Published online Apr 7, 2015. doi: 10.3748/wjg.v21.i13.3970
Peer-review started: September 4, 2014
First decision: October 14, 2014
Revised: November 2, 2014
Accepted: December 5, 2014
Article in press: December 8, 2014
Published online: April 7, 2015
AIM: To investigate transarterial chemoembolization (TACE) with hepatic infusion of oxaliplatin and 5-fluorouracil and Lipiodol chemoembolization in large hepatocellular carcinoma (HCC).
METHODS: In this retrospective study, 132 patients with unresectable HCCs larger than 10 cm were treated with hepatic infusion of oxaliplatin and 5-fluorouracil followed by Lipiodol chemoembolization. The primary endpoint was overall survival (OS). Sixteen-week disease-control rate, time to progression (TTP), and major complications were also studied. Univariate and multivariate analyses were performed to identify prognostic factors affecting OS and TTP.
RESULTS: A total of 319 procedures were performed in the 132 patients. Eleven (8.3%) patients received radical resection following TACE treatment (median time to initial TACE 4.3 ± 2.3 mo). The median OS and TTP were 10.3 and 3.0 mo respectively, with a 50.0% 16-wk disease-control rate. Major complications were encountered in 6.0% (8/132) of patients following TACE and included serious jaundice in 1.5% (2/132) patients, aleukia in 1.5% (2/132), and hepatic failure in 3.0% (4/132). One patient died within one month due to serious hepatic failure and severe sepsis after receiving the second TACE. The risk factor associated with TTP was baseline alpha-fetoprotein level, and vascular invasion was an independent factor related to OS.
CONCLUSION: Hepatic infusion of oxaliplatin and 5-fluorouracil followed by lipiodolized-chemoembolization is a safe and promising treatment for patients with HCCs larger than 10 cm in diameter.
Core tip: Treatment of large unresectable hepatocellular carcinomas (HCCs) with diameters exceeding 10 cm is clinically challenging due to the low response rate and high rate of major complications. In this study, the safety and efficacy of a transarterial chemoembolization modality that included a combination of oxaliplatin and 5-fluorourcil infusion followed by embolization with a mixture of mitomycin and Lipiodol was tested in patients with large HCCs. The results indicate that this modality is a promising treatment for certain patients with large HCCs.
- Citation: Li JH, Xie XY, Zhang L, Le F, Ge NL, Li LX, Gan YH, Chen Y, Zhang JB, Xue TC, Chen RX, Xia JL, Zhang BH, Ye SL, Wang YH, Ren ZG. Oxaliplatin and 5-fluorouracil hepatic infusion with lipiodolized chemoembolization in large hepatocellular carcinoma. World J Gastroenterol 2015; 21(13): 3970-3977
- URL: https://www.wjgnet.com/1007-9327/full/v21/i13/3970.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i13.3970
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide based on incidence[1]. Although patients at high risk of developing HCC can be monitored, only a small portion (20%-30%) of patients are eligible for curative treatments, such as resection, liver transplantation, and local ablation. Others may only receive palliative treatments, including transarterial chemoembolization (TACE)[2].
TACE is the main treatment option for unresectable HCC and is used in 58% of recurrent patients[2], and achieves partial responses in 15%-55% of patients with HCC who are not candidates for curative treatments[3]. Also, according to a recent prospective cohort study, TACE is safe and effective for elderly patients ≥ 75 years of age[4]. However, not all patients with unresectable HCC derive similar benefits from TACE. A systematic review in the Cochrane database showed that TACE or transarterial embolization (TAE) did not significantly increase survival for unresectable HCCs, particularly those with poor compensate liver function[5]. This may due in part to tumor heterogeneity, tumor burden, liver function, and the general condition of the individual patient. The heterogeneity for treatment modalities and for patients with different tumor stage or Child-Pugh classification of HCC may explain why some randomized, controlled trials of TACE failed to demonstrate prolonged survival in the patients[6].
Currently, the most widely used TACE modalities for unresectable HCC are conventional TACE, drug-eluting TACE beads (DB-TACE), and radioembolization[7]. There is increasing evidence supporting the safety and efficacy of DB-TACE, especially doxorubicin-eluting beads, and radioembolization, including iodine-131-labeled Lipiodol or microspheres containing yttrium-90[8-11]. However, conventional TACE remains the standard first-line choice. In addition, oxaliplatin shows promising efficiency and safety profiles in the treatment of HCC[12,13]. Thus, we evaluated the efficacy and safety of hepatic infusion of oxaliplatin in combination with Lipiodol embolization in patients with HCC.
Tumor size, especially a large tumor size, influences survival following TACE[14,15]. The difficulties related to treating a larger tumor include inadequate embolization of the tumor blood supply and major embolization-related complications, such as tumor lysis syndrome, tumor rupture, and hemolytic uremic syndrome. Several studies show that a tumor size larger than 10 cm is associated with poor prognosis following TACE[14-16]. However, one limitation of past studies was the relatively small number of patients with HCCs larger than 10 cm. Therefore, the safety and efficacy of TACE could not be analyzed. We performed this study to evaluate safety and efficiency of the protocol with local regional infusion of oxaliplatin and 5-fluorouracil (FU) followed with chemoembolization to treat large HCCs (≥ 10 cm), which is generally not considered as a good indication for TACE, because of the lack of complete tumor eradication[17].
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the institutional review board. All patients provided written informed consent for the treatment procedure.
This retrospective study review included 597 patients who were diagnosed with large HCCs (single nodule ≥ 10 cm or the largest nodule ≥ 10 cm for multiple nodules) and were consecutively treated with TACE at the Liver Cancer Institute, Zhongshan Hospital, Fudan University, from January 2008 to December 2012. Diagnosis was based on either histologic confirmation or non-invasive AASLD criteria[18,19]. The main inclusion criteria were the following: (1) patients received the defined TACE procedure in accordance with the treatment protocol described below; (2) liver function was maintained with Child-Pugh A or B; (3) performance was ECOG 0 to 2; (4) renal function was normal; and (5) there was adequate bone marrow function with a peripheral white blood cell count > 3.0 × 109/L and platelet count > 50 × 106/L. The patients were excluded in the cases of arterial-venous shunting identified by arterial angiography and lost follow-up after the first session of TACE. Thus, 132 patients were included in the study. Among these patients, 103 patients had well-documented follow-up imaging data [CT or magnetic resonance imaging (MRI)] to evaluate the objective response.
Angiography and chemoembolization were performed according to the standard operating protocol of the hospital. Briefly, a 5 F RH catheter was inserted into the common hepatic artery using the Seldinger method. Hepatic angiography was performed to evaluate the tumor-feeding artery. Doses of 100-150 mg oxaliplatin and 1000 mg 5-FU were diluted with 5% dextrose and normal saline, respectively, and slowly infused via the common hepatic artery. The catheter was selected and inserted into the arterial branch as close as possible to the tumor. The chemoembolization was performed with 10 mg mitomycin C mixed with 10-30 mL Lipiodol, which was slowly injected into tumor vessels. Gelatin-sponge particles were used in some of the patients (n = 50; 37.88%) with significant hypervascularization. The procedure was repeated with an interval of approximately 6 wk until complete necrosis of the tumor was shown with enhanced CT or MRI, tumor shrinkage was achieved and thus eligible for surgical resection, or treatment failure due to tumor progression. However, the procedure would be postponed or terminated in the case of impaired liver function or bone marrow function.
Patients received follow-up CT or MRI evaluation of tumor response and tumor markers [alpha-fetoprotein (AFP), carcinoembryonic antigen, and carbohydrate antigen 19-9] and routine biochemical and blood tests approximately one month after completing the first session of TACE. If a tumor showed enhancement by CT or MRI indicating viable tumor tissue, a repeat procedure was conducted with an interval of six weeks. This repeat procedure was postponed if impaired liver function had not recovered to an acceptable level compared to baseline. Patients received follow-up examinations for AFP, CT, or MRI every two or three months after the termination of TACE, while complete tumor necrosis was indicated by absence of artery enhancement by CT or MRI.
The primary endpoint was overall survival (OS) after the first TACE procedure. Other endpoints included 16-wk disease-control rate (16w-DCR) and time to progression (TTP). Target lesions were evaluated by measuring the longest diameter and using Response Evaluation Criteria in Solid Tumor (RECIST)[20].
TTP was calculated from the date of TACE to the date of radiologic disease progression or the last date on which imaging showed stable disease; data for patients who died were censored. The 16w-DCR was defined as the percentage of patients with complete response, partial response, or stable disease lasting until 16 wk after treatment. The best tumor response according to RECIST was evaluated. AFP response was also evaluated. AFP levels were measured before and after the first TACE (4 ± 2 wk), and the change in AFP levels was calculated. Patients were separated into three groups based on the change in serum AFP from that at baseline: > 25% AFP decline, > 25% AFP increase, and < 25% change in either direction. An AFP decrease > 25% was defined as an AFP response, whereas the other two were defined as a lack of AFP response. OS was measured from the first TACE treatment until death from any cause or until the last date of follow up. Data were censored for patients who remained alive at the end of the study.
Major complications were evaluated after treatment, defined as life-threatening events and events with medical importance requiring inpatient hospitalization or prolongation of existing hospitalization within 30 d after TACE. Criteria specific for the diagnosis of post-TACE syndrome include pain, fever, nausea, and vomiting.
Continuous variances are indicated as mean ± standard deviation. TTP and OS were analyzed by the Kaplan-Meier method, and survival curves were compared with the log-rank test. Multivariate analysis was performed with the Cox proportional hazards model. All analyses were performed with SPSS version 19.0 (IBM Corp., Armonk, NY, United States). A P < 0.05 was defined as statistically significant.
Detailed characteristics for the 132 patients are shown in Table 1. The median age was 53 ± 12.7 year (range: 20-83 year), and the female to male ratio was 1:11. Almost all patients (97.7%) had chronic HBV disease. Among these, there were 50 patients with HBV DNA > 104 copies/mL, but none of them were positive for HCV antibodies. The performance status was ECOG 0 for most patients. The majority of patients had intact liver function, with 97.7% of patients recorded as Child-Pugh A. The mean tumor diameter was 11.5 ± 2.2 cm (range: 10-20 cm), and 110 patients had a solitary lesion. Vascular invasion was detected in 56.8% of patients and included the portal and hepatic veins. Extrahepatic metastases were found in 30.3% of patients, affecting the lymph nodes in 34 patients, the lungs in 4 patients, and bone in 2 patients.
| Baseline characteristics | Value |
| Age, yr | |
| > 65 | 20 (15.2) |
| ≤ 65 | 112 (84.8) |
| Gender | |
| Male | 121 (91.7) |
| Female | 11 (8.3) |
| CLIP score | |
| 0 or 1 | 0 (0) |
| 2 | 38 (28.8) |
| 3 | 47 (35.6) |
| 4 | 47 (35.6) |
| BCLC | |
| A | 0 (0) |
| B | 53 (40.2) |
| C | 79 (59.8) |
| D | 0 (0) |
| ECOG Performance Status | |
| 0 | 87 (65.9) |
| 1 | 35 (26.5) |
| 2 | 10 (7.6) |
| AFP, ng/mL (mean ± SD) | 15038.8 ± 22661.5 |
| < 20 | 27 (20.5) |
| 20-400 | 23 (17.4) |
| > 400 | 82 (62.1) |
| Tumor nodules | |
| Solitary | 110 (83.3) |
| Multiple | 22 (16.7) |
| Vascular invasion | |
| With | 75 (56.8) |
| Without | 57 (43.2) |
| Extrahepatic metastasis | |
| With | 40 (30.3) |
| Without | 92 (69.7) |
| Child-Pugh stage | |
| A | 129 (97.7) |
| B | 3 (2.3) |
| HBV infection1 | |
| Yes | 129 (97.7) |
| No | 3 (2.3) |
| HBV DNA level > 104 copies/mL | 50 (37.9) |
| HCV antibody | |
| Positive | 0 |
| Negative | 132 (100) |
| Cirrhosis | |
| With | 124 (93.9) |
| Without | 8 (6.1) |
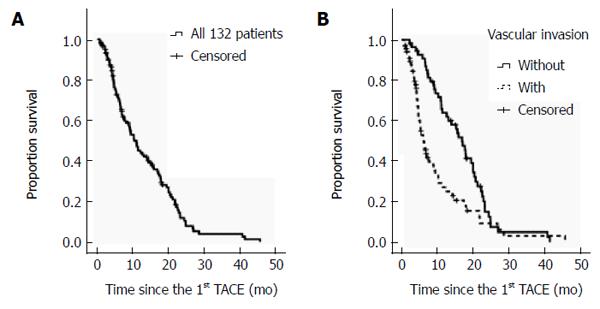
The median follow-up period was 12.0 mo (range: 1.0-48.0 mo). There were 98 deaths recorded by April of 2013. Among them, 83/98 (84.7%) patients died of tumor progression, 14/98 (14.3%) died of uncontrollable gastrointestinal bleeding, and 1/98 (1.0%) died of lung infection with subsequent respiratory failure. The median OS of all 132 patients was 10.3 ± 1.2 mo (range: 1.0-45.7) (Figure 1). The one-, two-, and three-year OS rates were 48, 15, and 5%, respectively. Among those who received TACE only, the median OS was 9.4 ± 1.4 mo (range: 1.0-45.7 mo), and the one-, two-, and three-year OS rates were 44, 14, and 6%, respectively. The median OS of those with BCLC stage B or BCLC stage C HCC was 14.2 and 7.4 mo, respectively.
Four potential prognostic variables were identified by univariate analysis (Table 2). The Clip score and BCLC staging of large HCCs were determined by baseline AFP, the presence of ascites, and extrahepatic metastasis. Thus the Clip score and BCLC staging were excluded from multivariate analysis. Only one factor significantly increased the hazard of reduced OS: the presence of vascular invasion before the TACE procedure (Table 3).
| Characteristic | Univariate | Multivariate | HR | 95%CI |
| Age ( ≤ 65 yr >) | 0.84 | |||
| Gender | 0.88 | |||
| Clip score | 0.05 | |||
| BCLC (B, C) | 0.02 | |||
| ECOG (0, 1, 2) | 0.59 | |||
| Cirrhosis | 0.79 | |||
| Solitary or multiple nodules | 0.50 | |||
| Vascular invasion | 0.01 | 0.30 | 0.75 | 0.44-1.29 |
| Extrahepatic spread | 0.04 | 0.26 | 0.73 | 0.42-1.27 |
| AFP-pre | 0.02 | |||
| < 20 ng/mL | 0.05 | |||
| 20-400 ng/mL | 0.04 | 0.55 | 0.30-0.99 | |
| > 400 ng/mL | 0.50 | 1.23 | 0.67-2.28 | |
| HBV DNA > 104 copies/mL | 0.66 | |||
| Gelatin-sponge application | 0.26 |
| Characteristic | Univariate | Multivariate | HR | 95%CI |
| Age ( ≤ 65 yr >) | 0.53 | |||
| Gender | 0.46 | |||
| Clip score | 0.29 | |||
| BCLC (B, C) | 0.03 | |||
| ECOG (0, 1, 2) | 0.57 | |||
| Cirrhosis | 0.73 | |||
| Solitary or multiple nodules | 0.74 | |||
| Vascular invasion | 0.00 | 0.00 | 2.36 | 1.41-2.94 |
| Extrahepatic spread | 0.04 | 0.34 | 1.27 | 0.78-2.10 |
| AFP-pre | 0.02 | |||
| < 20 ng/mL | 0.09 | |||
| 20-400 ng/mL | 0.19 | 0.71 | 0.43-1.20 | |
| > 400 ng/mL | 0.22 | 1.41 | 0.81-2.49 | |
| HBV DNA > 104 copies/mL | 0.21 | |||
| Gelatin-sponge application | 0.47 |
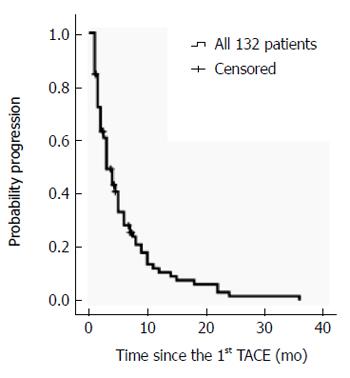
TTP was 3.00 ± 0.45 mo (95%CI: 2.11-3.89) in all 132 patients, and 3.00 ± 0.22 mo (95%CI: 2.57-3.43) in the 121 patients who received TACE only (Figure 2). Further analyses focused specifically on the 121 patients who received TACE only. Univariate analysis identified five potential factors related to TTP. Multivariate analysis revealed the AFP values at baseline were associated with TTP (Table 2).
Three hundred and nineteen sessions of TACE were performed in 132 patients with a mean of 2.4 sessions per patient (range: 1-8). Among the 132 patients, 11 (8.3%) received a secondary radical resection due to successful shrinking of the tumor. The median interval between the first TACE and resection was 4.3 ± 2.3 mo (range: 1.0-7.2 mo).
Excluding 29 patients who had no following-up image data and five patients who already received surgical resection within 16 wk after the first TACE, the 16w-DCR was 50.0% in the 98 patients. And among all 103 patients with well-documented follow-up imaging data (CT or MRI), partial response was achieved in 21.4%, while no complete response was observed. Around one month after the first TACE, 64.5% patients showed a decrease in AFP value, but the defined AFP response was achieved in 37.9% of patients (Table 4).
| Parameter | n (%) |
| 16-wk disease-control rate | 50% |
| Best response | |
| Complete response | 0 (0.0) |
| Partial response | 22 (21.4) |
| Stable disease | 58 (56.3) |
| Progressive disease | 23 (22.3) |
| AFP response rate (decrease > 25%) | 39 (37.9) |
| No AFP response rate | 64 (62.1) |
| Change < 25% | 39 (37.9) |
| Increase > 25% | 25 (24.3) |
The median OS of those with or without tumor response was 21.7 ± 3.1 and 9.4 ± 0.8 mo, respectively (P = 0.000). The median OS of those with or without an AFP response was 17.7 ± 1.6 and 10.2 ± 1.6 mo, respectively (P = 0.14). The difference was not statistically significant.
Among the 319 TACE procedures in 132 patients, only one patient died within the 30-d period following the procedure.
Post-TACE syndrome was the most common treatment-related adverse event, but was usually reversible and some patients received pain relievers or antipyretics. Increased enzyme levels were also common after TACE, but most cases were reversible and needed no specific treatment.
Major complications were encountered in 6.0% (8/132) of patients, including jaundice (n = 2), grade 3/4 liver dysfunction (n = 4; 1 patient died of hepatic failure and sepsis), and grade 3/4 aleukia (n = 2). One of the four patients who developed hepatic failure died on the 28th d after the second TACE, resulting in a procedure-related mortality rate of 0.76%.
The survival benefit of TACE has been explored in several randomized, controlled trials and meta-analyses, which are generally considered to be robust[21]. However, an optimal regimen has not yet been defined for conventional TACE, particularly for target lesions that are larger than 10 cm. These large HCCs cannot be completely embolized, and a high risk of complications accompanies the TACE procedure due to necrosis of the large tumor.
We tested the safety of a TACE protocol in patients with large HCCs that included a combination of oxaliplatin and 5-FU infusion followed by embolization with a mixture of mitomycin and Lipiodol. The toxicities of similar chemotherapeutic schedules have been shown to be tolerable during TACE of liver lesions in patients with advanced colorectal cancer[22,23] or intrahepatic cholangiocarcinoma[24,25]. Similarly, our study shows that the TACE protocol is safe and tolerable for patients with large HCCs. The most common adverse effects included tolerable post-embolism syndrome and temporarily elevated total bilirubin and alanine aminotransferase, which are generally reversible. Decreased platelet count and albumin were also common changes after therapy, but most grade 3/4 reductions were restricted to those who had low baseline values. One patient died of hepatic failure accompanied by suspicious infectious shock, giving a 0.75% 30-d mortality rate, which is acceptable as compared with other studies[26,27].
Considering the heavy tumor burden of the patients in our study, in which 59.8% belonged to BCLC-C, 56.8% had vascular invasion, and 30.3% had extrahepatic metastasis, the efficacy of the TACE protocol was acceptable to a certain extent. One recent study showed the median survival time was 6.6 months in patients with HCC lesions > 7 cm that were treated with TAE or TACE based on doxorubicin or cisplatin. These patients with large HCC lesions had double the risk of death and 60% reduction in median survival compared with those with tumors ≤ 7 cm[28]. In addition, similar overall (5.2 mo) and two-year (19.2%) survival data were reported previously in a study that recruited HCC patients with vascular invasion[29]. In that study, cisplatin and doxorubicin were used as chemoembolization drugs. Although one study focused on patients with BCLC-C HCC showed that DB-TACE achieved a better median OS (13.3 mo, 95%CI: 10.0-19.8)[30], most patients in that study were classified as BCLC-C based on ECOG score, rather than vascular invasion or metastasis.
Although vascular invasion was found to be a risk factor associated with OS, it may not be an absolute contraindication to conventional TACE. Based on the most recent EASL-EORTC guidelines, TACE is recommended for those with BCLC-B, which lacks vascular invasion of extrahepatic spread[18]. However, in a phase III trial of sorafenib in the Asia-Pacific[31], patients with HCC and vascular invasion or extrahepatic spread showed a similar median TTP (2.7 mo) and shorter median OS (5.6 mo) compared to the present study. These findings support the efficacy of our TACE protocol. Moreover, sorafenib does not induce tumor shrinkage[32], and our TACE protocol achieved cytoreduction in some patients. Radical resection was performed in 8.3% of patients following TACE. Thus, patients with BCLC-C HCC, including those with vascular invasion or extrahepatic spread, might also benefit from TACE if liver function and the general condition of the patient are intact. In fact, clinical practice guidelines proposed by some Asian countries noted that TACE was frequently performed in patients with minimal portal invasion[33,34].
AFP status was associated with TTP in patients with large HCCs. Previous studies demonstrated that a change in AFP during treatment might serve as a predictor of clinical outcome in advanced HCC[35,36]. However, the specific amount of change varied among studies. In our study, no significant associations were found between AFP response and survival proportion. Changes in other biochemical or hematologic results, such as total bilirubin and alanine aminotransferase, were also analyzed, but no links to treatment outcome were found.
Extrahepatic spread is not an independent predictor in our study. Intrahepatic tumor progression or liver failure was the main causes of death from HCC, rather than metastasis. In fact, two-thirds of patients with HCC die without metastasis. Thus, it is necessary to control intrahepatic tumors with loco-regional therapies[37].
Other factors that have been associated with outcome from TACE were also analyzed in our study. However, no significant relationships were found for ECOG score, HBV DNA copy number, or ascites. These may be due to differences in the patient population of each study.
Our study had several limitations. It was a retrospective study from a single institution. A multicenter, prospective study is desirable to validate our results. Combined TACE and sorafenib is also a promising strategy for patients with advanced HCC. In addition, most patients were Child-Pugh A, which may have led to more favorable outcomes as compared to the general population. Finally, ECOG status remains a highly subjective measurement with clinical variability.
In conclusion, TACE with hepatic infusion of oxaliplatin and 5-FU and Lipiodol embolization may be considered as a safe and promising treatment for patients with HCCs larger than 10 cm. Although systemic chemotherapy is usually recommended for advanced-stage patients, certain TACE regimens may be considered as adjuvant or sole therapies in a select group of patients.
The prognosis of hepatocellular carcinoma (HCC) is poor due, and only a small portion (20%-30%) of patients are eligible for curative treatments, such as resection, liver transplantation, and local ablation. Patients with unresectable HCC are typically treated with transarterial chemoembolization (TACE) or systemic therapy, and the long-term survival is far more unsatisfactory.
TACE is the most widely used standard treatment for unresectable HCC. However, treatment of large HCCs (> 10 cm in diameter) with TACE is clinically challenging due to the low response rate and high rate of major complications.
Treatment of large unresectable HCCs is clinically challenging, thus we tested the safety and efficacy of a certain TACE modality that included a combination of oxaliplatin and 5-fluorourcil infusion followed by embolization with a mixture of mitomycin and Lipiodol for these patients. The results show that this modality is a promising treatment for certain patients with large HCCs.
TACE with hepatic infusion of oxaliplatin and 5-fluorouracil and Lipiodol embolization may be a safe and promising treatment for patients with HCCs larger than 10 cm in diameter. Although systemic chemotherapy is usually recommended for advanced-stage patients, certain TACE regimens may be considered as adjuvant or sole therapies in a select group of patients.
Chemoembolization is a local regional therapy for unresectable HCC based on the principle of exposure of the HCC to a high concentration of chemotherapeutic agents via tumor feeding artery infusion followed by embolization of this artery, and the therapeutic benefit is achieved due to tumor necrosis.
There are many studies assessing the role of TACE for unresected HCC. However, this study focuses on HCC with a size larger than 10 cm. This subgroup of HCC is not common in Western countries and would be less in China due to the early diagnosis via regular screening. Thus the data provided by the manuscript is valuable.
P- Reviewer: Fu Q, Marcos R S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Liu XM
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25182] [Article Influence: 1937.1] [Reference Citation Analysis (3)] |
| 2. | Takayasu K. Transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: recent progression and perspective. Oncology. 2013;84 Suppl 1:28-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 383] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 4. | Cohen MJ, Bloom AI, Barak O, Klimov A, Nesher T, Shouval D, Levi I, Shibolet O. Trans-arterial chemo-embolization is safe and effective for very elderly patients with hepatocellular carcinoma. World J Gastroenterol. 2013;19:2521-2528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;CD004787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 6. | Cabibbo G, Tremosini S, Galati G, Mazza G, Gadaleta-Caldarola G, Lombardi G, Antonucci M, Sacco R. Transarterial chemoembolization and sorafenib in hepatocellular carcinoma. Expert Rev Anticancer Ther. 2014;14:831-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Dufour JF, Bargellini I, De Maria N, De Simone P, Goulis I, Marinho RT. Intermediate hepatocellular carcinoma: current treatments and future perspectives. Ann Oncol. 2013;24 Suppl 2:ii24-ii29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1063] [Cited by in F6Publishing: 1115] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 9. | Song MJ, Chun HJ, Song do S, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang UI, Yang JM. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244-1250. [PubMed] [Cited in This Article: ] |
| 10. | Kim DY, Park BJ, Kim YH, Han KH, Cho SB, Cho KR, Uhm SH, Choe JG, Choi JY, Chun HJ. Radioembolization With Yttrium-90 Resin Microspheres in Hepatocellular Carcinoma: A Multicenter Prospective Study. Am J Clin Oncol. 2013;Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 11. | Lintia-Gaultier A, Perret C, Ansquer C, Eugène T, Kraeber-Bodéré F, Frampas E. Intra-arterial injection of 131I-labeled Lipiodol for advanced hepatocellular carcinoma: a 7 years’ experience. Nucl Med Commun. 2013;34:674-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Rathore R, Safran H, Soares G, Dubel G, McNulty B, Ahn S, Iannitti D, Kennedy T. Phase I study of hepatic arterial infusion of oxaliplatin in advanced hepatocellular cancer: a brown university oncology group study. Am J Clin Oncol. 2010;33:43-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, Yang TS, Bhudhisawasdi V, Kang WK, Zhou Y. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 331] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 14. | Paul SB, Gamanagatti S, Sreenivas V, Chandrashekhara SH, Mukund A, Gulati MS, Gupta AK, Acharya SK. Trans-arterial chemoembolization (TACE) in patients with unresectable Hepatocellular carcinoma: Experience from a tertiary care centre in India. Indian J Radiol Imaging. 2011;21:113-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Hiraoka A, Horiike N, Yamashita Y, Koizumi Y, Doi H, Yamamoto Y, Ichikawa S, Hasebe A, Yano M, Miyamoto Y. Risk factors for death in 224 cases of hepatocellular carcinoma after transcatheter arterial chemoembolization. Hepatogastroenterology. 2009;56:213-217. [PubMed] [Cited in This Article: ] |
| 16. | Kim DY, Ryu HJ, Choi JY, Park JY, Lee DY, Kim BK, Kim SU, Ahn SH, Chon CY, Han KH. Radiological response predicts survival following transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:1343-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Zhou YM, Li B, Xu DH, Yang JM. Safety and efficacy of partial hepatectomy for huge (≥10 cm) hepatocellular carcinoma: a systematic review. Med Sci Monit. 2011;17:RA76-RA83. [PubMed] [Cited in This Article: ] |
| 18. | European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4059] [Cited by in F6Publishing: 4345] [Article Influence: 362.1] [Reference Citation Analysis (2)] |
| 19. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6338] [Article Influence: 487.5] [Reference Citation Analysis (1)] |
| 20. | Jang HJ, Kim BC, Kim HS, Kim JH, Song HH, Kim JB, Park JJ, Yoon SN, Woo JY, Zang DY. Comparison of RECIST 1.0 and RECIST 1.1 on computed tomography in patients with metastatic colorectal cancer. Oncology. 2014;86:117-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 391] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 22. | Ye T, Wang YH, Xia JL, Yang BW, Chen Y, Ge NL, Gan YH, Wang YH, Ren ZG. [Evaluation of the efficacy and prognostic factors for colorectal liver metastases treated with transcatheter arterial chemoembolization]. Zhonghua Zhongliu Zazhi. 2012;34:706-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 23. | Voigt W, Behrmann C, Schlueter A, Kegel T, Grothey A, Schmoll HJ. A new chemoembolization protocol in refractory liver metastasis of colorectal cancer--a feasibility study. Onkologie. 2002;25:158-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Poggi G, Amatu A, Montagna B, Quaretti P, Minoia C, Sottani C, Villani L, Tagliaferri B, Sottotetti F, Rossi O. OEM-TACE: a new therapeutic approach in unresectable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol. 2009;32:1187-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Poggi G, Quaretti P, Minoia C, Bernardo G, Bonora MR, Gaggeri R, Ronchi A, Saluzzo CM, Azzaretti A, Rodolico G. Transhepatic arterial chemoembolization with oxaliplatin-eluting microspheres (OEM-TACE) for unresectable hepatic tumors. Anticancer Res. 2008;28:3835-3842. [PubMed] [Cited in This Article: ] |
| 26. | Jang ES, Yoon JH, Chung JW, Cho EJ, Yu SJ, Lee JH, Kim YJ, Lee HS, Kim CY. Survival of infiltrative hepatocellular carcinoma patients with preserved hepatic function after treatment with transarterial chemoembolization. J Cancer Res Clin Oncol. 2013;139:635-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Gomes AS, Rosove MH, Rosen PJ, Amado RG, Sayre JW, Monteleone PA, Busuttil RW. Triple-drug transcatheter arterial chemoembolization in unresectable hepatocellular carcinoma: assessment of survival in 124 consecutive patients. AJR Am J Roentgenol. 2009;193:1665-1671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Kadalayil L, Benini R, Pallan L, O’Beirne J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24:2565-2570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 29. | Kim KM, Kim JH, Park IS, Ko GY, Yoon HK, Sung KB, Lim YS, Lee HC, Chung YH, Lee YS. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol. 2009;24:806-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Kalva SP, Pectasides M, Liu R, Rachamreddy N, Surakanti S, Yeddula K, Ganguli S, Wicky S, Blaszkowsky LS, Zhu AX. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2014;37:381-387. [PubMed] [Cited in This Article: ] |
| 31. | Cheng AL, Guan Z, Chen Z, Tsao CJ, Qin S, Kim JS, Yang TS, Tak WY, Pan H, Yu S. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. 2012;48:1452-1465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 32. | Zaanan A, Williet N, Hebbar M, Dabakuyo TS, Fartoux L, Mansourbakht T, Dubreuil O, Rosmorduc O, Cattan S, Bonnetain F. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: a large multicenter AGEO study. J Hepatol. 2013;58:81-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 549] [Cited by in F6Publishing: 630] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 34. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 732] [Cited by in F6Publishing: 813] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 35. | Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F, Carnaghi C, Pedicini V, Giordano L, Santoro A. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57:101-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 36. | Vora SR, Zheng H, Stadler ZK, Fuchs CS, Zhu AX. Serum alpha-fetoprotein response as a surrogate for clinical outcome in patients receiving systemic therapy for advanced hepatocellular carcinoma. Oncologist. 2009;14:717-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Zhao Y, Cai G, Zhou L, Liu L, Qi X, Bai M, Li Y, Fan D, Han G. Transarterial chemoembolization in hepatocellular carcinoma with vascular invasion or extrahepatic metastasis: A systematic review. Asia Pac J Clin Oncol. 2013;9:357-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |