients who were treated with two prior chemotherapy regimens, second-line chemotherapy included 5-FU plus cisplatin (75%) and capecitabine plus cisplatin (25%). More than 75.8% of the patients received prior palliative chemotherapy and 54.5% of the patients received prior radiotherapy. All patients had metastatic disease at the time of treatment with the most common site of metastasis being the lymph node (88%) followed by the lung (42%), and liver (18%).
| Characteristics | Patients |
| Total number of patients | 33 |
| Age (yr), median (range) | 59 (44-76) |
| Gender | |
| Male | 31 (94) |
| Female | 2 (6) |
| ECOG PS | |
| 1 | 32 (97) |
| 2 | 1 (3) |
| Anatomic site | |
| Upper thoracic | 1 (3) |
| Middle thoracic | 26 (79) |
| Distal | 6 (18) |
| Prior curative-aim treatment | |
| None | 7 (21) |
| Surgery | 23 (70) |
| Chemoradiotherapy | 3 (9) |
| Number of prior chemotherapy regimens | |
| 1 | 25 (76) |
| 2 | 8 (24) |
| Tumor grade | |
| Well differentiated | 3 (9) |
| Moderately differentiated | 18 (55) |
| Poorly differentiated or unknown | 12 (36) |
| Metastatic site (s) | |
| Lymph node | 29 (88) |
| Lung | 14 (42) |
| Liver | 6 (18) |
| Bone | 3 (9) |
Patients received a total of 157 treatment cycles (median 5, range 1-16). Nine (6%) of the 8th day doses of docetaxel and gemcitabine were delayed or skipped because of toxic effects, as per the protocol criteria, and dose reduction was required in 24 patients. In the majority of treatment discontinuation was caused by PD (n = 22). Another minor reasons were consent withdrawal (n = 6) and toxicity (n = 5). The planned dose intensities for docetaxel were 23 mg/m2 per week and gemcitabine were 667 mg/m2 per week, thus, the relative dose intensity of both drugs was 82% (95%CI: 65%-97%).
All eligible patients were assessable for adverse events. The treatment-related adverse events are shown in Table 2. The most commonly observed all-grade toxicity was anemia (97%), followed by asthenia/fatigue (64%), neutropenia (55%), alopecia (46%), and anorexia (39%). The major grade 3 or 4 toxicities were hematologic ones including neutropenia (39%), followed by anemia (9%), febrile neutropenia (9%) and thrombocytopenia (6%). Although these adverse events were generally tolerated and easily manageable, one patient, a 64-year-old male, died of respiratory failure after receiving the fourth cycle. His chest CT revealed bilateral pneumonitis while the lung and lymph node metastases remained a partial response (PR). The patient was treated with corticosteroids and antibiotics but did not benefit.
| NCI-CTC grade | ||||||
| 1 | 2 | 3 | 4 | 1-4 | 3/4 | |
| Hematologic | ||||||
| Anemia | 13 | 16 | 3 | 0 | 32 (97) | 3 (9) |
| Neutropenia | 0 | 5 | 10 | 3 | 18 (55) | 13 (39) |
| Thrombocytopenia | 6 | 2 | 1 | 1 | 10 (30) | 2 (6) |
| Febrile neutropenia | 0 | 0 | 3 | 0 | 3 (9) | 3 (9) |
| Non-hematologic | ||||||
| Asthenia/Fatigue | 13 | 4 | 4 | 0 | 21 (64) | 4 (12) |
| Myalgia | 5 | 1 | 0 | 0 | 6 (18) | 0 (0) |
| Anorexia | 10 | 2 | 1 | 0 | 13 (39) | 1 (3) |
| Nausea | 5 | 1 | 1 | 0 | 7 (21) | 1 (3) |
| Vomiting | 4 | 0 | 1 | 0 | 5 (15) | 1 (3) |
| Mucositis/Stomatitis | 6 | 2 | 0 | 0 | 8 (24) | 0 (0) |
| Diarrhea | 6 | 2 | 0 | 0 | 8 (24) | 0 (0) |
| Hand-foot syndrome | 2 | 2 | 0 | 0 | 4 (12) | 0 (0) |
| Peripheral neuropathy | 6 | 0 | 0 | 0 | 6 (18) | 0 (0) |
| Alopecia | 14 | 1 | 0 | 0 | 15 (46) | 0 (0) |
| Headache | 5 | 0 | 0 | 0 | 5 (15) | 0 (0) |
| Dysphagia | 7 | 1 | 0 | 0 | 8 (24) | 0 (0) |
| Rash | 4 | 2 | 0 | 0 | 6 (18) | 0 (0) |
| Cough | 4 | 1 | 1 | 0 | 6 (18) | 1 (3) |
| Sputum | 7 | 1 | 1 | 0 | 9 (27) | 1 (3) |
| Chest discomfort | 10 | 0 | 0 | 0 | 10 (30) | 0 (0) |
Clinical response evaluations were assessable for 32 patients who received at least two cycles of study treatment. Disease control (objective response and stable disease) in the ITT population was achieved in 88% of patients and the overall RR was 30% (95%CI: 15%-46%). Among the ten responders, one patient achieved a CR, which was maintained for 14 mo. The median duration of response in responders was 2 mo.
At the time of analysis, 30 patients were dead. With a median follow-up duration of 20 mo (95%CI: 18-21 mo), the median PFS and OS were 4.0 mo (95%CI: 3.4-4.6 mo) and 8.8 mo (95%CI: 7.8-9.8 mo), respectively. Kaplan-Meier curves for PFS and OS were depicted in Figure 1.
Although salvage treatment were not specified in the protocol, palliative radiotherapy was given to 4 patients with symptomatic progression in lung, bone, or lymph nodes. We performed salvage chemotherapy to 7 patients (21%) with irinotecan (n = 5) or pemetrexed (n = 2). Esophageal stenting to relieve obstructive symptoms was performed in 5 patients.
The objective of this phase II study was to assess the efficacy and safety of a non-platinum-based combination of docetaxel and FDR gemcitabine administered weekly to patients previously treated for metastatic esophageal SCC. Because of their antitumor activity as single agents and different mechanisms of action, docetaxel and gemcitabine combinations have been tested previously, although myelosuppression has been a serious problem[13]. In a previous phase II study involving weekly docetaxel and gemcitabine combination, a weekly regimen could be administered with acceptable toxicity to most patients[12]. The current study confirmed these results, the non-platinum combination of docetaxel 35 mg/m2 and FDR gemcitabine 1000 mg/m2 on days 1 and 8 every 3 wk had an acceptable toxicity profile.
In the second-line setting of esophageal SCC, docetaxel is one of the most frequently investigated, and the most widely used, regimens. Recently, a large retrospective study showed a moderated PFS advantage with docetaxel-based second-line chemotherapy in esophageal SCC[19,20]. Although 3-weekly docetaxel has proved to be active, it is associated with a significant incidence of severe neutropenia, often complicated by fever. Therefore, several clinical trials have examined docetaxel administered as weekly schedule, which demonstrated modest toxicity profiles with minimal myelosuppression[14]. In a randomized trial comparing weekly and 3-weekly schedules of docetaxel and cisplatin in patients with previously untreated NSCLC, the most frequent grade 3 or 4 toxicity was neutropenia in the 3-weekly regimen and fatigue in the weekly regimen[17]. Several phase I and II clinical trials have examined docetaxel administered in weekly doses of 30, 35, 40 mg/m2. The weekly docetaxel 35 mg/m2 chemotherapy group produced less myelosuppression, and better compliance and response rates than the 3-weekly docetaxel or other weekly dose groups[21,22]. Our clinical trial administered docetaxel at a weekly dose of 35 mg/m2. Gemcitabine also had been tested in phase I and II clinical trials[8], and the combination of docetaxel and gemcitabine has been considered a rational approach for salvage setting esophageal SCC. An explanation for the high response rates observed may be that docetaxel and gemcitabine exhibit true in vivo synergy, and the combination has complementary mechanisms of action, docetaxel promoting cell death and gemcitabine inducing cell cycle arrest[23].
In addition to the weekly schedule of docetaxel, we employed a FDR schedule for gemcitabine infusion. To render gemcitabine more effective, longer exposures to the drug may be associated with greater antitumor effects by maximizing the amounts of gemcitabine that can accumulate intracellularly in a given time period. FDR infusion of gemcitabine is a term that refers to gemcitabine infusion at a rate that maintains gemcitabine concentration at levels that optimize the incorporation of the active gemcitabine metabolite, gemcitabine triphosphate, into DNA. Pre-clinical data have shown that the maintenance of gemcitabine triphosphate concentration at 20 μmol/L, optimizes in vivo cell killing[24,25]. The issue of whether prolonged-infusion gemcitabine (10 mg/m2 per minute) results in higher clinical response rates compared to bolus infusions has been addressed in a randomized trial in pancreatic cancer[10]. Grade 3 and 4 myelosuppression occurred with both the FDR infusion and the bolus infusion group. This result seemed to be more toxic with the FDR infusion. However, a higher incidence of dose modification or discontinuation of gemcitabine was not observed[10]. The use of FDR infusion of gemcitabine as a combination treatment for esophageal cancer has been documented in a few phase I and II clinical trials[26].
Recently, several studies on combination chemotherapy for second-line treatment of previously treated metastatic esophageal cancer have been conducted. Among them, there are two reports of combination chemotherapy including docetaxel as a second-line regimen in metastatic esophageal squamous cell carcinoma. Shim et al[27] did a phase II study on docetaxel and cisplatin chemotherapy, which showed a response rate of 34.2%, a median PFS of 4.5 mo, and a median OS of 7.4 mo. However, this regimen showed toxicity with grade 3 or 4 neutropenia at 52.6%, asthenia at 31.6%, nausea at 18.4%, and neuropathy at 15.8%. In another phase II study using docetaxel and nedaplatin for patients previously treated with cisplatin and fluorouracil by Jin et al[28], the reported response rates were 27.1%, the median PFS was 3.1 mo, and the median OS was 5.9 mo. This regimen showed toxicity of grade 3 or 4 of neutropenia at 19.6%, grade 1 to 4 anorexia at 47.8%, fatigue at 41.3%, and nausea/vomiting at 32.6%. The docetaxel-platinum based chemotherapy present similar response rates and survival data compared with the current study. However, it is important to recognize that docetaxel and gemcitabine combination chemotherapy is potentially associated with significant toxicity. There are several cumulative platinum induced toxicities observed after platinum-based chemotherapy is used as a first-line treatment in esophageal SCC, including emesis, decrease in glomerular filtration rate, and neurotoxicity.
In the current study, combination of weekly docetaxel plus FDR gemcitabine was well tolerated with only three episodes of febrile neutropenia. Unfortunately, one patient died of bilateral pneumonitis and respiratory failure after receiving the forth cycle. Some cases reported pulmonary toxicities associated with both docetaxel and gemcitabine, although not very common[29,30]. Because of the low incidence of severe myelosuppression, this combination of weekly docetaxel and FDR gemcitabine can be applied to a salvage setting, especially if the patient is not anticipated to tolerate a platinum-based chemotherapy.
In conclusion, a combination of weekly docetaxel with a FDR of gemcitabine showed promising antitumor activity in previously treated, metastatic esophageal SCC and a tolerable toxicity profile. A phase III trial comparing this combination therapy to combination with other novel agents should be considered in esophageal cancer.
Esophageal squamous cell carcinoma (SCC) is a highly lethal disease with poor prognosis. Palliative chemotherapy is a treatment option in unresectable or metastatic esophageal SCC. In this paper, the authors conducted a phase II study on combination chemotherapy of weekly docetaxel and fixed-dose-rate (FDR) gemcitabine in previously treated metastatic esophageal SCC.
There is limited data on effective cytotoxic agents for the treatment of patients with metastatic esophageal SCC, particularly when patients have failed first-line chemotherapy with fluoropyrimidine and platinum.
The results demonstrated that the combination of weekly docetaxel and FDR gemcitabine show promising antitumor activity and tolerable toxicity as a second-line therapy for patients with previously treated metastatic esophageal SCC.
The combination of weekly docetaxel and FDR gemcitabine is active and well tolerated as a second-line chemotherapy for patients previously treated with fluoropyrimidine and platinum for metastatic esophageal SCC.
FDR infusion of gemcitabine is a term that refers to infusion of gemcitabine at a rate that maintains gemcitabine concentration levels that optimize incorporation of the active gemcitabine metabolite, gemcitabine triphosphate, into DNA.
This is a good descriptive study in which the authors assess the efficacy and safety of weekly docetaxel plus a FDR of gemcitabine in metastatic esophageal SCC. The results are interesting and suggest that the combination of the above two drugs is an active second-line therapy with tolerable toxicity for previously treated esophageal SCC.
P- Reviewer: Ameratunga M, Chen GS, Chen XL, Wu AB S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Melhado RE, Alderson D, Tucker O. The changing face of esophageal cancer. Cancers (Basel). 2010;2:1379-1404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 3. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2219] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 4. | Scheithauer W. Esophageal cancer: chemotherapy as palliative therapy. Ann Oncol. 2004;15 Suppl 4:iv97-i100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Grünberger B, Raderer M, Schmidinger M, Hejna M. Palliative chemotherapy for recurrent and metastatic esophageal cancer. Anticancer Res. 2007;27:2705-2714. [PubMed] |
| 6. | Ajani JA. Contributions of chemotherapy in the treatment of carcinoma of the esophagus: results and commentary. Semin Oncol. 1994;21:474-482. [PubMed] |
| 7. | Muro K, Hamaguchi T, Ohtsu A, Boku N, Chin K, Hyodo I, Fujita H, Takiyama W, Ohtsu T. A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol. 2004;15:955-959. [PubMed] |
| 8. | Sandler AB, Kindler HL, Einhorn LH, Mitchell E, Masters G, Kraut M, Nicol S, Raghavan D. Phase II trial of gemcitabine in patients with previously untreated metastatic cancer of the esophagus or gastroesophageal junction. Ann Oncol. 2000;11:1161-1164. [PubMed] |
| 9. | Pourquier P, Gioffre C, Kohlhagen G, Urasaki Y, Goldwasser F, Hertel LW, Yu S, Pon RT, Gmeiner WH, Pommier Y. Gemcitabine (2’,2’-difluoro-2’-deoxycytidine), an antimetabolite that poisons topoisomerase I. Clin Cancer Res. 2002;8:2499-2504. [PubMed] |
| 10. | Tempero M, Plunkett W, Ruiz Van Haperen V, Hainsworth J, Hochster H, Lenzi R, Abbruzzese J. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402-3408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 366] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 11. | Laufman LR, Spiridonidis CH, Pritchard J, Roach R, Zangmeister J, Larrimer N, Moore T, Segal M, Jones J, Patel T. Monthly docetaxel and weekly gemcitabine in metastatic breast cancer: a phase II trial. Ann Oncol. 2001;12:1259-1264. [PubMed] |
| 12. | Park SH, Hong J, Kim YS, Kim Y, Kyung SY, An CH, Lee SP, Park JW, Jeong SH, Park J. Phase II trial of weekly docetaxel and gemcitabine for previously untreated, advanced non-small cell lung cancer. Lung Cancer. 2008;62:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Hainsworth JD, Burris HA, Erland JB, Thomas M, Greco FA. Phase I trial of docetaxel administered by weekly infusion in patients with advanced refractory cancer. J Clin Oncol. 1998;16:2164-2168. [PubMed] |
| 14. | Hensley ML, Maki R, Venkatraman E, Geller G, Lovegren M, Aghajanian C, Sabbatini P, Tong W, Barakat R, Spriggs DR. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol. 2002;20:2824-2831. [PubMed] |
| 15. | Baker SD, Zhao M, Lee CK, Verweij J, Zabelina Y, Brahmer JR, Wolff AC, Sparreboom A, Carducci MA. Comparative pharmacokinetics of weekly and every-three-weeks docetaxel. Clin Cancer Res. 2004;10:1976-1983. [PubMed] |
| 16. | Schuette W, Nagel S, Blankenburg T, Lautenschlaeger C, Hans K, Schmidt EW, Dittrich I, Schweisfurth H, von Weikersthal LF, Raghavachar A. Phase III study of second-line chemotherapy for advanced non-small-cell lung cancer with weekly compared with 3-weekly docetaxel. J Clin Oncol. 2005;23:8389-8395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Park SH, Choi SJ, Kyung SY, An CH, Lee SP, Park JW, Jeong SH, Cho EK, Shin DB, Hoon Lee J. Randomized phase II trial of two different schedules of docetaxel plus cisplatin as first-line therapy in advanced nonsmall cell lung cancer. Cancer. 2007;109:732-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21598] [Article Influence: 1349.9] [Reference Citation Analysis (1)] |
| 19. | Song Z, Zhang Y. Second-line docetaxel-based chemotherapy after failure of fluorouracil-based first-line treatment for advanced esophageal squamous cell carcinoma. Onco Targets Ther. 2014;7:1875-1881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Albertsson M, Johansson B, Friesland S, Kadar L, Letocha H, Frykholm G, Wagenius G. Phase II studies on docetaxel alone every third week, or weekly in combination with gemcitabine in patients with primary locally advanced, metastatic, or recurrent esophageal cancer. Med Oncol. 2007;24:407-412. [PubMed] |
| 21. | Chen YM, Shih JF, Perng RP, Tsai CM, Whang-Peng J. A randomized trial of different docetaxel schedules in non-small cell lung cancer patients who failed previous platinum-based chemotherapy. Chest. 2006;129:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Tanaka Y, Yoshida K, Sanada Y, Osada S, Yamaguchi K, Takahashi T. Biweekly docetaxel, cisplatin, and 5-fluorouracil (DCF) chemotherapy for advanced esophageal squamous cell carcinoma: a phase I dose-escalation study. Cancer Chemother Pharmacol. 2010;66:1159-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Smorenburg CH, Sparreboom A, Bontenbal M, Verweij J. Combination chemotherapy of the taxanes and antimetabolites: its use and limitations. Eur J Cancer. 2001;37:2310-2323. [PubMed] |
| 24. | Grunewald R, Kantarjian H, Keating MJ, Abbruzzese J, Tarassoff P, Plunkett W. Pharmacologically directed design of the dose rate and schedule of 2’,2’-difluorodeoxycytidine (Gemcitabine) administration in leukemia. Cancer Res. 1990;50:6823-6826. [PubMed] |
| 25. | Houlihan WJ, Munder PG, Handley DA, Nemecek GA. Preclinical pharmacology and possible mechanism of action of the novel antitumor agent 5-(4’-piperidinomethylphenyl)-2,3-dihydroimidazo [2,1-a]isoquinoline. Arzneimittelforschung. 1995;45:1133-1137. [PubMed] |
| 26. | Attia S, Morgan-Meadows S, Holen KD, Bailey HH, Eickhoff JC, Schelman WR, Traynor AM, Mulkerin DL, Campbell TC, McFarland TA. Dose-escalation study of fixed-dose rate gemcitabine combined with capecitabine in advanced solid malignancies. Cancer Chemother Pharmacol. 2009;64:45-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Shim HJ, Cho SH, Hwang JE, Bae WK, Song SY, Cho SB, Lee WS, Joo YE, Na KJ, Chung IJ. Phase II study of docetaxel and cisplatin chemotherapy in 5-fluorouracil/cisplatin pretreated esophageal cancer. Am J Clin Oncol. 2010;33:624-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Jin J, Xu X, Wang F, Yan G, Liu J, Lu W, Li X, Tucker SJ, Zhong B, Cao Z. Second-line combination chemotherapy with docetaxel and nedaplatin for Cisplatin-pretreated refractory metastatic/recurrent esophageal squamous cell carcinoma. J Thorac Oncol. 2009;4:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |