Published online Jan 7, 2015. doi: 10.3748/wjg.v21.i1.373
Peer-review started: April 14, 2014
First decision: May 13, 2014
Revised: June 12, 2014
Accepted: July 11, 2014
Article in press: July 11, 2014
Published online: January 7, 2015
Processing time: 268 Days and 19.4 Hours
Herein, we report a new technique that consists of placing two 125I seed strands and two stents in the right and left intrahepatic bile ducts for the treatment of hilar cholangiocarcinoma. A 75-year-old man presented with jaundice and was diagnosed with Bismuth type IV Klatskin tumor. Abdominal computed tomography (CT) showed intrahepatic and extrahepatic bile duct dilatation and a soft tissue mass in the hepatic hilum. Because curative surgical resection was not possible, we placed 125I seed strands and stents in the right and left intrahepatic bile ducts. Three months later, abdominal CT showed less intrahepatic and extrahepatic bile duct dilatation than before the procedure. This technique was feasible and could be considered for the treatment of patients with Bismuth type IV tumors.
Core tip: Treatment of Klatskin tumor remains difficult. Although brachytherapy using 125I seed strands was reported for treatment of cholangiocarcinoma, to our knowledge, the use of 125I seed strands has seldom been reported for the treatment of type IV Klatskin tumors. Herein, we describe a patient with a Bismuth type IV Klatskin tumor who was treated via the placement of two 125I seed strands and two stents in the right and left intrahepatic bile ducts, which provided both brachytherapy and biliary drainage. This technique may provide a novel method for prolonging survival in these patients.
- Citation: Zhang W, Yang ZQ, Shi HB, Liu S, Zhou WZ, Zhao LB. Placement of 125I seed strands and stents for a type IV Klatskin tumor. World J Gastroenterol 2015; 21(1): 373-376
- URL: https://www.wjgnet.com/1007-9327/full/v21/i1/373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i1.373
The treatment of hilar cholangiocarcinoma (Klatskin tumor) remains difficult, particularly in Bismuth type IV tumors that have invaded the right and left intrahepatic bile ducts[1]. Malignant bile duct obstruction is often treated through surgery, the placement of metal stents, or radiation therapy. Brachytherapy with the implantation of 125I seeds has been reported for the treatment of hepatocellular carcinoma with intrahepatic portal vein tumor thrombus[2], and 125I seed strands have been used to treat tumor thrombus in the main portal vein[2]. Chen et al[3,4] reported that the placement of 125I seeds was a safe method for treating cholangiocarcinoma. However, to our knowledge, the use of 125I seed strands has seldom been reported for the treatment of type IV Bismuth tumors. Herein, we describe a patient with a type IV Bismuth tumor who was treated via the placement of 125I seed strands and stents in the right and left intrahepatic bile ducts.
Our institutional review board approved the reporting of this case. In July 2013, a 75-year-old man was admitted to the interventional radiology department with a 2-wk history of jaundice and upper abdominal pain. He was diagnosed with hilar cholangiocarcinoma based on abdominal computed tomography (CT) and magnetic resonance imaging (MRI) findings. There was marked dilatation of the intrahepatic bile ducts, although the patient’s vital signs were normal and the physical examination was unremarkable except for jaundice. Laboratory testing showed a total bilirubin (TBIL) level of 151.7 μmol/L (8.9 mg/dL) and a direct bilirubin (DBIL) level of 99.4 μmol/L (5.8 mg/dL).
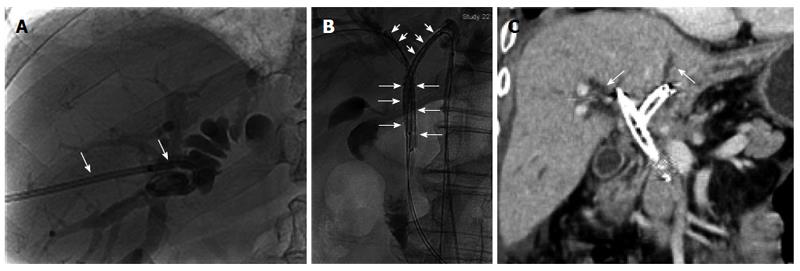
After obtaining informed consent, we performed percutaneous transhepatic biliary drainage and cholangiography via the right intrahepatic bile duct on the second day after admission. A type IV Bismuth tumor was diagnosed based on the cholangiography findings (Figure 1A). An 8.5-F catheter (Cook, Bloomington, IL, United States) was placed for external drainage, and the cylindrical titanium capsules of the 125I seeds (Xingke Company, Shanghai, China) were 0.8 mm in diameter and 4.5 mm in length, as previously described[2].
Under local anesthesia, the dilated left intrahepatic bile duct was punctured transhepatically using a 22-gauge Chiba needle (Cook, Bloomington, IL, United States) under fluoroscopic guidance. After confirmation of placement in the biliary tract, a 0.018-inch wire (Cook, Bloomington, IL, United States) was advanced into the left intrahepatic bile duct, and a 6-F sheath (Neff set, Cook) was introduced over the wire. After wire exchange, the 6-F sheath was replaced with an 8-F sheath (Cordis, United States). Two 0.035-inch, 260-cm-long stiff wires (Terumo, Tokyo, Japan) were then passed through the obstructed segment of the left intrahepatic duct and the common bile duct into the duodenum, with the guidance of a 5-F H1 catheter via the 8-F sheath. The 8.5-F external drainage catheter in the right intrahepatic bile duct was removed and replaced with an 8-F sheath over a 0.035-inch wire. Two 0.035-inch, 260-cm-long stiff wires were then passed through the obstructed segment of the right intrahepatic bile duct and the common bile duct into the duodenum via the 8-F sheath. According to the length of the bile duct obstructions, we placed 12 125I seeds in one 4-F sterile catheter and 13 in another catheter to construct 125I seed strands. The ends of the 4-F catheter were closed using 5-0 sutures. The 8-F sheaths in the right and left intrahepatic bile ducts were removed, leaving the wires in place, and a 5-F, 30-cm sheath and stent delivery system was introduced via the common bile duct into each of the right and left intrahepatic bile ducts (Figure 1B). In each of the right and left intrahepatic bile ducts, the stent was deployed across the obstructed segment, and a 125I seed strand was placed through the 5-F sheath. When the sheath was withdrawn, the radioactive seed strand was fixed in place between the stent and the bile duct. Finally, the transhepatic puncture was occluded with a sponge.
The patient refused further medical treatment after the procedure for personal reasons. Three months later, abdominal CT showed less intrahepatic and extrahepatic bile duct dilatation than before the procedure and the presence of biliary pneumatosis and peritoneal metastasis (Figure 1C). In addition, laboratory testing showed a TBIL level of 57.0 μmol/L (3.3 mg/dL) and a DBIL level of 41.0 μmol/L (2.4 mg/dL).
Patients with hilar cholangiocarcinoma have a poor prognosis, with a life expectancy of less than 3 mo if they do not receive aggressive treatment[1,5]. Radical surgical resection represents an effective treatment for hilar cholangiocarcinoma.
We encountered a patient with a type IV Bismuth tumor that was too advanced for surgical resection. He was treated via the placement of 125I seed strands and self-expandable metal stents in the right and left intrahepatic bile ducts, which relieved the biliary tract obstruction, and treated the tumor with brachytherapy.
Kroger et al[6] reported that sustained low-dose irradiation causes cell death by apoptosis and that this type of low-dose irradiation slows tumor growth. In particular, the placement of 125I seed strands in the bile ducts delivers sustained irradiation to the tumor and can oxygenize hypoxic cells, which makes the tumor cells more sensitive to irradiation.
The radiation dose delivered by 125I seeds is inversely proportional to the distance from the seeds. The seeds can deliver a high radiation dose to the target tissues that drops rapidly with the distance from the seeds, thereby resulting in low radiation doses to the surrounding tissues. As a result, the use of 125I seeds enables the delivery of a high radiation dose to tumor tissues while minimizing injury to normal tissues. The half-life of the 125I seeds used in our patient was 59.4 d, and this long half-life resulted in increased injury to the tumor compared with the use of external irradiation. However, the 125I seed strands could not be replaced or removed after isotopic radiation energy attenuation in half a year.
Although there was a concern that the stents and seed strands would obstruct the bile ducts, this did not occur. Thus, we consider this technique to be an effective method for ensuring effective biliary drainage. The biliary pneumatosis observed after the procedure may have been caused by biliary tract infection, tumor cell necrosis, or damage to the normal hepatic tissues. Because the patient refused further medical treatment, we were unable to obtain more information about the long-term results of treatment. There is currently no standard guideline regarding the number of seeds that should be placed according to the tumor size.
In conclusion, the placement of two 125I seed strands and two stents in the right and left intrahepatic bile ducts represents a feasible treatment for patients with type IV Bismuth tumors, providing both brachytherapy and biliary drainage. Thus, this technique may provide a novel method for prolonging survival in these patients.
A 75-year-old man who presented with jaundice and upper abdominal pain was diagnosed with a Bismuth type IV Klatskin tumor. The placement of two 125I seed strands and two stents in the right and left intrahepatic bile ducts was shown to be feasible for this patient, providing both brachytherapy and biliary drainage.
Malignant obstructive jaundice.
Gallbladder carcinoma, choledocholithiasis, and primary sclerosing cholangitis.
The total bilirubin level was 151.7 μmol/L(8.9 mg/dL), and the direct bilirubin level was 99.4 μmol/L(5.8 mg/dL).
Computed tomography showed bile duct dilatation and a soft tissue mass in the hepatic hilum, and the cholangiography suggested a type IV Bismuth tumor.
Pathological examination was not performed.
125I seed strands and stents were placed in the intrahepatic left and right bile ducts.
The placement of two 125I seed strands and two stents in the right and left intrahepatic bile ducts was shown to be feasible for relieving the jaundice caused by a Bismuth type IV cholangiocarcinoma.
This article describes a new technique involving the placement of 125I seed strands and stents in the right and left intrahepatic bile ducts for treating a patient with hilar cholangiocarcinoma. This approach was feasible for the treatment of Bismuth type IV tumors and may therefore provide a new, effective, and low-risk technique for the treatment of type IV Bismuth tumors.
P- Reviewer: Di Costanzo GG, Tziomalos K, Zhu F S- Editor: Ding Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Ramia JM. Hilar cholangiocarcinoma. World J Gastrointest Oncol. 2013;5:113-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Luo J, Yan Z, Liu Q, Qu X, Wang J. Endovascular placement of iodine-125 seed strand and stent combined with chemoembolization for treatment of hepatocellular carcinoma with tumor thrombus in main portal vein. J Vasc Interv Radiol. 2011;22:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Chen Y, Wang XL, Yan ZP, Wang JH, Cheng JM, Gong GQ, Li GP. Damage to pig bile duct caused by intraluminal brachytherapy using a (125)I ribbon. Acta Radiol. 2013;54:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Chen Y, Wang XL, Yan ZP, Wang JH, Cheng JM, Gong GQ, Luo JJ. The use of ¹²5I seed strands for intraluminal brachytherapy of malignant obstructive jaundice. Cancer Biother Radiopharm. 2012;27:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Iwatsuki S, Todo S, Marsh JW, Madariaga JR, Lee RG, Dvorchik I, Fung JJ, Starzl TE. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 1998;187:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 169] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Kroger LA, DeNardo GL, Gumerlock PH, Xiong CY, Winthrop MD, Shi XB, Mack PC, Leshchinsky T, DeNardo SJ. Apoptosis-related gene and protein expression in human lymphoma xenografts (Raji) after low dose rate radiation using 67Cu-2IT-BAT-Lym-1 radioimmunotherapy. Cancer Biother Radiopharm. 2001;16:213-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |