Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4210
Peer-review started: November 5, 2014
First decision: November 26, 2014
Revised: January 10, 2015
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: April 14, 2015
Processing time: 161 Days and 11.1 Hours
AIM: To evaluate the feasibility of using multiphoton microscopy (MPM) to assess a tumor regression grading (TRG) system.
METHODS: Fresh specimens from seven patients with colorectal carcinoma undergoing neoadjuvant radiochemotherapy at the Fujian Medical University Union Hospital were obtained immediately after proctectomy. Specimens were serially sectioned (10 µm thickness) and used for MPM or stained with hematoxylin and eosin for comparison. Sections were imaged by MPM using 810 nm excitation, and images were collected in two wavelength channels corresponding to second-harmonic generation (SHG) and two-photon excited fluorescence (TPEF) signals. The ratio of these signal intensities was used to distinguish fibrosis from normal mucosal and serosal tissues.
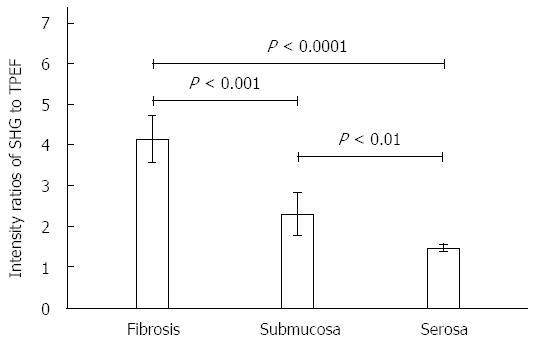
RESULTS: TRG of specimens assessed by MPM were in complete agreement with histologic grading performed by a consulting pathologist. SHG and TPEF images clearly revealed collagen fibers and fragmented elastic fibers in the muscularis propria specimens following neoadjuvant radiochemotherapy. Additionally, blood vessel hyperplasia was observed as thickening and fibrosis of the intima and media, which was accompanied by minimal inflammatory cell infiltration. Furthermore, the SHG/TPEF ratio in stromal fibrosis (4.15 ± 0.58) was significantly higher than those in the normal submucosal (2.31 ± 0.52) and serosal (1.47 ± 0.10) tissues (P < 0.001 for both). Analysis of emission spectra from cancerous tumor cells revealed two peaks corresponding to nicotinamide adenine dinucleotide hydrogen and flavin adenine dinucleotide signals; the ratio of these values was 1.19 ± 0.02, which is close to a normal metabolic state.
CONCLUSION: MPM can be used to perform real-time diagnosis of tumor response after neoadjuvant treatment, and can be applied to evaluate TRG.
Core tip: This study evaluated the feasibility of using multiphoton microscopy for the assessment of a tumor regression grading system. Multiphoton microscopy allows diagnostic features of colorectal carcinoma treated with neoadjuvant therapy to be visualized. Quantitative image analyses can be used to distinguish fibrotic tissue from normal submucosal and serosal tissues. This is the first study demonstrating the application of multiphoton microscopy for tumor regression grading.
- Citation: Li LH, Chen ZF, Wang XF, Zhuo SM, Li HS, Jiang WZ, Guan GX, Chen JX. Multiphoton microscopy for tumor regression grading after neoadjuvant treatment for colorectal carcinoma. World J Gastroenterol 2015; 21(14): 4210-4215
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4210.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4210
For patients with locally advanced gastrointestinal cancer, neoadjuvant therapy followed by surgery or perioperative treatment provides a survival benefit over surgery alone, particularly for patients with complete or subtotal tumor regression[1-5]. As such, assessment of therapeutic response and evaluation of residual disease are very important. The response to therapy can be evaluated histologically via a tumor regression grading (TRG) system, which has been shown to correlate with survival[6,7]. The TRG system aims to categorize the extent of regressive changes with consideration of the percentage of the tumor that is residual and the degree of therapy-induced fibrosis[7-10]. This system provides valuable prognostic information, and may serve as a morphologic indicator for neoadjuvant treatment and surgery[11,12].
Histopathologic evaluation of resected specimens can be subject to crush artifacts and sampling error, and involves time-consuming pathologic procedures[13,14]. In contrast, multiphoton microscopy (MPM), which relies on the nonlinear optical processes of second-harmonic generation (SHG) and two-photon excited fluorescence (TPEF), provides high resolution visualization of cell morphology and tissue architecture without the use of exogenous contrast agents[15,16]. The value of this method for use in the TRG system has not been examined. Therefore, the purpose of this study was to evaluate the accuracy and feasibility of MPM for optical diagnoses with the TRG system.
Fresh specimens were obtained immediately after proctectomy from seven patients with colorectal carcinoma undergoing neoadjuvant radiochemotherapy at the Fujian Medical University Union Hospital. Normal tissue specimens were also obtained 6 cm away from the cancer margin. This investigation was approved by the Institutional Review Board of the Fujian Medical University Union Hospital. Written informed consent was obtained before study participation from patients.
Specimens were sectioned (10 μm thickness) and five serial slices were selected. The middle section was stained with hematoxylin and eosin (HE) for histologic comparison and imaged under a standard bright-field light microscope (Eclipse Ci-L; Nikon Corp., Tokyo, Japan) with a CCD (DS-Fi2; Nikon). The remaining four sections were used for MPM imaging.
MPM was performed using an LSM 510 META imaging system (Carl Zeiss AG, Jena, Germany) equipped with a femtosecond Ti:sapphire laser (110 fs, 76 MHz, Mira 900-F; Coherent Inc., Santa Clara, CA, United States) mode-locked at a wavelength of 810 nm as described previously[17,18]. A Plan-Apochromat oil immersion objective (× 63, numerical aperture = 1.4) was used for image acquisition to collect the backscattered intrinsic SHG and TPEF signals. SHG signals were collected in one channel with a wavelength range of 387-419 nm, and TPEF signals were collected in another channel with a wavelength range of 430-698 nm.
TRG from MPM was performed by two independent investigators who were blinded to the results. Tumor regression was classified into five histologic grades according to vital tumor tissue at the ratio of fibrosis[8]: TRG-1: fibrosis without detectable tumor tissue (complete regression); TRG-2: fibrosis with scattered tumor cells; TRG-3: fibrosis and tumor cells with preponderance of fibrosis; TRG-4: fibrosis and tumor cells with preponderance of tumor cells; TRG-5: tumor tissue without regression. TRG scores were confirmed by comparison with corresponding HE-stained sections that were reviewed by a consulting pathologist (Table 1).
| Patient | Sex | Age (yr) | Cancer classification | TRG score | |
| HE | MPM | ||||
| 1 | Male | 44 | Adenocarcinoma | TRG-1 | TRG-1 |
| 2 | Male | 38 | Adenocarcinoma | TRG-2 | TRG-2 |
| 3 | Female | 67 | Adenocarcinoma | TRG-2 | TRG-2 |
| 4 | Female | 59 | Adenocarcinoma | TRG-1 | TRG-1 |
| 5 | Male | 59 | Adenocarcinoma | TRG-2 | TRG-2 |
| 6 | Male | 57 | Adenocarcinoma | TRG-3 | TRG-3 |
| 7 | Male | 47 | Adenocarcinoma | TRG-2 | TRG-2 |
All quantitative analyses were performed by two individuals experienced in identification of TPEF/SHG images. Collagen and elastin changes and metabolic status after neoadjuvant radiochemotherapy were quantified as SHG/TPEF and redox ratios.
One-way analyses of variance were conducted to compare differences using SPSS, version 15.0 statistical software (SPSS Inc., Chicago, IL, United States). Data are presented as mean ± SD, and a P-value < 0.05 was considered significant.
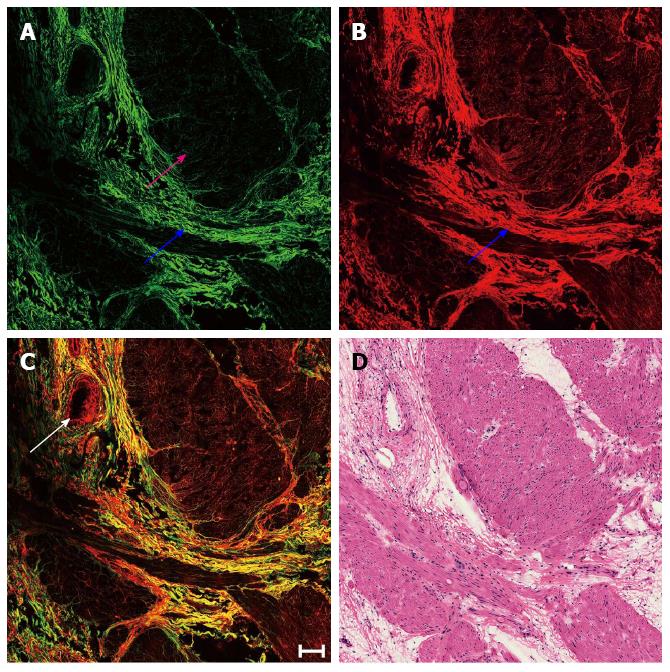
Representative MPM images depicting the predominant fibrosis with minimal inflammatory cell infiltration replacing large parts of a previous tumor in the muscularis propria following neoadjuvant radiochemotherapy are shown in Figure 1. The smooth muscle was divided due to prior cancer invasion, and the tumor underwent complete regression and was replaced by fibrous tissue mainly composed of collagen fibers, which simultaneously generated SHG (blue arrow in Figure 1A) and TPEF (blue arrow in Figure 1B) signals. Additionally, blood vessel hyperplasia was observed as thickening and fibrosis of the intima and media accompanied by minimal inflammatory cell infiltration (white arrow in Figure 1C).
Although fibrosis can easily be differentiated from muscular tissues (pink arrow in Figure 1A) by strong SHG signals, the submucosa and serosa also contain connective tissue comprised of collagen. Therefore, the ratio of SHG to TPEF intensity was calculated to distinguish fibrosis from normal submucosal and serosal tissues, as well as to quantitatively describe the change in fibrous tissue. SHG/TPEF ratio in stromal fibrosis was significantly higher than those in the submucosa and serosa (P < 0.001 for both) (Figure 2).
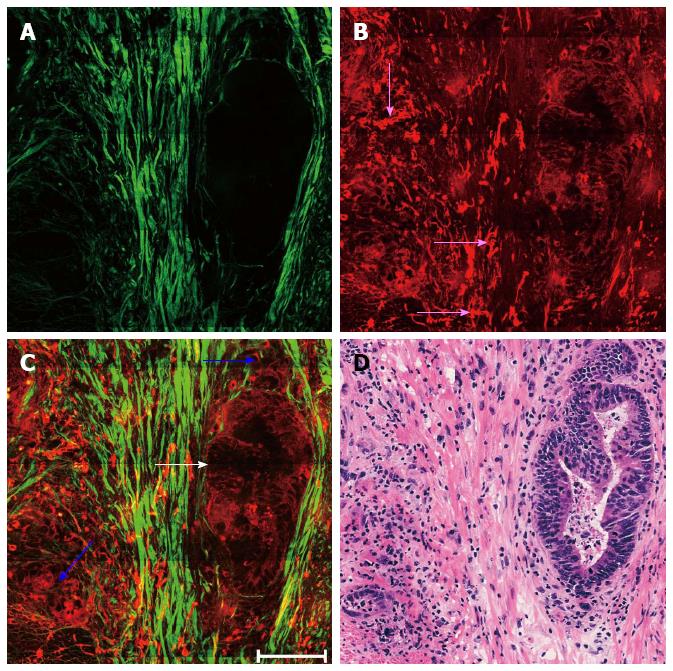
Representative MPM images depicting remaining malignant glands dispersed within the muscularis propria with minimal inflammatory cell infiltration are shown in Figure 3. Tumor cells in post-treatment rectal carcinoma may show marked changes and these altered tumor cells may become more solid (blue arrows in Figure 3C) or still have a glandular growth pattern (white arrow in Figure 3C). The elastic fibers became fragmented (pink arrows in Figure 3B), while there was an increase in collagen fibers because of stromal fibrosis (Figure 3A).
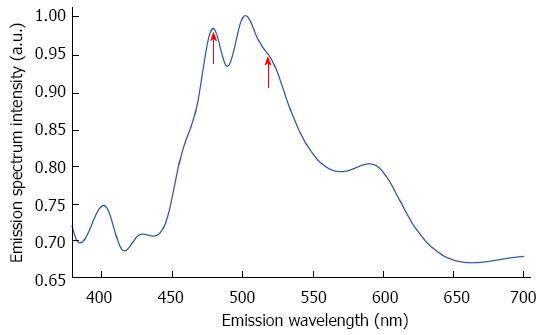
To further characterize the residual tumors, an image-guide spectral analysis method was used to obtain emission spectra of the cancerous cells, revealing two peaks at 470 and 530 nm (red arrows in Figure 4). These peaks were used to calculate the redox ratio of nicotinamide adenine dinucleotide hydrogen (NADH) to flavin adenine dinucleotide (FAD), represented by fluorescence at 470 and 530 nm, respectively[19,20]. The NADH/FAD ratio was 1.19 ± 0.02.
A recent meta-analysis found that partial tumor regression is associated with improvement in disease-free survival[21]. Furthermore, TRG can serve as an independent predictor of disease-free and metastases-free survivals[6,11,22]. The prognostic value of this measure may even exceed that of currently used staging systems (e.g., tumor-node-metastasis staging), which are based on characteristics of untreated tumors[12]. The evaluation criteria of the TRG system incorporate the ratio of tumor cells to fibrosis. However, evaluating TRG using current approaches such as computerized tomography, magnetic resonance imaging, and positron emission computed tomography is challenging, as these medical imaging technologies lack sufficient resolution[23].
MPM relies on nonlinear optical processes to achieve high resolution imaging of biologic tissues, and can detect cellular and subcellular tissue microstructures. Compared with its single-photon counterpart, TPEF offers an inherent optical sectioning property and deep penetration, and the nonlinear scattering from non-centrosymmetric structures provides complementary information to visualize endogenous structures in intact tissues. Residual tumor cells are detected by the TPEF signal, and the SHG signal is used to detect fibrotic tissue. The SHG/TPEF ratio can be used to distinguish fibrosis from submucosal and serosal tissues, as well as to quantify the fibrotic change, which has been proposed as a diagnostic indicator for gastrointestinal diseases[24,25].
Neoadjuvant radiochemotherapy can result in significant morphologic changes, including disrupted muscles, tumor regression, and extensive fibrosis[26]. Moreover, tumor cells in post-treatment rectal carcinoma can become more solid or retain a glandular growth pattern similar to untreated colorectal adenocarcinoma[27]. These alterations were readily detected in the specimens evaluated by MPM in this study. Also observed was the division and damage of muscular tissue by invasion of the adenocarcinoma, which can cause destruction or elimination of collagen and elastic fibers[24].
The redox ratio is an indicator of cellular metabolic state[28]. As this is known to be accelerated in cancerous cells, the redox ratio can be used to quantitatively monitor tumor regression[29]. In this work, the redox ratio in specimens was close to the metabolic state of normal cells[20]. This result supports the notion that abnormal cells undergo significant regression after neoadjuvant radiochemotherapy, which can be assessed via MPM.
In conclusion, MPM was used to evaluate TRG in colorectal cancer after neoadjuvant treatment. Spectral analyses provided quantitative evaluations of tumor regression and fibrosis, which corresponded to TRG via histopathologic investigation. Given the advantages of this method, including the capacity to produce real-time, label-free images that can be acquired in the near-infrared range, MPM may represent a valuable tool to evaluate TRG after neoadjuvant therapy to treat colorectal carcinoma. This is the first demonstration of the use of MPM to estimate TRG of colorectal carcinoma following neoadjuvant treatment. The results suggest that MPM has a promising future for real-time optical biopsy diagnosis of tumor regression.
Tumor regression grading (TRG) can provide important prognostic information and should be included in histopathologic reports of colorectal carcinoma after neoadjuvant treatment. However, the resolution of current imaging approaches, such as computerized tomography, magnetic resonance imaging, and positron emission tomography, is insufficient for accurate and easy assessment. The purpose of this study was to evaluate the feasibility of using multiphoton microscopy (MPM) to obtain optical diagnoses for the TRG system.
The TRG system provides highly valuable prognostic information for evaluating colorectal cancer after neoadjuvant treatment.
This is the first study evaluating the use of MPM for optical diagnosis of tumor regression.
These results are essential and significant for developing MPM for TRG, and to perform real-time diagnosis of tumor response after neoadjuvant treatment.
MPM incorporates nonlinear optical processes to generate second-harmonic generation and two photon excited fluorescence signals.
The article has good characteristics, value, and significance.
P- Reviewer: Gassler N S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Hwang MR, Park JW, Park S, Yoon H, Kim DY, Chang HJ, Kim SY, Park SC, Choi HS, Oh JH. Prognostic impact of circumferential resection margin in rectal cancer treated with preoperative chemoradiotherapy. Ann Surg Oncol. 2014;21:1345-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4057] [Article Influence: 312.1] [Reference Citation Analysis (0)] |
| 3. | Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 471] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 4. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1494] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 5. | Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem JG. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 305] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 6. | Abdul-Jalil KI, Sheehan KM, Kehoe J, Cummins R, O’Grady A, McNamara DA, Deasy J, Breathnach O, Grogan L, O’Neill BD. The prognostic value of tumour regression grade following neoadjuvant chemoradiation therapy for rectal cancer. Colorectal Dis. 2014;16:O16-O25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688-8696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 950] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 8. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1060] [Article Influence: 37.9] [Reference Citation Analysis (1)] |
| 10. | Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, Böttcher K, Siewert JR, Höfler H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 585] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 11. | Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94:1121-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment. Front Oncol. 2013;3:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Ying M, Zhuo S, Chen G, Zhuo C, Lu J, Zhu W, Xie S, Chen J, Yan J. Real-time noninvasive optical diagnosis for colorectal cancer using multiphoton microscopy. Scanning. 2012;34:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Wu X, Chen G, Lu J, Zhu W, Qiu J, Chen J, Xie S, Zhuo S, Yan J. Label-free detection of breast masses using multiphoton microscopy. PLoS One. 2013;8:e65933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Hoover EE, Squier JA. Advances in multiphoton microscopy technology. Nat Photonics. 2013;7:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 16. | Campagnola PJ, Loew LM. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat Biotechnol. 2003;21:1356-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 765] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 17. | Chen J, Zhuo S, Chen G, Yan J, Yang H, Liu N, Zheng L, Jiang X, Xie S. Establishing diagnostic features for identifying the mucosa and submucosa of normal and cancerous gastric tissues by multiphoton microscopy. Gastrointest Endosc. 2011;73:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Chen J, Xu J, Kang D, Xu M, Zhuo S, Zhu X, Jiang X. Multiphoton microscopic imaging of histological sections without hematoxylin and eosin staining differentiates carcinoma in situ lesion from normal oesophagus. Appl Phys Lett. 2013;103:183701. [DOI] [Full Text] |
| 19. | Monici M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol Annu Rev. 2005;11:227-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 530] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 20. | Zhuo S, Chen J, Luo T, Jiang X, Xie S, Chen R. Two-layered multiphoton microscopic imaging of cervical tissue. Lasers Med Sci. 2009;24:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Lee YC, Hsieh CC, Chuang JP. Prognostic significance of partial tumor regression after preoperative chemoradiotherapy for rectal cancer: a meta-analysis. Dis Colon Rectum. 2013;56:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE, Soubrane O. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 393] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 23. | Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1963] [Cited by in RCA: 1644] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 24. | Liu N, Chen J, Chen G, Yan J, Zhuo S, Jiang X. Detecting the imaging characteristics of colorectal carcinoma invading the muscularis propria with multiphoton microscopy. Laser Phys Lett. 2012;9:155-159. [DOI] [Full Text] |
| 25. | Vidal Bde C, Mello ML. Optical anisotropy of collagen fibers of rat calcaneal tendons: An approach to spatially resolved supramolecular organization. Acta Histochem. 2010;112:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | O’Neil M, Damjanov I. Histopathology of colorectal cancer after neoadjuvant chemoradiation therapy. Open Pathol J. 2009;3:91-98. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Shia J, Guillem JG, Moore HG, Tickoo SK, Qin J, Ruo L, Suriawinata A, Paty PB, Minsky BD, Weiser MR. Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol. 2004;28:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Skala MC, Riching KM, Gendron-Fitzpatrick A, Eickhoff J, Eliceiri KW, White JG, Ramanujam N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc Natl Acad Sci USA. 2007;104:19494-19499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 684] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 29. | Tiede LM, Rocha-Sanchez SM, Hallworth R, Nichols MG, Beisel K. Determination of hair cell metabolic state in isolated cochlear preparations by two-photon microscopy. J Biomed Opt. 2007;12:021004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |